Abstract
Plasmodium falciparum infections remain among the leading causes of morbidity and mortality in holoendemic transmission areas. Located within region 5q31.1, the colony-stimulating factor 2 gene (CSF2) encodes granulocyte–macrophage colony-stimulating factor (GM-CSF), a hematopoietic growth factor that mediates host immune responses. Since the effect of CSF2 variation on malaria pathogenesis remains unreported, we investigated the impact of two genetic variants in the 5q31.1 gene region flanking CSF2:g-7032 G > A (rs168681:G > A) and CSF2:g.64544T > C (rs246835:T > C) on the rate and timing of malaria and severe malarial anemia (SMA, Hb < 5.0 g/dL) episodes over 36 months of follow-up. Children (n = 1654, aged 2–70 months) were recruited from a holoendemic P. falciparum transmission area of western Kenya. Decreased incidence rate ratio (IRR) for malaria was conferred by inheritance of the CSF2:g.64544 TC genotype (P = 0.0277) and CSF2 AC/GC diplotype (P = 0.0015). Increased IRR for malaria was observed in carriers of the CSF2 AT/GC diplotype (P = 0.0237), while the inheritance of the CSF2 AT haplotype increased the IRR for SMA (P = 0.0166). A model estimating the longitudinal risk of malaria showed decreased hazard rates among CSF2 AC haplotype carriers (P = 0.0045). Investigation of all-cause mortality revealed that inheritance of the GA genotype at CSF2:g-7032 increased the risk of mortality (P = 0.0315). Higher risk of SMA and all-cause mortality were observed in younger children (P < 0.0001 and P = 0.0015), HIV-1(+) individuals (P < 0.0001 and P < 0.0001), and carriers of HbSS (P = 0.0342 and P = 0.0019). Results from this holoendemic P. falciparum area show that variation in gene region 5q31.1 influences susceptibility to malaria, SMA, and mortality, as does age, HIV-1 status, and inheritance of HbSS.
Similar content being viewed by others
Introduction
In 2020, the estimated number of malaria cases reported worldwide was 241 million [1]. A large proportion (95%; 228 million) of the cases occurred in the African region, mainly attributed to Plasmodium falciparum (P. falciparum) infections (> 99.8% of the total cases) [1]. Globally, there were ~ 627,000 malaria-related deaths, for which the most vulnerable population were children under 5 years of age [1]. Accordingly, 77% of the global malaria mortalities were children residing in the World Health Organization (WHO) African region [1]. In western Kenya, a region holoendemic for P. falciparum transmission, malaria is one of the primary causes of childhood morbidity and mortality [2, 3]. In this region, severe P. falciparum infections manifest as severe malarial anemia [SMA, hemoglobin (Hb) < 5.0 g/dL] [4, 5]. Our previous investigations in the study area show that HIV-1 significantly increases the cross-sectional risk of SMA [6].
The pathophysiology of SMA is complex, and includes the destruction of malaria parasite-infected and non-infected erythrocytes, as well as decreased erythropoiesis [7]. We have demonstrated that variability in genes that encode immune-response proteins plays a key role in the pathogenesis of SMA, largely through imparting changes in soluble mediators of inflammation [8]. However, the causal molecular basis of the disease has not been fully elucidated.
Granulocyte monocyte-colony stimulating factor (GM-CSF) is a hematopoietic growth factor that facilitates the differentiation of progenitor cells into three lineages in the bone marrow, namely the lymphoid, myeloid, and erythroid progenies [9,10,11]. GM-CSF has been shown to promote growth and differentiation of leucocytes, and enhance release of other cytokines, which are central mediators of host immune responses [12, 13]. GM-CSF is secreted by an array of cell types including mast cells, B cells, activated T cells, fibroblasts, macrophages, vascular endothelial cells, and various oncogenic cells [9]. Secretion of GM-CSF is often accompanied by the release of additional inflammatory mediators, such as the granulocyte colony-stimulating factor (G-CSF) [10, 12], which in turn, modulate GM-CSF production through feedback regulatory mechanisms [14, 15].
The importance of GM-CSF in host immune response to both non-infectious and infectious diseases has been reported for tumor growth and metastasis, Crohn’s disease (CD), and tuberculosis [16,17,18]. With regard to malaria, studies utilizing murine models have reported: (1) a reduction in the levels of erythropoietic-related cytokines, including GM-CSF; (2) a negative correlation between GM-CSF concentrations and enhanced pathology in malarial anemia; and (3) elevated levels of GM-CSF in lethal malaria [19, 20]. In the context of human malaria, the toll-like receptors (TLR) 7/8 stimulated production of GM-CSF was elevated in cord blood cells of infants with evidence of past placental malaria [21], suggesting a profound effect on the fetal immune system, with the differential alternations in innate immune responses predicting the risk of malaria during the first year of life [21]. Moreover, elevated serum levels of GM-CSF have been reported in cases of severe P. falciparum malaria [22]. Previous investigations in our laboratories identified elevated levels of GM-CSF in children with SMA compared to those with non-SMA, and elevated GM-CSF levels in children with P. falciparum and HIV-1 co-infection relative to children with malaria alone [23, 24].
The gene that encodes GM-CSF is colony-stimulating factor 2 (CSF2), which is located on the human chromosome at 5q23-31, in close proximity to interleukin 3 (IL3) within a T helper type 2-associated gene cluster [25]. CSF2 spans ~ 2.5 kb in length, and encompasses 4 exons and 3 introns [26]. CSF2 expression is regulated at both the transcriptional and post-transcriptional stages [27,28,29]. Elements in the proximal promoter of CSF2 were shown to contribute to transcriptional regulation of CSF2 by multiple transcription factors such as the AP-1, ZEB1, NF-AT, and GATA [27,28,29]. At the post-transcriptional regulation level, a stretch of AU-rich elements (ARE) in the 3′ untranslated region (3′-UTR) of CSF2 mRNA mediates the binding of ARE-binding proteins to CSF2 mRNA, causing degradation of this transcript [28, 29]. Interestingly, various distal enhancers were shown to modulate transcription of human target genes such as CCR5, BRN3A, CDKN1C, VEGFA, ADGB, C-myb, SOX-9, SCNA, C-myc, cPLA2α gene (PLA2G4A) and renin gene (REN) by forming cognate enhancer–promoter loops with the target gene promoter site [30,31,32,33,34,35,36]. Some of these distal enhancers are located at varied distances upstream of the transcription start site (TSS) of the corresponding gene: ~ 552 kb for CCR5, ~ 55 kb for BRN3A, over 100 kb for CDKN1C, ~ 157 kb for VEGFA, ~ 34 kb for C-myb, ~ 635 kb for SOX-9, ~ 9.5 kb for PLA2G4A, and ~ 5.3 kb for REN [31, 32, 34, 35]. In contrast, other distal enhancers are located at varied distances downstream of corresponding TSS of the gene: 216 kb for ADGB, 85 kb for SCNA, and 1.43 Mb for C-myc [33, 36]. Collectively, these studies show that the enhancers can be located either up- or downstream at varied distances of target genes.
A number of studies have found associations between polymorphic variability in the CSF2 gene and outcomes of various disease [37,38,39,40,41,42], while other studies observed no relationships [43, 44]. In order to understand the influence of CSF2 variants on susceptibility to malaria and SMA, the current study investigated the impact of two SNPs flanking the CSF2:g.-7032 G > A (rs168681:G > A) and CSF2:g.64544T > C (rs246835:T > C). The first SNP, rs168681:G > A is about 6 kb upstream of the 1 kb proximal promoter of CSF2 and has recently emerged among the genetic biomarkers for prediction of the urinary nicotine metabolite ratio in multiethnic samples [45]. The second SNP, rs246835:T > C has not been linked to any diseases. We selected these two SNPs based on their characteristics of (1) minor allele frequencies (MAF) ≥ 10% in African populations and (2) the ability to impart functional changes in transcription-factor binding sites (TFBS) within corresponding distal enhancers [27, 28]. The relationship between the SNPs (and their haplotypes and diplotypes) on susceptibility to malaria, SMA and mortality were investigated longitudinally over a 36-month follow-up in a cohort of children (n = 1654; age 2–70 months at enrollment) resident in a P. falciparum holoendemic region of Kenya.
Methods
Study area
This study was conducted at Siaya County Referral Hospital (SCRH), located in a holoendemic P. falciparum transmission area in western Kenya. Details of the study site have previously been published [46]. One of the primary causes of childhood mortality and morbidity in the Siaya community is P. falciparum malaria [47], whose transmission has remained stable, despite malaria control efforts [48]. SMA accounts for one of the highest percentages of hospital-bed occupancies in SCRH, and results in significant in-hospital morbidity and mortality [4]. Individuals inhabiting the study area are predominantly from the Luo ethnic group (> 96%), a culturally homogeneous population [49].
Study participants
Children presenting with suspected malaria infections and those reporting for routine vaccinations were recruited at SCRH. After screening for malaria parasites, children (aged 2–70 months) with varying severities of malarial anemia (n = 1319) and aparasitemic controls (n = 335) were enrolled. Children were excluded from the study if they presented with non-falciparum parasite strains, had confirmed cerebral malaria, were previously hospitalized for any reason, or had reported use of antimalarial therapy in the two preceding weeks. The current study utilized two cohorts recruited and followed with identical parameters across a temporal continuum: cohort 1 (2003–2005; n = 777) and cohort 2 (2007–2012; n = 877).
Longitudinal follow-ups
After enrollment (Day 0), children (n = 1654) were scheduled for follow-up visits on day 14 (if they were febrile upon enrollment) and quarterly over 36 months [50,51,52]. Parents/guardians who failed to return for scheduled quarterly follow-up visits were traced by the study team at their residence to check the child’s health status, including mortality. Each residence was identified by a GIS/GPS surveillance system. In addition, parents/guardians were asked to return to the hospital any time their child was febrile [acute febrile episode(s)]. Physical evaluations and laboratory tests required for comprehensive clinical management of the patients were performed at enrollment, day 14, and each acute and quarterly visit (complete blood counts [CBC], malaria parasitemia measures, and evaluation of bacteremia where indicated). For all acute episodes and scheduled visits, children were managed according to the Ministry of Health-Kenya guidelines.
Laboratory procedures
Heel and/or finger-prick blood samples (< 100 µL) and venipuncture blood (1–3 mL) were obtained and used to determine parasite densities. Giemsa-stained thin blood smears were prepared, examined, and asexual malaria parasite densities determined as previously published [46]. Complete blood counts (CBCs) were determined using a Beckman-Coulter AcT diff2 (Beckman-Coulter Corporation, Brea, CA, USA). At enrollment, based on Hb concentrations, children with any density parasitemia, were stratified into either non-SMA (Hb ≥ 5.0 g/dL; n = 1029) or SMA (Hb < 5.0 g/dL; n = 290), according to the WHO definition of SMA [53]. Aparasitemic children (n = 335; P. falciparum negative blood smear), recruited from vaccination clinics at SCRH, were age and gender-matched.
Since co-infections influence the severity of malarial anemia in Siaya, all children were tested for HIV-1 and bacterial infections as previously described [6, 54]. Parents/legal guardians of participating children received pre- and post-test HIV&AIDS counseling. At the time of enrollment, none of the children were on antiretroviral therapy for HIV-1.
To further discern chronic anemia resulting from genetic factors, sickle cell traits and α3.7-thalassemia deletions were investigated. Sickle cell status was determined using the alkaline cellulose acetate electrophoresis on Titan III plates (Helena BioSciences, Sunderland, UK). The α3.7-thalassemia deletion variants were determined as previously described [55].
CSF2 genotyping
The BuccalAmp™ DNA extraction kit (Epicentre Biotechnologies, Madison, WI, USA) was used to extract genomic DNA from buccal swabs collected from each of the 1654 study participants described above. The resulting genomic DNA was then amplified using the Genomiphi DNA amplification kit (GE Healthcare, Life Sciences, Marlborough, MA, USA), according to the manufacturer’s instructions. Genetic variants rs168681:G > A and rs246835:T > C were genotyped using the TaqMan 5′ allelic discrimination Assay-By-Design (assay IDs C_3285157_20 and C_2397167_10, respectively). Allelic discrimination was performed using the StepOne™ Software Version 2.3 (Thermo Fisher Scientific, Carlsbad, CA, USA).
Amplification was conducted in a total reaction volume of 20 µL, with the following conditions: initial denaturation at 60 °C for 30 s and 95 °C for 10 min., followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min., and a final extension (60 °C for 30 s). To validate results obtained with the Taqman® real-time genotyping assays, 10% of the samples were randomly selected and genotyped using restriction fragment length polymorphism PCR. There was 100% agreement between the two methods.
Data analyses
Data were analyzed using R version 3.1.4 [56]. Data from both cohorts (1: 2003–2005, n = 777, and 2: 2007–2012, n = 877) were combined into one data set. However, cohort was kept as a categorical covariate to account for potential changing malaria incidence across time. Metric variables upon enrollment were compared across aparasitemic, non-SMA, and SMA patients. For metric variables, boxed plots, histograms, and Q–Q-plots were used to identify normally distributed variables. The median (interquartile range) and mean (standard deviation) were calculated for each variable. For normally distributed variables, one-way ANOVAs and two-sample T-tests were used. If normality was violated, Kruskal–Wallis tests and Mann–Whitney U tests were used. The distribution of categorical variables was compared between the groups (aparasitemic, non-SMA, and SMA) using a Chi-square test for homogeneity. If cell count was < 20, a generalized Fisher’s exact test was performed. If numerically not feasible, P-values were approximated using a Monte Carlo method, with B = 50,000 contingency tables.
Haplotypes and diplotypes were constructed to investigate the effects of the genetic architecture determined by the CSF2:g.-7032 G > A (rs168681:G > A) and CSF2:g.64544T > C (rs246835:T > C) loci. The top-level was the individual diplotypes, consisting of 2 of the 4 possible haplotypes; GT, GC, AT, and AC. At the marginal levels, the diplotypes gave rise to the (1-locus) genotypes at CSF2:g.-7032 G > A (GG, GA, AA) and CSF2:g.64544T > C (TT, TC, CC). For all generalized linear and hazard models performed, the effects of the genetic architecture were coded as 0/1 variables that reflected: (1) per locus contributions determined by the mutant homozygotes and heterozygotes (indicating additive and dominant effects), (2) haplotype contributions (reflecting epistatic interactions), and (3) diplotype contributions (reflecting positional effects). The respective wild types were subsumed as a baseline factor. Hence, we coded the following 0/1 variables: (1) genotypes GA, AA for CSF2:g.-7032 G > A and TC, CC for CSF2:g.64544T > C; (2) haplotypes GC, AT, AC; and (3) diplotypes GT/GC, GT/AT, GT/AC, GC/GC, GC/AC, AT/AT, AT/AC, AC/AC. We tested for differences in the frequency distributions between the aparasitemic, non-SMA, and SMA groups with the generalized Fisher’s exact test with simulated P-values. Exact tests for deviations from Hardy–Weinberg equilibrium (HWE) at the rs168681:G > A and the rs246835:T > C loci were performed as previously described [57]. Furthermore, due to low cell counts for some genotypes, Fisher’s exact test was performed to estimate departures from linkage-equilibrium between the two SNPs. Linkage disequilibrium (LD) of these two SNPs was determined by HaploView (version 4.2, Broad Institute, Cambridge, MA, USA).
The effect of covariates on the rate of malaria and SMA episodes was investigated using Poisson regression, with the model of best fit determined using Akaike’s information criterion (AIC). CSF2 genetic variants, age, sex, cohort, hemoglobinopathies (sickle cell traits and α3.7-thalassemia deletions), and co-infections (HIV-1 and bacteremia) were included as covariates. Only co-variables present in more than two samples were retained in the model.
An ordered multiple-outcome-per-subject Cox proportional hazard model was used to investigate the longitudinal influence of covariates (as listed above) on the occurrence of malaria and SMA episodes. Independent increments were used according to Anderson–Gill and data were right-censored to account for cases in which a patient was malaria or SMA free upon clinical presentation.
The influence of covariates on the risk of mortality was investigated using a Cox proportional hazard model. For each patient, either mortality or survival occurred at the last hospital visit. The last recorded hospital visit was the time to the event, or censoring, in the case of survival. Age (at the last hospital visit) was included as a covariate. The model was fit using all-cause mortality as the outcome variable.
Results
Demographic, clinical, and laboratory characteristics
Children (n = 1654) were categorized into aparasitemic (n = 335), and parasitemic (n = 1319) stratified into non-severe malarial anemia (non-SMA, hemoglobin; Hb ≥ 5.0 g/dL, n = 1029), and severe malarial anemia (SMA, Hb < 5.0 g/dL, n = 290) groups. The demographic, clinical, and laboratory characteristics of study participants are presented in Table 1. The distribution of sex was comparable (P = 0.6991) across the groups. Although the distribution of SMA cases were equal in cohort 1 (2003–2005) and cohort 2 (2007–2012), they differed significantly between aparasitemic and non-SMA groups (P = 5.5311 × 10–11). Children with SMA were younger (P = 0.0152) relative to aparasitemic and non-SMA groups, but the difference was not significant after correction for multiple testing. Axillary temperature differed across the groups and was higher in children with non-SMA (P = 1.4420 × 10–14), compared to those with parasitemia or SMA. Children were stratified a priori based on Hb concentrations. As such, relative to both the aparasitemic and non-SMA groups, children with SMA presented with significantly decreased hematocrit levels (P = 1.4732 × 10–174). White blood cell counts progressively increased across the groups (P = 4.3233 × 10–12), as did the mean corpuscular volume (P = 7.9395 × 10–21), with the SMA group having the highest levels (Table 1). Parasite densities were lower in children with SMA relative to non-SMA (P = 2.9626 × 10–25). The presence of HIV-1 did not significantly differ across groups (P = 0.0729), nor did bacteremia status (P = 0.1583). However, the distribution of sickle cell genotypes differed across groups (P = 1.9990 × 10–06), with a lower frequency of HbAS carriage in children with non-SMA and lowest frequencies among children with SMA. The distribution of α3.7-thalassemia deletion variants were also different across the groups (P = 0.0433), with the highest frequencies of single and double-deletions in children SMA, however the difference is not significant after correction for multiple testing.
Linkage disequilibrium
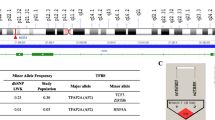
The location of the two SNPs selected for investigation in the CSF2 gene region (5q31.1) [58] is shown in Fig. 1a and b. The chromosomal location of rs168681:G > A is 5:132066757, 7032 bp upstream of TSS of CSF2, and 1279 bp downstream of LOC108449898. The chromosomal location of rs246835:T > C SNP is 5:132138332, 64544 bp downstream of the TSS of CSF2, 34642 bp downstream of LOC112997558, and 46544 bp upstream of the human P4HA2-AS1 gene [58] (Fig. 1a, b).
CSF2 (5q31.1) region, transcription factor binding sites and linkage disequilibrium. a and b Location of the CSF2 region on chromosome 5q31.1. CSF2 is 9379 bp and is composed of four exons. Chromosome position (build GRCh38.p13) for the selected SNPs under investigation; rs168681:G > A is located at 132066757, rs246835:T > C located at 132138332 [58]. c Linkage disequilibrium between the selected CSF2 SNPs rs168681:G > A and rs246835:T > C (D′ = 0.416, LOD = 4.32, r2 = 0.01). d CSF2 genotypes allele frequencies from the International HapMap project for Yoruba (YRI; Nigeria). Allele frequencies from the 1000 Genome project shows African (AFR) ancestry. Transcription factor binding analyses of the CSF2 gene variants. AFR African, YRI Yoruba, ER-alpha estrogen receptor alpha, ZEB1 zinc finger E-box binding homeobox 1, NF-X3 nuclear factor I-X3, USF2 upstream transcription factor 2, GATA GATA binding protein-1
Linkage disequilibrium analysis revealed a weak association between the two CSF2 variants (D′ = 0.416, LOD = 4.32, r2 = 0.01; Fig. 1c). Departures from linkage equilibrium (LE) were significant for the overall sample and stratified by aparasitemic, non-SMA, and SMA groups. However, the departure from LE was not significant in the SMA group after multiple testing, suggesting that these departures are marginal and only identified as significant due to the large sample size.
Selection of CSF2 SNPs
As recently described [50], a pilot high-throughput genotyping experiment was performed using the Human Immunochip® (coated with > 196 K markers, Illumina®, San Diego, CA, USA) in a subset of children representing “polarized extremes” of severe and non-severe malaria: cases (SMA with Hb < 5.0 g/dL, n = 70) vs. controls (non-SMA with Hb > 8.0 g/dL, n = 74). Immunochip® data were analyzed using logistic regression analysis, with an additive mode of inheritance and identified an intergenic SNP rs246835:T > C within the CSF2 haploblock that was associated with increased risk to SMA [odds ratio (OR) = 2.771, 95% confidence interval (CI) = 0.904–8.494, P = 0.059]. We selected rs246835:T > C since this SNP is part of a potential distal enhancer with the loss of TFBS for USF2 and creation of TFBS for GATA-1 by switch of T allele to C allele (Fig. 1d). rs246835:T > C had a minor allele frequency (MAF) of 0.10 in the African (AFR) population (1000 Genome Project), and a MAF of 0.14 in the Yoruban (YRI) population (HapMap) (Fig. 1d). Though not in the Immunochip®, SNP rs168681:G > A had desirable characteristics for further exploration, including: (1) a MAF of 0.20 (1000 Genome Project) in the AFR population and a MAF of 0.18 in the YRI population (HapMap), and (2) potential functional effects, where the wild-type G allele creates TFBS for ER-alpha and ZEB1, and transition to the minor A allele ablates such binding and creating a TFBS for NF-X3. These TFBSs are part of a potential distal enhancers (Fig. 1d).
Distribution of the CSF2 genotypes, haplotypes, and diplotypes
Following genotyping, SNP calling was successful for rs168681:G > A and rs246835:T > C variants, with both genotypes present in 1203 out of 1654 study participants recruited into cohorts 1 and 2. To increase the power of analysis, no imputations were performed to infer genotypes, haplotypes or diplotypes. As such, only data with both SNPS present for each participant were utilized for subsequent statistical analyses (Table 2).
The distribution of CSF2 genotypes, haplotypes, and diplotypes in aparasitemic, non-SMA, and SMA groups is presented in Table 2. The genotypic proportions for rs168681:G > A (P = 0.9972), and rs246835:T > C (P = 0.2202) were comparable across the groups. The allele frequencies for the study population is comparable to those from the International HapMap Project and 1000 Genomes Project (Fig. 1d). The HWE analyses showed a departure for the rs246835:T > C for the overall population, and in the aparasitemic, non-SMA, and SMA groups. There was no departure from HWE for the rs168681:G > A polymorphic variant (Table 2). The distribution of haplotypes (P = 0.5565), and diplotypes (P = 0.3255) were not significantly different across the study groups.
Relationship between CSF2 variants and the rate of malaria episodes
In malaria holoendemic regions, such as western Kenya, children experience multiple episodes of malaria from infancy onward, with occasional life-threatening complications of SMA before the development of naturally acquired malaria immunity [7]. We, therefore, investigated the impact of the CSF2 variants on malaria episodes over a follow-up period of 36 months. Factors associated with the rate of malaria episodes were determined using a generalized linear model (Poisson regression). Results for the covariates that emerged for the 6029 recorded malaria episodes over 36 months of follow-up are shown in Table 3. Age at enrollment was an important variable for the rate of malaria episodes, with older children presenting a decreased risk over time [incidence rate ratio (IRR; for a delta of 1 month) = 0.967 (95% CI 0.963–0.970), P < 0.0001)]. Children who were HIV-1 positive had a lower incidence rate of malaria episodes (P = 0.0023), relative to HIV-1 negative participants, as did female study participants (P = 0.0013), compared to their male counterparts. Carriage of the homozygous double deletion (-α3.7/-α3.7) for α+-thalassemia also conferred a protective effect against malaria episodes (P = 0.0068) relative to homozygous αα/αα carriers. Protection against multiple bouts of malaria over time was also associated with co-inheritance of homozygosity for the sickle cell disease (Hb SS, P < 0.0001) and heterozygosity for the sickle cell trait [Hb AS, (P < 0.0001). Protective effects against the longitudinal risk of malaria were also present in heterozygous carriers of the rs246835 TC genotype (P = 0.0277) relative to the wild type genotype TT. Similarly, the positional interaction of the CSF2 AC/GC diplotype was associated with decreased malaria episodes (P = 0.0015) compared to wild-type diplotype GT/GT. Conversely, co-inheritance of the CSF2 AT/GC diplotype increased the rate of malaria episodes (P = 0.0237). There was also a trend towards more malaria episodes in carriers of the CSF2 GC/GT diplotype, however, the trend was not significant (P = 0.0663) (Table 3).
Relationship between CSF2 variants and the rate of SMA episodes
The effect of the CSF2 variants on the rate of SMA episodes over a follow-up period of 36 months was investigated using a Poisson regression as described above. Factors associated with the rate of SMA for the 297 episodes that occurred over the 36 months of follow-up are shown in Table 3. Consistent with the results observed for susceptibility to malaria, children who were older at admission into the study had decreased incidence rates for SMA (P < 0.0001). Similarly, children who were recruited into cohort 2 (2007–2012) had lower incidences of SMA (P = 0.0013), relative to those enrolled in study cohort 1 (2003–2005). Unlike the protection observed against malaria in HIV-1-positive children, being positive for HIV-1 markedly increased the risk of SMA (P < 0.0001). Inheritance of the double mutation that confers sickle cell disease (Hb SS) increased the rate of SMA (P = 0.0024), whereas carriage of sickle cell trait (Hb AS) protected against SMA episodes (P = 0.0026). The only CSF2 variant found to influence the rate of SMA episodes was carriage of the CSF2 AT haplotype, which increased the risk of SMA episodes (P = 0.0166) compared to the wild-type GT haplotype.
Impact of CSF2 variants on the longitudinal risk of malaria infections
After determining the impact of CSF2 variants on the rate of malaria and SMA episodes, we then determined the effect of the variants on the time between events using an ordered-events, generalized Cox proportional hazard model. Shown in Table 4 are the covariates that emerged from the model. Children who were older upon enrollment had a reduced hazard risk (HR) for malaria (P < 0.0001). Although retained in the model, cohort (P = 0.1159), HIV-1 positivity (P = 0.1711), sex (P = 0.0580) and -α3.7/-α3.7 (P = 0.0874) were not significant predictors for the time between malaria infections (Table 4). However, inheritance of either the Hb SS mutant genotype (P < 0.0001) or the Hb AS variant (P = 0.0001) decreased the longitudinal hazard for malaria infections. Carriage of the CSF2 AC haplotype was also protective against malaria infections (P = 0.0045) relative to wild-type haplotype (GT), while co-inheritance of the CSF2 GC/GT diplotype showed a non-significant trend towards a lower risk of malaria (P = 0.1108) compared to wild type (GT/GT) (Table 4).
Impact of CSF2 variants on the longitudinal risk of SMA infections
Cox proportional hazard model was also used to examine the influence of CSF2 variants rs168681:G > A and rs246835:T > C on the time-to-event for malaria infections that culminated in the development of SMA (Table 4). Consistent with the Poisson models examining the rates of SMA, time-to-event modeling revealed that older children (at enrollment) had a lower risk of SMA (P < 0.0001, i.e., the hazard decreases by a factor of 0.954 for each additional month of enrollment age) as did children enrolled in cohort 2 (P = 0.0036). Co-infection with malaria and HIV-1 was a strong predictor of increased susceptibility to SMA (P < 0.0001), as was carriage of the Hb SS genotype (P = 0.0342). Conversely, inheritance of the Hb AS variant decreased the longitudinal risk of SMA (P = 0.0196). Although non-significant, there was a trend towards increased risk of SMA in children with the CSF2 AT haplotype (P = 0.0809).
Influence of CSF2 variants on the longitudinal risk of all-cause mortality
The influence of covariates on the risk of all-cause mortality was investigated using a Cox proportional hazard model (Table 4). Older children (at enrollment) had a reduced risk of mortality (P = 0.0015), whereas children with HIV-1 had a 16-times higher risk of mortality (P < 0.0001). Children with Hb SS also had a marked increase in the risk of mortality (P = 0.0019). Conversely, carriage of sickle cell trait (Hb AS) conferred a lower hazard risk for mortality (P = 0.0427). Inheritance of the rs168681 GA genotype significantly increased the risk of dying during the follow-up period (P = 0.0315), as did carriage of the CSF2 GC/GC diplotype, although not significant (P = 0.0592).
Discussion
Advances in human gene mapping, along with an increased understanding of the molecular mechanisms of protective immunity, illustrate that susceptibility to malaria and its clinical outcomes is conditioned by genotypic variation [8]. The work presented here is focused on gaining an improved understanding of the molecular basis of clinical immunity to SMA in pediatric populations living under intense P. falciparum transmission [8]. In such holoendemic regions, clinical immunity to malaria is mediated, at least in part, by the progressive generation of antibodies following repeated malaria infections [7, 24]. However, prior to the development of naturally acquired malarial immunity, children are reliant upon innate immune responses and can experience life-threatening complications that include severe anemia, hyperparasitemia, and respiratory distress [7, 8]. To better understand the pathogenesis of SMA, and as a consequence, reduce mortality, our studies primarily focus on genes and gene pathways involved in innate immunity. Consistent with this strategy, the current study investigated the influence of genetic variants flanking CSF2 (rs168681:G > A and rs246835:T > C) on the rate and timing of malaria and SMA over a 36-month longitudinal follow-up period during the developmental phase of naturally acquired malarial immunity. The impact of CSF2 gene variants on all-cause mortality was also investigated. Since other factors can influence the development of malaria, SMA, and mortality, we also determined the influence of important covariates in all statistical models.
Previous studies from our laboratories have shown that the host releases both pro- and anti-inflammatory cytokines, chemokines, growth factors, and effector molecules as part of the innate immune response to malaria infections [7, 8]. Among the growth factors is GM-CSF, which is highest among children with uncomplicated malaria relative to those with non-SMA and SMA [24]. However, children with SMA have higher levels of circulating GM-CSF than individuals with non-SMA, suggesting a complicated pattern of production during acute infection [24]. Studies by others have shown divergent results on clinical outcomes in malaria. For example, elevated GM-CSF levels are associated with severe malaria complications (i.e., splenomegaly and leukocytosis) in some investigations, while others have found a protective role for GM-CSF [59]. Recent studies suggest that the production of TLR 7/8-driven GM-CSF in cord blood is an independent predictor of enhanced malaria risk over the first year of life, suggesting that GM-CSF indeed plays an important role in malarial immunity [21]. To expand knowledge on the role of GM-CSF in malaria, we examined the impact of genetic variations around CSF2 on susceptibility to malaria and its severe disease manifestations (i.e., SMA and mortality) in children as they progressively develop immunity to clinical malaria.
Selection of the polymorphic variants was based on interrogating SNPs (and their combinations) that are previously unexplored in malaria and have the potential to impart functional changes on GM-CSF production. For example, the presence of the wild-type G allele at the rs168681:G > A locus within one distal enhancer produce TFBSs for ER-alpha and ZEB1, whereas transition to the A allele creates a binding site for NF-X3. Variation at rs246835:T > C locus within another distal enhancer of CSF2 has a TFBS for USF2 in the presence of the T allele and a TFBS for GATA-1 in carriers of the C allele. Previous studies have confirmed that CSF contains consensus elements for ZEB1 and GATA [27, 28]. Since transcription factors are adaptor molecules that detect regulatory sequences in the DNA and target the assembly of protein complexes that control gene expression, mutations within the transcription factor binding sites (TFBS) can alter gene expression [60]. The allele frequencies for the two SNPs in the study cohort were comparable to those in the Yoruba population (HapMap Project) and the African population (1000 Genome Project), suggesting that the two loci have been steadily maintained in ethnic groups of African descent [61]. The rs168681:G > A variant displayed HWE, whereas the rs246835:T > C locus had a significant departure from HWE in the overall population, and in each of the clinical groups investigated. Although a departure from HWE at rs246835:T > C locus could be attributed to historical pressure from malaria [62], there were comparable frequencies of the genotypes, as well as haplotypes and diplotypes in combination with rs168681:G > A across the clinical groups (i.e., aparasitemic, non-SMA, and SMA), suggesting an influence of a large sample size. This finding suggests that the two variants selected for investigation are likely not under strong selection pressure. The LD measures for the two loci with a D′ = 0.416 and r2 = 0.01 indicate that the two variants are not strongly linked [63].
Analysis of the interacting covariates revealed that the children’s age, cohort (2003–2005 vs 2007–2012), sex (female vs male), and HIV-1 status (+ vs −) altered the incidences and risk of malaria and SMA over the longitudinal follow-up of 36 months. Further, co-inheritance of genetic variants; α+-Thalassemia and/or sickle cell traits influenced longitudinal susceptibility to malaria and SMA infections and mortality outcomes. These results parallel those of previous studies that reported variability in the degree of protection conferred by hemoglobinopathies against mild and severe malaria (reviewed in [64]). In addition, the selected CSF2 homozygous and heterozygous variants (genotypes, haplotypes, and diplotypes) altered the incidence rate and hazard ratios to malaria and SMA over the follow-up period. Results presented here complement previous studies illustrating that children living in regions with high transmission rates for P. falciparum experience multiple infections prior to developing malarial immunity, with the greatest burden of severe disease manifesting in children less than 5 years of age [8, 50,51,52]. As such, longitudinal studies have a greater ability to detect genetic factors, and covariates that can influence both the rate and timing of repeated malaria episodes and the development of severe disease over time.
A Poisson regression model estimating the rate of malaria episodes over 36 months revealed that the presence of the rs246835 TC heterozygote genotype and CSF2 AC/GC diplotype decreased the incidence of malaria, while the CSF2 AT/GC diplotype increased the incidence of malaria episodes. Additional Poisson analysis identified significantly more episodes of SMA among children who inherited the CSF2 AT haplotype. To further elucidate the impact of the CSF2 genetic variants on the longitudinal risk of malaria and SMA, a Cox proportional hazard model was fit with independent increments according to the Anderson–Gill method. Carriage of the CSF2 AC haplotype was associated with a lower hazard risk for malaria infections. Additionally, inheritance of the CSF2 AT haplotype that significantly increased the incidence rate for SMA was associated with an elevated longitudinal hazard for SMA, albeit non-significant. Results obtained in this study support our previous findings that showed innate immune response genes influence longitudinal susceptibility to malaria and subsequent development of SMA in this P. falciparum holoendemic region [50,51,52]. Moreover, results presented here illustrate that CSF2 genetic variants are associated with both the rate and timing of malaria and SMA episodes during the development of naturally acquired malarial immunity in children living in a high malaria transmission region. Although transcript and protein levels in circulation for CSF2/GM-CSF were not measured in the current study due to sample availability, we hypothesize that the variants alter the gene products and, thereby, influence susceptibility to malaria and SMA, as demonstrated in our previous studies for other genetic variants [50, 61].
We also investigated predictors of all-cause mortality across 36 months using Cox proportional hazard modeling and found that younger children and those with HIV-1 had an increased risk of death. In addition, carriage of the Hb AS trait protected against mortality, whereas inheritance of the Hb SS homozygous mutant imparted 6.5 times higher risk of dying. Several CSF2 variants were also associated with increased susceptibility to all-cause mortality, including carriage of the rs168681 GA genotype (1.8 times higher) and the CSF2 GC/GC diplotype (2.6 times higher). Interestingly, neither of these variants significantly impacted susceptibility to either malaria or SMA, suggesting that their impact on mortality may be due to non-malaria-related causes.
Conclusions
In summary, although the two variants investigated correspond to distal enhancer regions within the 5q31.1, and are associated with altered susceptibility to malaria, SMA, and all-cause mortality, the underlying molecular mechanism(s) for the disease associations remain largely unknown. To our knowledge, this is the first study examining the impact of two genetic variants flanking the 5q31.1 gene region on longitudinal malaria disease outcomes and all-cause mortality. It will be important for future studies to establish the precise role of CSF2, and the soluble inflammatory mediator it encodes, GM-CSF, on susceptibility to malaria, and the subsequent development of severe disease once an individual becomes infected.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its additional information files].
References
WHO. World malaria report 2021. Geneva: World Health Organization; 2021:Licence: CC BY-NC-SA 3.0 IGO.
Amek NO, Odhiambo FO, Khagayi S, Moige H, Orwa G, Hamel MJ, et al. Childhood cause-specific mortality in rural Western Kenya: application of the InterVA-4 model. Glob Health Action. 2014;7:25581.
Amek NO, Van Eijk A, Lindblade KA, Hamel M, Bayoh N, Gimnig J, et al. Infant and child mortality in relation to malaria transmission in KEMRI/CDC HDSS, Western Kenya: validation of verbal autopsy. Malar J. 2018;17(1):37.
Obonyo CO, Vulule J, Akhwale WS, Grobbee DE. In-hospital morbidity and mortality due to severe malarial anemia in western Kenya. Am J Trop Med Hyg. 2007;77(6 Suppl):23–8.
Zucker JR, Perkins BA, Jafari H, Otieno J, Obonyo C, Campbell CC. Clinical signs for the recognition of children with moderate or severe anaemia in western Kenya. Bull World Health Organ. 1997;75(Suppl 1):97–102.
Otieno RO, Ouma C, Ong’echa JM, Keller CC, Were T, Waindi EN, et al. Increased severe anemia in HIV-1-exposed and HIV-1-positive infants and children during acute malaria. AIDS. 2006;20(2):275–80.
Perkins DJ, Were T, Davenport GC, Kempaiah P, Hittner JB, Ong’echa JM. Severe malarial anemia: innate immunity and pathogenesis. Int J Biol Sci. 2011;7(9):1427–42.
Perkins DJ, Were T, Anyona SB, Hittner JB, Kempaiah P, Davenport GC, et al. The global Burden of severe falciparum malaria: an immunological and genetic perspective on pathogenesis. Dynamic models of infectious diseases: Springer; 2013:231–83.
Becher B, Tugues S, Greter M. GM-CSF: from growth factor to central mediator of tissue inflammation. Immunity. 2016;45(5):963–73.
Shi Y, Liu CH, Roberts AI, Das J, Xu G, Ren G, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don’t know. Cell Res. 2006;16(2):126–33.
van de Laar L, Coffer PJ, Woltman AM. Regulation of dendritic cell development by GM-CSF: molecular control and implications for immune homeostasis and therapy. Blood. 2012;119(15):3383–93.
Barreda DR, Hanington PC, Belosevic M. Regulation of myeloid development and function by colony stimulating factors. Dev Comp Immunol. 2004;28(5):509–54.
Hamilton JA. GM-CSF in inflammation and autoimmunity. Trends Immunol. 2002;23(8):403–8.
Miyashita H, Katayama N, Fujieda A, Shibasaki T, Yamamura K, Sugimoto Y, et al. IL-4 and IL-10 synergistically inhibit survival of human blood monocytes supported by GM-CSF. Int J Oncol. 2005;26(3):731–5.
Lukens JR, Barr MJ, Chaplin DD, Chi H, Kanneganti TD. Inflammasome-derived IL-1beta regulates the production of GM-CSF by CD4(+) T cells and gammadelta T cells. J Immunol. 2012;188(7):3107–15.
Gathungu G, Zhang Y, Tian X, Bonkowski E, Rowehl L, Krumsiek J, et al. Impaired granulocyte-macrophage colony-stimulating factor bioactivity accelerates surgical recurrence in ileal Crohn’s disease. World J Gastroenterol. 2018;24(5):623–30.
Hong IS. Stimulatory versus suppressive effects of GM-CSF on tumor progression in multiple cancer types. Exp Mol Med. 2016;48(7): e242.
Szeliga J, Daniel DS, Yang CH, Sever-Chroneos Z, Jagannath C, Chroneos ZC. Granulocyte-macrophage colony stimulating factor-mediated innate responses in tuberculosis. Tuberculosis (Edinb). 2008;88(1):7–20.
Owhashi M, Kirai N, Asami M. Temporary appearance of a circulating granulocyte-macrophage colony-stimulating factor in lethal murine malaria. Southeast Asian J Trop Med Public Health. 1996;27(3):530–4.
Xu L, Zheng X, Berzins K, Chaudhuri A. Cytokine dysregulation associated with malarial anemia in Plasmodium yoelii infected mice. Am J Transl Res. 2013;5(2):235–45.
Natama HM, Moncunill G, Rovira-Vallbona E, Sanz H, Sorgho H, Aguilar R, et al. Modulation of innate immune responses at birth by prenatal malaria exposure and association with malaria risk during the first year of life. BMC Med. 2018;16(1):198.
Ringwald P, Peyron F, Vuillez JP, Touze JE, Le Bras J, Deloron P. Levels of cytokines in plasma during Plasmodium falciparum malaria attacks. J Clin Microbiol. 1991;29(9):2076–8.
Davenport GC, Hittner JB, Were T, Ong’echa JM, Perkins DJ. Relationship between inflammatory mediator patterns and anemia in HIV-1 positive and exposed children with Plasmodium falciparum malaria. Am J Hematol. 2012;87(7):652–8.
Ong’echa JM, Davenport GC, Vulule JM, Hittner JB, Perkins DJ. Identification of inflammatory biomarkers for pediatric malarial anemia severity using novel statistical methods. Infect Immun. 2011;79(11):4674–80.
van Leeuwen BH, Martinson ME, Webb GC, Young IG. Molecular organization of the cytokine gene cluster, involving the human IL-3, IL-4, IL-5, and GM-CSF genes, on human chromosome 5. Blood. 1989;73(5):1142–8.
Miyatake S, Otsuka T, Yokota T, Lee F, Arai K. Structure of the chromosomal gene for granulocyte-macrophage colony stimulating factor: comparison of the mouse and human genes. EMBO J. 1985;4(10):2561–8.
Katsura A, Tamura Y, Hokari S, Harada M, Morikawa M, Sakurai T, et al. ZEB1-regulated inflammatory phenotype in breast cancer cells. Mol Oncol. 2017;11(9):1241–62.
Linnemann AK, O’Geen H, Keles S, Farnham PJ, Bresnick EH. Genetic framework for GATA factor function in vascular biology. Proc Natl Acad Sci U S A. 2011;108(33):13641–6.
Masuda ES, Tokumitsu H, Tsuboi A, Shlomai J, Hung P, Arai K, et al. The granulocyte-macrophage colony-stimulating factor promoter cis-acting element CLE0 mediates induction signals in T cells and is recognized by factors related to AP1 and NFAT. Mol Cell Biol. 1993;13(12):7399–407.
Bickford JS, Beachy DE, Newsom KJ, Barilovits SJ, Herlihy JD, Qiu X, et al. A distal enhancer controls cytokine-dependent human cPLA2alpha gene expression. J Lipid Res. 2013;54(7):1915–26.
Croft B, Ohnesorg T, Hewitt J, Bowles J, Quinn A, Tan J, et al. Human sex reversal is caused by duplication or deletion of core enhancers upstream of SOX9. Nat Commun. 2018;9(1):5319.
Dahan S, Sharma A, Cohen K, Baker M, Taqatqa N, Bentata M, et al. VEGFA’s distal enhancer regulates its alternative splicing in CML. NAR Cancer. 2021;3(3):zcab029.
Koay TW, Osterhof C, Orlando IMC, Keppner A, Andre D, Yousefian S, et al. Androglobin gene expression patterns and FOXJ1-dependent regulation indicate its functional association with ciliogenesis. J Biol Chem. 2021;296: 100291.
Li M, Jiang P, Cheng K, Zhang Z, Lan S, Li X, et al. Regulation of MYB by distal enhancer elements in human myeloid leukemia. Cell Death Dis. 2021;12(2):223.
Moore N, Dicker P, O’Brien JK, Stojanovic M, Conroy RM, Treumann A, et al. Renin gene polymorphisms and haplotypes, blood pressure, and responses to renin-angiotensin system inhibition. Hypertension. 2007;50(2):340–7.
Yashiro-Ohtani Y, Wang H, Zang C, Arnett KL, Bailis W, Ho Y, et al. Long-range enhancer activity determines Myc sensitivity to Notch inhibitors in T cell leukemia. Proc Natl Acad Sci U S A. 2014;111(46):E4946–53.
Burkhardt J, Kirsten H, Wolfram G, Quente E, Ahnert P. Differential allelic expression of IL13 and CSF2 genes associated with asthma. Genet Mol Biol. 2012;35(3):567–74.
Chen WC, Wei JC, Lu HF, Wong HS, Woon PY, Hsu YW, et al. rs657075 (CSF2) is associated with the disease phenotype (BAS-G) of ankylosing spondylitis. Int J Mol Sci. 2017;18(1):83.
Cotterchio M, Lowcock E, Bider-Canfield Z, Lemire M, Greenwood C, Gallinger S, et al. Association between variants in atopy-related immunologic candidate genes and pancreatic cancer risk. PLoS ONE. 2015;10(5): e0125273.
Hardikar S, Johnson LG, Malkki M, Petersdorf EW, Galloway DA, Schwartz SM, et al. A population-based case-control study of genetic variation in cytokine genes associated with risk of cervical and vulvar cancers. Gynecol Oncol. 2015;139(1):90–6.
Johnson LG, Schwartz SM, Malkki M, Du Q, Petersdorf EW, Galloway DA, et al. Risk of cervical cancer associated with allergies and polymorphisms in genes in the chromosome 5 cytokine cluster. Cancer Epidemiol Biomarkers Prev. 2011;20(1):199–207.
Natividad A, Hull J, Luoni G, Holland M, Rockett K, Joof H, et al. Innate immunity in ocular Chlamydia trachomatis infection: contribution of IL8 and CSF2 gene variants to risk of trachomatous scarring in Gambians. BMC Med Genet. 2009;10:138.
Brown SM, Grissom CK, Rondina MT, Hoidal JR, Scholand MB, Wolff RK, et al. Polymorphisms in key pulmonary inflammatory pathways and the development of acute respiratory distress syndrome. Exp Lung Res. 2015;41(3):155–62.
He JQ, Shumansky K, Connett JE, Anthonisen NR, Pare PD, Sandford AJ. Association of genetic variations in the CSF2 and CSF3 genes with lung function in smoking-induced COPD. Eur Respir J. 2008;32(1):25–34.
Bergen AW, McMahan CS, McGee S, Ervin CM, Tindle HA, Le Marchand L, et al. Multiethnic prediction of nicotine biomarkers and association with nicotine dependence. Nicotine Tob Res. 2021;23(12):2162–9.
Ong’echa JM, Keller CC, Were T, Ouma C, Otieno RO, Landis-Lewis Z, et al. Parasitemia, anemia, and malarial anemia in infants and young children in a rural holoendemic Plasmodium falciparum transmission area. Am J Trop Med Hyg. 2006;74(3):376–85.
McElroy PD, ter Kuile FO, Hightower AW, Hawley WA, Phillips-Howard PA, Oloo AJ, et al. All-cause mortality among young children in western Kenya. VI: the Asembo Bay Cohort Project. Am J Trop Med Hyg. 2001;64(1–2 Suppl):18–27.
Okiro EA, Alegana VA, Noor AM, Snow RW. Changing malaria intervention coverage, transmission and hospitalization in Kenya. Malar J. 2010;9:285.
Bloland PB, Boriga DA, Ruebush TK, McCormick JB, Roberts JM, Oloo AJ, et al. Longitudinal cohort study of the epidemiology of malaria infections in an area of intense malaria transmission II. Descriptive epidemiology of malaria infection and disease among children. Am J Trop Med Hyg. 1999;60(4):641–8.
Achieng AO, Hengartner NW, Raballah E, Cheng Q, Anyona SB, Lauve N, et al. Integrated OMICS platforms identify LAIR1 genetic variants as novel predictors of cross-sectional and longitudinal susceptibility to severe malaria and all-cause mortality in Kenyan children. EBioMedicine. 2019;45:290–302.
Anyona SB, Hengartner NW, Raballah E, Ong’echa JM, Lauve N, Cheng Q, et al. Cyclooxygenase-2 haplotypes influence the longitudinal risk of malaria and severe malarial anemia in Kenyan children from a holoendemic transmission region. J Hum Genet. 2019;65:99.
Raballah E, Anyona SB, Cheng Q, Munde EO, Hurwitz IF, Onyango C, et al. Complement component 3 mutations alter the longitudinal risk of pediatric malaria and severe malarial anemia. Exp Biol Med (Maywood). 2021;247:672.
WHO. Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Trans R Soc Trop Med Hyg. 2000;94(Suppl 1):S1-90.
Were T, Davenport GC, Hittner JB, Ouma C, Vulule JM, Ong’echa JM, et al. Bacteremia in Kenyan children presenting with malaria. J Clin Microbiol. 2011;49(2):671–6.
Liu YT, Old JM, Miles K, Fisher CA, Weatherall DJ, Clegg JB. Rapid detection of alpha-thalassaemia deletions and alpha-globin gene triplication by multiplex polymerase chain reactions. Br J Haematol. 2000;108(2):295–9.
Team RC. R: A language and environment for statistical computing. R Foundation for statistical computing V, Austria. 2013. URL http://www.R-project.org. 2013.
Hartl DL, Clark AG. Principles of population genetics: Sinauer associates Sunderland; 1997.
Sherry ST, Ward M, Sirotkin K. dbSNP-database for single nucleotide polymorphisms and other classes of minor genetic variation. Genome Res. 1999;9(8):677–9.
Kumaratilake LM, Ferrante A, Jaeger T, Rzepczyk C. GM-CSF-induced priming of human neutrophils for enhanced phagocytosis and killing of asexual blood stages of Plasmodium falciparum: synergistic effects of GM-CSF and TNF. Parasite Immunol. 1996;18(3):115–23.
Zaret KS, Carroll JS. Pioneer transcription factors: establishing competence for gene expression. Genes Dev. 2011;25(21):2227–41.
Anyona SB, Kempaiah P, Raballah E, Ouma C, Were T, Davenport GC, et al. Functional promoter haplotypes of interleukin-18 condition susceptibility to severe malarial anemia and childhood mortality. Infect Immun. 2011;79(12):4923–32.
Eybpoosh S. Hardy Weinberg equilibrium testing and interpretation: focus on infection. J Med Microbiol Infect Dis. 2018;6(1):35–6.
Hosking L, Lumsden S, Lewis K, Yeo A, McCarthy L, Bansal A, et al. Detection of genotyping errors by Hardy-Weinberg equilibrium testing. Eur J Hum Genet. 2004;12(5):395–9.
Taylor SM, Parobek CM, Fairhurst RM. Haemoglobinopathies and the clinical epidemiology of malaria: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(6):457–68.
Acknowledgements
The authors gratefully acknowledge the assistance of the University of New Mexico-Kenya team: Nicholas Otieno Ondiek, Vincent Odhiambo Otieno, Anne A Ong’ondo, Chrispine Wasonga Ochieng, Everlyne A Modi, Joan L A Ochieng, Joseph Oduor, Moses Epungure, Moses Lokorkeju, Rodney B Mongare, and Vincent Omanje. We are also grateful to all of the parents, guardians, and children who participated in the study.
Funding
The work was supported by National Institutes of Health (NIH) Research Grants R01AI130473 and R01AI51305 (DJP) and NIH Fogarty International Center Grants D43TW05884 (DJP) and D43TW010543 (SBA, DJP).
Author information
Authors and Affiliations
Contributions
LEK participated in SNP selection, generated genotyping and GM-CSF levels determination, data analyses, and manuscript writing. ER, EOM, JMO and CO: project supervision, and manuscript review and editing. QC, KC and PK participated in SNP selection technical support, and manuscript review and editing. BHM, NWH, NL, CGL and KAS performed longitudinal data analysis, statistical interpretations, wrote portions of R code, and manuscript review and editing. DJP designed clinical and experimental studies, data analyses, manuscript writing and editing, provided clinical samples and data, and supplied reagents, materials, and analyses tools. SBA provided technical support, performed longitudinal data analyses, statistical interpretations, manuscript writing and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the ethics and scientific review committee of the Kenya Medical Research Institute (KEMRI), Maseno University Ethics Review Committee (MUERC), and University of New Mexico Institutional Review Board. Parents/legal guardians of children participating in the study provided written informed consent. All research was performed according to stipulated guidelines and regulations of the approving and participating institutions.
Consent for publication
Parents/legal guardians of children participating in the study provided consent for publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kisia, L.E., Cheng, Q., Raballah, E. et al. Genetic variation in CSF2 (5q31.1) is associated with longitudinal susceptibility to pediatric malaria, severe malarial anemia, and all-cause mortality in a high-burden malaria and HIV region of Kenya. Trop Med Health 50, 41 (2022). https://doi.org/10.1186/s41182-022-00432-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41182-022-00432-5