Abstract
Background
A prevalence study of Wuchereria bancrofti infection was carried out in 2014 at 4 study sites in northern Uganda using antigen and microfilaria tests. Each study site consists of a primary school and surrounding communities. These sites are inside the filariasis endemic area and have been covered by mass drug administration under the national elimination programme. However, no prevalence study had been conducted there before the present study. Without information on past and present endemicity levels, our study was meant to be an independent third-party investigation to know the latest filariasis situation.
Results
A total of 982 people including 570 schoolchildren (7–19 years) and 412 community people (7–25 years) were examined, all of them for filarial antigen and 695 for microfilariae. The study revealed that all subjects were negative by both methods.
Conclusions
It was considered that annual mass drug administrations together with anti-malarial activities such as indoor residual spraying had contributed to the reduction of the filarial infection. However, based on the past data obtained near our study sites, we cannot exclude the possibility that filarial prevalence rates in our study sites were very low or even zero originally. During the study, we encountered several patients with lower leg edema and pachydermic (elephant skin-like), mossy skin lesion of the foot. Judging from clinical features and bare-footed life-style of people in the area, non-filarial elephantiasis, possibly podoconiosis, was suspected. This elephantiasis has been reported in areas where filariasis is not endemic.
Similar content being viewed by others
Background
Lymphatic filariasis (LF), which is one of the neglected tropical diseases (NTDs) in the world, is caused by Wuchereria bancrofti in Africa and transmitted mostly by Anopheles and Culex mosquitoes [1]. The adult worms parasitize the human lymphatic system and cause dilation of lymph vessels. The vessel dilation accompanied by inflammation impairs the normal drainage of lymph fluid and leads to the accumulation of the fluid or lymphedema. The edematous skin is liable to bacterial infections which can trigger an acute febrile symptom or fever attack, and repeated attacks will result in thickening of the skin and eventually disfiguring lesions known as elephantiasis [2, 3]. In the scrotum, dead adult worms may block the lymph vessel and cause accumulation of lymph fluid in the tunica vaginalis, a membranous pouch surrounding the testis. This will produce a swelling of the scrotum or hydrocele [4].
Before 2010, it was estimated that 120 million people in 81 countries were infected with LF and 40 million incapacitated or disfigured by lymphedema (including elephantiasis) and/or hydrocele [5], and World Health Organization (WHO) ranked LF as the no. 2 cause of permanent and long-term disability [6]. The Global Programme to Eliminate Lymphatic Filariasis was launched by WHO in 2000 targeting the elimination by 2020. The strategy is to give 4–6 annual mass drug administrations (MDAs) with ivermectin (or diethylcarbamazine) and albendazole covering all eligible people living in endemic areas regardless of filarial infection. An endemic area was defined as having an infection rate of ≥1% using antigen (or microfilaria) test. Approximately 1.34 billion people globally were expected to participate in the annual treatment [5].
Uganda is one of the countries in Africa, south of the Sahara, with extensive elimination activities for LF. The first epidemiological study was conducted in 1998 in Lira, Soroti, and Katakwi districts of northern and eastern Uganda. The antigen and microfilaria (mf) prevalence rates for the three districts were reported to be 18.3–30.1% and 9.7–25.5%, respectively. Similarly, the prevalence rates of hydrocele and limb elephantiasis in adults aged ≥20 years were 7.0–28.7% and 3.7–9.7%, respectively [7]. A country-wide distribution of LF in Uganda was subsequently assessed in Oct. 2000–Apr. 2003 with schoolchildren at 76 locations based on antigen assay. The survey showed that W. bancrofti infections were concentrated in the north of the Victoria Nile and the Lake Kyoga. Utilizing the same data, the first nation-wide filariasis distribution and prevalence map was prepared for the national elimination program. Further analysis, based on the map and the population (2002) data, estimated that 8.7 million people (35.3% of the national population) live in areas where antigen-positive rate of school-aged children is >1% [8].
In our study sites, which are under the national filariasis elimination program, no filariasis survey had been done before this study. A pre-treatment mf survey carried out in 2008 at two sites which are located relatively close to our study sites documents that no positives were found (unpublished report). An attempt to predict the pre-treatment prevalence based on published prevalence maps was not successful because the results varied so much by map. Also, we could not obtain enough information on regular annual MDAs and drug coverage and actual intake at local government offices. Under these circumstances, it was considered important to conduct an independent study, which is separate from the government initiative, to determine recent prevalence and intensity of infection. Another impetus to start this study was unconfirmed information by local medical officers that they see many cases of elephantiasis.
Meanwhile, in a study conducted in Oct./Nov. 1998 in the Mt. Elgon area of Uganda, the presence of non-filarial elephantiasis, most likely podoconiosis, was reported [9]. The disease is found in the high mountain area, where filariasis does not exist. Three cases of podoconiosis were also reported in the south-western highland in Uganda [10]. In the course of the study, we encountered several “unusual” elephantiasis cases among people walking barefoot. The findings are documented in this report.
Methods
Study sites
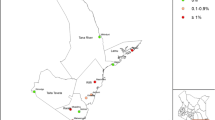
The study was carried out at four study sites selected one from each of four districts of Oyam, Nwoya, Amuru, and Gulu in northern Uganda (Fig. 1). Each study site consists of a primary school and surrounding village communities. Before the site selection, the information on LF situation and elimination activities was first collected at each District Office with assistance of the District Health Officer (DHO) and District Vector Control Officer (DVCO), and one sub-county, 4–16 of which constitute one district in this study area, was selected. Then, at the sub-county office, 5–7 candidate schools were listed for blood test. All the candidate schools were visited and 4th to 7th grade schoolchildren were questioned as a class and recorded individually for their knowledge of drugs used for MDA, actual intake of the drugs and the number of clinical cases in their villages. Finally, based on summarized answers, one study school with relatively more clinical cases and lower drug intake rate was selected for the antigen and mf survey. Several communities surrounding the selected school were also included as part of the study site with advice from village leaders, village health team, and teachers. Hereafter, the study sites are thus designated using the names of the selected schools: Barromo, Goro, Oloyotong, and Labworomor (Fig. 1).
Study subjects
At the selected school, male and female children of grades 1–7 were recruited. A minimum of 20 children per grade were sampled by drawing individually Yes/No lots of folded papers to get a total of >140 subjects/school. The official age to start schooling is at 6 years old, but in reality, the age per grade varies widely. In this study, children’s ages ranged from 7 to 19 years. The lowest age of 7 years was fixed by us. In the communities, >50 children aged 7–13 years, who do not go to school, and >60 people aged 14–25 years were targeted. Thus, >250 subjects/study site or >1000 subjects for the whole study were included. The subjects were first examined for antigen and only those checked for antigen were included in the mf study.
Diagnostic methods
Blood collections were carried out at each of the selected school for enrolled children and a convenient point for community people. For antigen test, BinaxNow® Filariasis antigen test (AlereScarborough, Inc.) was used in the daytime following manufacturer’s instructions: 100 μl of heparinized finger-prick blood was applied to the sample pad and the result was read after 10 min. For mf test, approximately 60 μl of blood was collected after 8:30 pm, smeared on a glass slide which was later stained with 10% Giemsa for 10 min.
Results
The field study was carried out in Oct.–Nov. 2014. This is in the rainy season in northern Uganda, and the work was often interrupted with strong showers. A total of 982 volunteer subjects (570 schoolchildren and 412 community people) were examined for the filarial antigen in the four study sites (Table 1A). Analyzed by age, 94.6% (n = 539) of schoolchildren were 7–15 years, and the rest between 16–19 years. Adults aged 20–25 years accounted for 26.5% (n = 109) of all community subjects. The results of the antigen test were unexpected: all of the subjects were negative.
For mf test, a total of 695 subjects, which is 70.8% of those tested for antigen, were examined at night from 8:30 pm to midnight (Table 1B). Schoolchildren and community children aged 7–13 years participated in the mf survey at the rates of 76.3 and 74.4%, respectively, but for community youths and adults (14–25 years), participation was only 55.0% of the total tested for antigen. Only 29 adults (20–25 years) were examined for mf. All slides examined were negative for W. bancrofti mf, and no mf of another species, Mansonella perstants, which is distributed in Uganda [11], were found in any of the blood smears either.
During the blood surveys, about 7–8 male and female adults visited us asking advice for their “filarial” elephantiasis. The lesions were basically similar: lower leg edema, and fine pachydermic (which is like a rough elephant skin) or mossy skin change on the feet. The photographs of three cases (a, b, and c) are shown in Fig. 2. The edema is slight or moderate and confined below the knee (Fig. 2a1–c1). The skin lesion, which is typically bilateral and severer at one side, is most prominent around toes (Fig. 2a2–c2). In case a, some toe nails are unrecognizable (Fig. 2a2, arrows). In case b, a pachydermic lesion is observed along the side of the foot (Fig. 2b). Most people in these areas walk and work barefooted. Based on this life style, clinical picture and negative antigen/mf results (with the assumption that our sites are non-endemic originally), we conclude that a possibility of non-filarial endemic elephantiasis, podoconiosis, should be considered.
Three cases (A, B, and C) with leg edema and pachydermic skin changes. In case A, the right lower leg is slightly edematous and the dorsal skin of the right foot is whitish in appearance (A1). The skin of the foot is pachydermic and mossy: the skins of the right toes are rather spiny, and the nails of the left 2nd and 3rd toes are unrecognizable (arrows) (A2). In case B, foot edema and pachydermic skin change along the side of the foot are recognized (B). Mild edema is observable in the left lower leg of case C (C1), and the skin around the left toes is pachydermic (C2)
Discussion
A total of 982 people were examined with the antigen test, and 695 of them (70.8%) were also examined for mf, despite the fact that the second blood collection was done at night with unpredictable downpours. The results of the blood tests turned out to be all negative. This can be explained, in general, by the effect of anti-filarial MDAs. They have been implemented in Oyam district since 2005 when the district was a part of Apac district and in the other three districts since 2009. Before our study in 2014, 8, 4, and 3 rounds of annual MDAs were conducted by the national program in Oyam, Amuru and Gulu, and Nwoya districts, respectively. The treatment coverage was satisfactory (>65%) in Oyam, Amuru and Nwoya, but in Gulu three of four rounds resulted in low coverage rates of 34–58%. However, specific rates for our study sites are unknown. Various anti-malarial activities might have also influenced the result, though quantitative effects of these interventions to reduce the prevalence of filarial infection are unknown. Between 2009 and 2014, our four districts were covered by the intensive indoor residual spraying (IRS) program for malaria control [12]. It is reported that five rounds of IRS, especially using bendiocarb, reduced malaria morbidity significantly in Apac district [13], implying that IRS would reduce FL transmission as the two different parasites share the same vector species of Anopheles. A mass distribution campaign of long-lasting insecticidal nets (LLINs) initially focused on pregnant women and children under 5 years in 2009–2010 and then expanded in 2013–2014 to nationwide universal distribution of LLINs. In Mid-North region including our study districts, each household was reported to own an average of 2.7 LLINs [12, 14]. The possible supportive effect of LLINs in anti-filarial MDAs is reported [15].
To understand the totally negative results, crucial information is the pre-MDA infection level in our study sites. In order to have rough estimates of the pre-MDA rates, the geographical positioning system (GPS) data of our study sites (recorded at the schools) were first plotted on the antigen prevalence map made by Onapa et al. [8], which gave a rate of >5% at all four sites (Fig. 3a). Since the map by Onapa et al. was created based on a limited number of samples (n = 76) scattered across the whole country, the prevalence map could not be accurate for our purpose. However, based on the predicted estimate (>5%), we still anticipated some positives in our study even after treatment. As for the comparability between predicted rates from the map and antigen rates by our study, the average number of subjects examined and the age range in Onapa et al. [8] are 231 persons/school and 5–19 years, and ours are 218 persons/site and 7–19 years excluding adults aged 20–25 years. Interestingly, by the use of a newly published (2015) pre-MDA antigen prevalence map for Uganda [16], we predicted high rates of 21–30% in Barromo and 11–20% in the other three sites (Fig. 3b). Surprisingly, another filariasis distribution map of Uganda published in 2007 for the integrated control of NTDs [17] excludes three of our districts, Nwoya, Amuru, and Gulu from the LF endemic area (Fig. 3c). In fact, in the pre-treatment mf survey carried out in July–October 2008 by the national elimination program, no positives were recorded for two sites, Koch Goma in Nwoya district (n = 442) and Opit in Gulu district (n = 354) [previously mentioned unpublished report]. The former site is located 10 km from our Goro site and the latter 29 km from our Labworomor site. Then it could be inferred, based on the relative proximity, that our two sites had very low infection rates when MDA started or that the sites are non-endemic from the beginning. This conclusion, if confirmed, will be a challenge to the reliability of computer-based mapping. Barromo site belongs to a more endemic region, and its pre-treatment prevalence might be higher than those of the other three sites. Prolonged and more intensive MDAs could explain the zero prevalence we obtained there.
Three published maps showing prevalence or endemicity of bancroftian filariasis in Uganda. Our four study sites are plotted on each map. O Oloyotong, G Goro, L Labworomor, B Barromo. a The map reported by Onapa et al. [8]. All of our four study sites are in the >5.0% endemic zone. b The map is from Global Atlas of Helminth Infections (GAHI). The original map is reported by Moraga et al. [16]. Three of our sites are in the 11–20% prevalence zone, and one in the 21–30% zone. c The map is reported by Kolaczinski et al. [17]. Three of our sites are in the districts classified as non-endemic, and one in the endemic district
During this study, we encountered patients with unique elephantiasis: the leg edema is mild and restricted below the knee, but mossy skin change around their toes is obvious. We also noticed that most people walk and work barefooted. Thus, non-filarial elephantiasis such as podoconiosis was suspected. Podoconiosis is a well-known non-filarial endemic elephantiasis found in high mountain areas (1000–2800 m) in Ethiopia, where weathered volcanic ash containing silicon predominates in soil and people live barefoot in a cool and relatively rainy (annual precipitation of >1000 mm) environment [18]. A pathological study revealed amorphous and crystalline silicon compounds in macrophages of femoral lymph nodes [19]. In Uganda, podoconiosis cases were reported from highlands of >1500 m [9, 10], while LF distribution was limited in areas lower than 1300 m [8]. In Ethiopia, however, potential distribution overlap of these two different types of elephantiasis is reported at an altitude of 1225−1698 m [20], and one report clearly mentions the co-endemicity in western Ethiopia [21]. Our study sites are located at 1086–1119 m, which is lower than the reported endemic range. However, a recent report that quartz, crystallized silicon dioxide, is closely related to the occurrence of podoconiosis [22] may suggest wider distribution of podoconiosis because quartz is ubiquitous in the environment, and its microscopic particles may be absorbed through the foot skin. Another supportive reason to suspect podoconiosis is found in the previously mentioned unpublished report from Koch Goma and Opit of 931 people examined clinically, 13 elephantiasis (1.40%), and 1 hydrocele (0.11%) cases were found (p < 0.002). It is quite unusual to have a significantly higher elephantiasis rate than a hydrocele rate in LF endemic areas [23, 24]. In 2011, WHO identified podoconiosis as one of NTDs and its elimination has been considered [25], and it will be important for us to confirm the diagnosis, though the definite diagnosis of podoconiosis is not easy [26]. If we can confirm the absence of LF in the area, it can be a strong supportive evidence for podoconiosis, and for this, W. bancrofti antibody test may be valuable.
Although silicon compounds play an essential role, the exact pathogenesis of podoconiosis is yet unknown. The skin lesions we observed are mild compared with those reported in various articles [27,28,29]. It may be possible that we encountered early stage cases. It is also possible that the skin lesion is influenced by the difference in the concentration and constitution of silicon compounds in soil. The severity may also be influenced by genetic difference between people/population [30].
Conclusions
Two of the computer-based maps predicted relatively high prevalence of LF infections in our study sites, but no antigen/mf positives were detected by our study. Also, in another study carried out in nearby areas before MDAs, no mf positives were found. Thus, we suspected the possibility that our area was originally non-endemic (or very low endemic). The present study also suggests a possibility that non-filarial elephantiasis or probable podoconiosis could be co-endemic with filarial elephantiasis in Uganda. It is necessary to confirm the diagnosis and investigate the clinical and epidemiological significance of “unusual” elephantiasis in Uganda.
Change history
20 September 2017
An erratum to this article has been published.
Abbreviations
- IRS:
-
Indoor residual spraying
- LF:
-
Lymphatic filariasis
- LLINs:
-
Long-lasting insecticidal nets
- MDAs:
-
Mass drug administrations
- Mf :
-
Microfilaria
- NTDs:
-
Neglected tropical diseases
- WHO:
-
World Health Organization
References
WHO (WHO/HTM/NTD/PCT/2013.10). Lymphatic filariasis: a handbook of practical entomology for national lymphatic filariasis elimination programmes; 2013.
Dreyer G, Addiss D, Roberts J, Norões J. Progression of lymphatic vessel dilatation in the presence of living adult Wuchereria bancrofti. Trans R Soc Trop Med Hyg. 2002;96:157–61.
Dreyer G, Medeiros Z, Netto MJ, Leal NC, de Castro LG, Piessens WF. Acute attacks in the extremities of persons living in an area endemic for bancroftian filariasis: differentiation of two syndromes. Trans R Soc Trop Med Hyg. 1999;93:413–7.
Norões J, Addiss D, Cedenho A, Figueredo-Silva J, Lima G, Dreyer G. Pathogenesis of filarial hydrocele: risk associated with intrascrotal nodules caused by death of adult Wuchereria bancrofti. Trans R Soc Trop Med Hyg. 2003;97:561–6.
WHO (WHO/HTM/NTD/PCT/2010.6). Progress report 2000-2009 and strategic plan 2010-2020 of the global programme to eliminate lymphatic filariasis: halfway towards eliminating lymphatic filariasis; 2010.
WHO. The World Health Report 1995: The report of The Director General (http://www.who.int/whr/1995/en/). Bridging the gaps. Geneva; 1995
Onapa AW, Simonsen PE, Pedersen EM, Okello DO. Lymphatic filariasis in Uganda: baseline investigations in Lira, Soroti and Katakwi Districts. Trans R Soc Trop Med Hyg. 2001;95:161–7.
Onapa AW, Simonsen PE, Baehr I, Pedersen EM. Rapid assessment of the geographical distribution of lymphatic filariasis in Uganda, by screening of schoolchildren for circulating filarial antigens. Ann Trop Med Parasitol. 2005;99:141–53.
Onapa AW, Simonsen PE, Pedersen EM. Non-filarial elephantiasis in the Mt. Elgon area (Kapchorwa district) of Uganda. Acta Trop. 2001;78:171–6.
Dwek P, Kong LY, Wafer M, Cherniak W, Pace R, Malhamé I, et al. Case report and literature review: podoconiosis in southwestern Uganda. Int J Trop Dis Health. 2015;9:1–7.
Stensgaard AS, Vounatsou P, Onapa AW, Utzinger J, Pedersen EM, Kristensen TK, et al. Ecological drivers of Mansonella perstans infection in Uganda and patterns of co-endemicity with lymphatic filariasis and malaria. PLoS Negl Trop Dis. 2016;10:e0004319.
Yeka A, Gasasira A, MpimbazaA, Nsobya S, Staedke SG et al. Malaria in Uganda: Challenges to control on the long road to elimination;1. Epidemiology and current control effort. Acta Trop. 2012;121(3):184–95.
Kigozi R, Baxi SM, Gasasira A, Sserwanga A, Kakeeto S, Nasr S, et al. Indoor residual spraying of insecticide and malaria morbidity in a high transmission intensity area of Uganda. PLoS ONE. 2012;7:e42857.
The Republic of Uganda. Universal coverage of LLINs in Uganda––insights into the campaign implementation; 2014.
Ashton RA, Kyabayinze DJ, Opio T, Auma A, Edwards T, Matwale G, et al. The impact of mass drug administration and long-lasting insecticidal net distribution on Wuchereria bancrofti infection in humans and mosquitoes: an observational study in northern Uganda. Parasit Vectors. 2011;4:134.
Global Atlas of Helminth Infections (GAHI). Predicted antigenaemia prevalence and probability that prevalence is >1% prior to large-scale MDA-based interventions (1990-onwards) Uganda. Based on Moraga P, Cano J, et al. Modelling the distribution and transmission intensity of lymphatic filariasis in sub-Saharan Africa prior to scaling up interventions: integrated use of geostatistical and mathematical modelling. Parasit Vectors. 2015;8:560.
Kolaczinski JH, Kabatereine NB, Onapa AW, Ndyomugyenyi R, Kakembo ASL, Brooker S. Neglected tropical diseases in Uganda: the prospect and challenge of integrated control. Trends Parasitol. 2007;23:485–93.
Deribe K, Cano J, Newport MJ, Golding N, Pullan R, Sime H, et al. Mapping and modelling the geographical distribution and environmental limits of podoconiosis in Ethiopia. PLoS Negl Trop Dis. 2015;9:e0003946.
Price EW, Henderson WJ. Silica and silicates in femoral lymph nodes of barefooted people in Ethiopia with special reference to elephantiasis of the lower legs. Trans R Soc Trop Med Hyg. 1979;73:640–7.
Sime H, Deribe K, Assefa A, Newport MJ, Enquselassie F, Gebretsadik A, et al. Integrated mapping of lymphatic filariasis and podoconiosis: lessons learnt from Ethiopia. Parasit Vectors. 2014;7:397.
Yimer M, Hailu T, Mulu W, Abera B. Epidemiology of elephantiasis with special emphasis on podoconiosis in Ethiopia: a literature review. J Vector Borne Dis. 2015;52:111–5.
Molla YB, Wardrop NA, Le Blond JS, Baxter P, Newport MJ, Atkinson PM, et al. Modelling environmental factors correlated with podoconiosis: a geospatial study of non-filarial elephantiasis. Int J Health Geogr. 2014;13:24.
Gyapong JO, Webber RH, Bennett S. The potential role of peripheral health workers and community key informants in the rapid assessment of community burden of disease: the example of lymphatic filariasis. Trop Med Int Health. 1998;3:522–8.
Meyrowitsch DM, Simonsen PE, Makunde WH. Bancroftian filariasis: analysis of infection and disease in five endemic communities of north-eastern Tanzania. Ann Trop Med Parasitol. 1995;89:653–63.
Deribe K, Wanji S, Shafi O, Tukahebwa EM, Umulisa I, Molyneux DH, et al. The feasibility of eliminating podoconiosis. Bull World Health Organ. 2015;93:712–8.
Padovese V, Marrone R, Dassoni F, Vignally P, Barnabas GA, Morrone A. The diagnostic challenge of mapping elephantiasis in the Tigray region of northern Ethiopia. Int J Dermatol. 2016;55:563–70.
Wendemagegn E, Tirumalae R, Böer-Auer A. Histopathological and immunohistochemical features of nodular podoconiosis. J Cutan Pathol. 2015;42:173–81.
Morrone A, Padovese V, Dassoni F, Pajno MC, Marrone R, Franco G, et al. Podoconiosis: an experience from Tigray, northern Ethiopia. J Am Acad Dermatol. 2011;65:214–5.
Visser BJ, Korevaar DA, van der Zee J. Images in Clinical Tropical Medicine: a 24-year-old Ethiopian farmer with burning feet. Am J Trop Med Hyg. 2012;87:583.
Ayele FT, Adeyemo A, Finan C, Hailu E, Sinnott P, Burlinson ND, et al. HLA class II locus and susceptibility to podoconiosis. N Engl J Med. 2012;366:1200–8.
Acknowledgements
We would thank schoolchildren and the communities in our study sites for their cooperation in blood collection, especially late at night. Without hard work by school teachers, village health team members, and community leaders, the study could not have been done. We are grateful for the assistance by the District Health Officers and District Education Officers. District Vector Control Officers worked always with us until late at night.
Funding
This work was supported by funds for Integrated Promotion of Social System Reform and Research and Development (MEXT: Strategic Promotion of International Cooperation to Accelerate Innovation in Africa) (15651988); Grant for Translational Research Network Program (AMED) and Global Health Innovative Technology Fund (G2013-105; G2014-109) to TH.
Availability of data and materials
All essential data generated or analyzed during this study are included in this article.
Endnote
Not applicable.
Author information
Authors and Affiliations
Contributions
Authors’ contributions
EIOA, EK, planed and designed the study. TH provided the fund for the study. AO, KJL, EIOA, EN, YO, EK, AMA, GE, GM mobilized and recruited the schoolchildren and the community in the study. AMA, GE, GM performed the antigen tests and made smears for microfilaria. AO and EIOA stained and examined the slides. EN and YO checked the records and analyzed the data. EIOA and EK drafted the manuscript, and all the authors read and approved it for submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval for the study was obtained from St. Mary’s Hospital Lacor Institutional Research and Ethics Committee (LHIREC 011/03/14). Uganda National Council for Science and Technology (UNCST) also approved the study (HS 1629), and the Research Secretariat in the Office of the President wrote an introductory letter (ADM154/212/01) to the Resident District Commissioners (RDCs) in the proposed sites allowing us to carry out the study. The field work was carried out per district with cooperation of the RDC. At each school, the headmaster signed and stamped consent form to allow us to study the pupils in the school. Schoolchildren aged ≥18 years consented to the study, while for those <18 years, their older brothers or sisters in the same school, or their guardians/parents consented to participate in the study in addition to personal assent. In the community, written consent/assent was obtained individually.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional information
The original publication of this article has been updated: table 1, references 5 and 6 and the caption of figure 2 have been updated.
An erratum to this article is available at https://doi.org/10.1186/s41182-017-0068-3.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Odongo-Aginya, E.I., Olia, A., Luwa, K.J. et al. Wuchereria bancrofti infection at four primary schools and surrounding communities with no previous blood surveys in northern Uganda: the prevalence after mass drug administrations and a report on suspected non-filarial endemic elephantiasis. Trop Med Health 45, 20 (2017). https://doi.org/10.1186/s41182-017-0060-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41182-017-0060-y