Abstract
Background
Bisphosphonates have a high adsorption on calcified tissues and are commonly used in the treatment of bone disorder diseases. Conjugates of bisphosphonates with macrocyclic chelators open new possibilities in bone targeted radionuclide imaging and therapy. Subsequent to positron emission tomography (PET) examinations utilizing 68Ga-labelled analogues, endoradiotheraphy with 177Lu-labelled macrocyclic bisphosphonates may have a great potential in the treatment of painful skeletal metastases.
Methods
Based on the established pharmaceuticals pamidronate and zoledronate two new DOTA-α-OH-bisphosphonates, DOTAPAM and DOTAZOL(MM1.MZ) were successfully synthesized. The ligands were labelled with the positron emitting nuclide 68Ga and the β- emitting nuclide 177Lu and compared in in vitro studies and in ex vivo biodistribution studies together with small animal PET and single photon emission computed tomography (SPECT) studies against [18F]NaF and a known DOTA-α-H-bisphosphonate conjugate (BPAPD) in healthy Wistar rats.
Results
The new DOTA-bisphosphonates can be labelled in high yield of 80 to 95 % in 15 min with post-processed 68Ga and >98 % with 177Lu. The tracers showed very low uptake in soft tissue, a fast renal clearance and a high accumulation on bone. The best compound was [68Ga]DOTAZOL (SUV Femur = 5.4 ± 0.6) followed by [18F]NaF (SUV Femur = 4.8 ± 0.2), [68Ga]DOTAPAM (SUV Femur = 4.5 ± 0.2) and [68Ga]BPAPD (SUV Femur = 3.2 ± 0.3). [177Lu]DOTAZOL showed a similar distribution as the diagnostic 68Ga complex.
Conclusion
The 68Ga labelled compounds showed a promising pharmacokinetics, with similar uptake profile and distribution kinetics. Bone accumulation was highest for [68Ga]DOTAZOL, which makes this compound probably an interesting bone targeting agent for a therapeutic approach with 177Lu. The therapeutic compound [177Lu]DOTAZOL showed a high target-to-background ratio. SPECT experiments showed concordance to the PET scans in healthy rats. [68Ga/177Lu]DOTAZOL appears to be a potential theranostic combination in the management of disseminated bone metastases.
Similar content being viewed by others
Background
Radionuclide bone scintigraphy is usually applied to locate skeletal lesions from various cancer origins. The findings of the scintigraphy reflect the status of metabolic activity of the bone tissue as a result of tumour invasion. Positron emission tomography (PET) has one of the highest diagnostic sensitivity and specificity for the detection of skeletal metastases (Hamaoka et al. 2004), when used with the cyclotron produced [18F]NaF. Actually more common is the use of 99mTc-bisphosphonate compounds in the whole body scintigraphy (Evan-Sapir et al. 2006). The positron emitter 68Ga has several advantages, which makes it an interesting object in the development of radiopharmaceuticals. First of all it is the cyclotron independent availability based on the 68Ge/68Ga generator system—similar to the availability of 98mTc, and second, it is the intense emission of positrons—similar to 18F. Finally, it is the principle of the bifunctional chelating agent to design radiometal, in particular *Me(III), based pharmaceuticals. Substitution of β+ by β- or α-particle emitters leads in principle to a theranostic concept, wherein diagnosis and therapy is combined in one precursor molecule. Since bisphosphonates (BP) show a high adsorption rate on bone, a number of new DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) based bone targeting molecules were described in the literature (Ogawa & Saji 2011), including conjugates of alendronic acid (Ogawa et al. 2009) (4-aminobutyl-1-hydroxy-1,1-bis[phosphonic acid]) and other α-H-bisphosphonates (Vitha et al. 2008). First promising human applications reported for [68Ga]BPAMD showed PET scans of equal quality to [18F]NaF and particularly a higher SUVmax in seleceted bone lesions (Fellner et al. 2010). In addition Passah et al. reported the use of a bisphosphonate conjugated to NOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) chelator to detect bone metastases efficiently (Passah et al. 2014; Pfannkuchen et al. 2015).
The evolution of modern osteoporosis drugs went from α-H bisphosphonates to α-OH and N-heteroaromatic bisphosphonates (Russell et al. 2008). Actually, the synthesis of DOTA-conjugated α-OH-bisphosphonates is more difficult compared to DOTA-conjugated α-H-bisphosphonates. But DOTA-α-OH-bisphosphonates may have higher skeletal retentions compared to DOTA-α-H-bisphosphonates. Consequently, we synthesized a pamidronate (3-aminopropyl-1-hydroxy-1,1-bis[phosphonic acid]) and a zoledronate derivative (([1-hydroxy-2-(1H-imidazol-1-yl)-ethylidene]bisphosphonic acid) as DOTA conjugates (Chart 1), labelled both with 68Ga and compared them in in vivo small animal PET and in ex vivo organ distribution studies in healthy Wistar rats with the known DOTA-α-H-bisphosphonate of similar structure (BPAPD) (for structures see Chart 1) and with [18F]NaF. Since DOTA offers the possibility of stable complexation of various radiometals, including isotopes relevant to therapy, the compounds discussed in this manuscript are potential precursors for a theranostic treatment of metabolic bone disorders, like skeletal metastases. Therefore, the best structure from the 68Ga experiments was further investigated in its 177Lu complex in organ distribution studies ex vivo and in small animal SPECT imaging studies in vivo in healthy Wistar rats.
Results
Synthesis
Synthesis of BPAPD is published in the literature (Vitha et al. 2008) as well as the synthesis of pamidronate (Kieczykowski et al. 1995). Compound (4) was prepared by a standard method for the synthesis of hydroxyl-bisphosphonates from carboxylic acids (Widler et al. 2002). The cleavage of the amide to form the primary amine was achieved in the same step, based on the strong acidic condition after hydrolysis of phosphorus trichloride. The imidazole derivative (3) was obtained from ω-N-acetylhistamine (1) after alkylation with benzyl bromoacetate and hydrogenation. Only the 1,4-(H-2 and H-5) imidazole (2) was formed during this reaction. A convenient method to discriminate between the 1,4- and the 1,5-alkylation product is described in the literature by the different coupling constants of the aromatic imidazole ring protons (Amino et al. 1989). (2) showed a coupling constant of J 2,5 = 1.3 Hz, which is characteristic for the 1,4-product (range from 1.1 to 1.5 Hz). The final conjugation of the hydroxy-bisphosphonates (DOTAPAM and DOTAZOL) was performed via a modified route described by Ogawa et al. (2009). The compounds were first purified by preparative HPLC workup, and the remaining DOTA impurities were removed by solid phase extraction (SPE) using a weak anion exchanger resin (Scheme 1).
Radiolabelling with n.c.a. 68Ga and n.c.a. 177Lu as well as quality control
Labelling with n.c.a. 68Ga was performed in sodium acetate buffer under heating (95 °C) on a thermo shaker for 15 min. Radiochemical yields (RCY) of the 68Ga-DOTA-bisphosphonate complexes were in the range between 80 and 95 %. After SPE using again a weak anion exchanger, the labelled compounds were present in 2 mL phosphate buffered saline (PBS) with a purity of over 98 %, ready for injection. Yields and purities were measured by radio thin layer chromatography (TLC) and HPLC (Additional file 1: Figure S1). For this HPLC quality controls aliquots of the purified reaction solutions were quenched with desferoxamine (DFO). Uncomplexed 68Ga remaining was instantly trapped by DFO to form [68Ga]DFO, which could be discriminated on RP-HPLC from the 68Ga-DOTA-BPs. This technique was not seen as a perfect HPLC method, since the radio labelled 68Ga-DOTA-bisphosphonates showed no retention on common RP-HPLC columns when using acetronitrile/water mixtures as solvents. Therefore, the radio HPLC method was further developed for the Lu-177 analogous using an ion pair reagent as running buffer. The method was later successfully adapted for the Ga-68 complexes (further details about radio-HPLC please refer to Additional file 1 section). Quantitative complexation of 177Lu(III) with DOTAZOL was obtained within 30 min at 98 °C. Radiochemicals yields were determined by radio-TLC and radio-HPLC.
Adsorption experiments on apatite
Simple adsorption experiments on hydroxy apatite (HAP) proved the high accumulation of the 68Ga-labelled compounds on calcified surfaces like bone tissue, while [68Ga]DOTA as a control showed almost no adsorption. The hydroxy bisphosphonates [68Ga]DOTAPAM (91.2 ± 2.7 %) and [68Ga]DOTAZOL (92.7 ± 1.3 %) showed an identical accumulation in this experiment (Fig. 1). Thus, binding of the pamidronate and zoledronate conjugates was superior compared to the α-H-bisphosphonate [68Ga]BPAPD (83.0 ± 0.8 %).
Ex vivo biodistribution: 68Ga
The results of the ex vivo organ distribution in healthy Wistar rats of [68Ga]DOTAPAM, [68Ga]DOTAZOL, [68Ga]BPAPD and [18F]NaF are presented in Tables 1 and 2, respectively. All 68Ga-labelled bisphosphonate compounds showed high accumulation in bone and low uptake in soft tissue. After 60 min the estimated activity in the skeleton was in the range of 40 to 50 % of the injected dose (ID) for [68Ga]BPAPD (38.8 ± 5.0 %ID), [68Ga]DOTAPAM (48.4 ± 4.7 %ID) and [68Ga]DOTAZOL (42.7 ± 5.2 %ID), which is comparable to that of [18F]NaF (43.7 ± 1.9 %ID). Compared to the bisphosphonates, [18F]NaF showed a slight faster renal clearance and an overall lower concentration of activity in other organs, besides the bone. The bone-to-blood ratio determined from biodistribution were 3.7 for [68Ga]BPAPD, 7.6 for [68Ga]DOTAPAM, 11.5 for [68Ga]DOTAZOL and 97.4 for [18F]NaF.
In vivo biodistribution: 68Ga
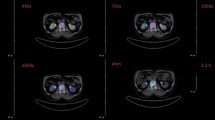
Uptake kinetics and images from in vivo μPET studies are presented in Fig. 2. [68Ga]BPAPD, [68Ga]DOTAPAM and [68Ga]DOTAZOL showed a rapid bone accumulation, reaching a plateau level at 45 min p. i. and a fast blood elimination. Distribution kinetics as time activity curves, Figs. 2 and 3, are very similar for all 68Ga-tracers. Variation in the kidney profile might be a result of differing response of the rats to anesthesia and diverging kidney activity. After 60 min all 68Ga-labelled bisphosphonates gave clearly visible bone scans. [18F]NaF PET showed in small animals a slight better quality and resolution.
Ex vivo biodistribution: 177Lu
Because [68Ga]DOTAZOL appeared to be the compound with the highest bone accumulation among all the 68Ga-bisphosphonates studied in this manuscript, the 177Lu-analoque compound was investigated. The results of the organ distribution of [177Lu] DOTAZOL are represented in Table 3. The compound showed fast and high uptake in the skeleton and low accumulation in soft tissue. [177Lu]DOTAZOL showed the same skeletal retention as [68Ga]DOTAZOL. Blood activity was lower for the 177Lu-complex, but renal clearance time showed to be longer. The activity in the skeleton was constant for 8 days, which clearly reveals the long biological half-life of [177Lu]DOTAZOL in the targeted tissue (Fig. 4). The complex was found to be intact in blood serum and urine samples over 1 h (radio-HPLC in Additional file 1: Figure S3).
In vivo evaluation: 177Lu
SPECT image of [177Lu]DOTAZOL is exemplified in Fig. 5. The images show a good target to soft tissue ratio (49) with high skeletal accumulation (3.43 ± 0.40 %ID/g) and low background activity (blood: 0.07 ± 0.01 %ID/g; muscle: 0.02 ± 0.00 %ID/g).
Discussion
The synthetic route for the DOTA-α-OH-bisphosphonate derivatives is distinctly different from that of the compound BPAPD. As a consequence, the α-H-bisphosphonate are definitely in a more easy way to synthesize. The coupling of the primary amine of the pamidronate or imidazole moiety in water via the N-hydroxysuccinimide ester of DOTA results in lower yields and the formation of problematic side products from ester hydrolysis, which have to be removed. Ogawa et al. (2009) separated the [90Y]DOTA-alendronate derivative from [90Y]DOTA by semipreparative HPLC. A combination of semipreparative HPLC and SPE showed the best purification results after the amide formation. Other groups reported the synthesis of protected alendronate, where the hydroxy bisphosphonate part is esterified by ethyl groups and a tert-butyldimethylsilyl ether (Vachal et al. 2006), leading to a new route for the developing of chelator α-OH- bisphosphonate conjugates (Suzuki et al. 2011). Nevertheless, this reaction seemed to be limited for compounds like alendronate. We were not able to prepare other derivatives with different alkyl or aromatic residues on the geminal hydroxy bisphosphonate by this synthetic pathway. The rearranged phosphonic acid derivative was the main product (Ruel et al. 1995; Brittelli 1985).
Complex formation with n.c.a. 68Ga was comparable for BPAPD, DOTAPAM or DOTAZOL in terms of labelling yields and kinetics. Yields between 80 to 95 % were achieved after 15 min. Complex formation with n.c.a. 177Lu resulted in a quantitative radio-nuclide incorporation with DOTAZOL within 30 min reaction time.
Data from in vitro adsorption studies indicate a higher adsorption rate towards apatite for the DOTA-α-OH-bisphosphonate compared to DOTA-α-H-bisphosphonate, which could be further verified in ex vivo and in in vivo experiments. The DOTA pamidronate conjugate [68Ga]DOTAPAM (SUV = 4.5 ± 0.2) showed a higher accumulation in bone compared to the DOTA bisphosphonate conjugate [68Ga]BPAPD (SUV = 3.2 ± 0.3). This proves the expectation that bisphosphonates containing an α-OH group have an enhanced accumulation on calcified surfaces, like it is discussed in the common literature. This leads to an almost doubled bone to blood ratio of 7.6 for [68Ga]DOTAPAM compared to 3.7 for [68Ga]BPAPD. According to previous studies nitrogen-containing hydroxy bisphosphonates, especially N-heteroaromatic compounds showed an enhanced accumulation on bone. The N-heteroaromatic [68Ga]DOTAZOL showed the best bone to blood ratio of 11.5 and the best bone uptake (SUV = 5.4 ± 0.6) of all tested bisphosphonates. The overall skeleton accumulation is very similar for all tracers and it is comparable to that of [18F]NaF, which is in the range of 40 to 50 %ID.
In rats, all DOTA-based bisphosphonates showed PET images of good quality, which proves their potential as useful skeletal imaging agents. Basically, bisphosphonates are known to have a long biological half-life in bone. Since DOTA compounds are easy to label with β- or α-particle emitters like 177Lu, 213Bi or 225Ac, the tracers discussed in this paper seemed to be excellently suitable for bone targeted radionuclide therapy. Therefore, we investigated the biodistribution of the best performed compound with its 177Lu analogous. [177Lu]DOTAZOL showed to have the same skeletal retention, but a faster blood clearance. The kidney uptake was found to be higher for the 177Lu-complex (1.78 ± 0.13 %ID/g) compared to the 68Ga-complex (0.24 ± 0.02 %ID/g). The results show that the incorporated radionuclide can have a significant influence on the organ distribution. The reason therefore can be explained by the different complexes, the six-dentate 68Ga-complex and the seven-dentate 177Lu-complex. The overall change of the Me(III)-DOTA-moiety may contribute to the pharmacology of the whole molecule. Nevertheless, the compound DOTAZOL maybe very well suited for the use of the theranostic pair 68Ga/177Lu to both detect and treat bone diseases which has to be further evaluated in dosimetry and tumor uptake studies.
It is also to mention that [68Ga]EDTMP was evaluated by Fellner et al. (2011) and Mitterhauser et al. (2007) as a PET imaging agent against [18F]NaF. They concluded that [68Ga]EDTMP has no advantage in image quality. In fact it was distinctly inferior and EDTMP is not suited as a bone targeting complexing agent for 68Ga3+ , in contrast to 153Sm3+ or 177Lu3+. Yousefnia et al. recently compared [177Lu]EDTMP against [177Lu]BPAMD (Yousefnia et al. 2016). His results substantiate the advantage of using macrocyclic conjugates of bisphosphonates. In contrast to the well-known bone targeting compounds [18F]NaF and [177Lu/153Sm]EDTMP DOTA-bisphosphonates can be used as a therapy and imaging agents, when labelled with β- and positron emitting radio metals.
Conclusion
In conclusion, α-OH and N-heteroaromatic bisphosphonates like DOTAZOL are more difficult to synthesize than simple α-H-bisphosphonates like BPAPD but show a significant improved accumulation in the skeleton. The new DOTA conjugated bisphosphonates allow for high radiochemical yields (80–95 %) with 68Ga after 15 min and a quantitative radiochemical yields with 177Lu after 30 min. Data from in vivo small animal PET/SPECT and ex vivo biodistribution showed fast blood clearance, low uptake in soft tissue and a high accumulation in the skeleton with [68Ga/177Lu]DOTAZOL being the best leading compound. In order to avoid problems with the nomenclature of this compound, in the following this molecule will be referred to also as [68Ga/177Lu]MM1.MZ.
Methods
General methods
Chemicals and solvents were commercially available in analytical, HPLC or grade and were purchased from Sigma-Aldrich (TraceSELECT®) or Merck KGaA. DOTA-NHS ester (1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid mono(N-hydroxysuccinimidyl ester)) was synthesized according to a procedure described in the literature (Li et al. 2006) or purchased from Chematech (Dijon, France). Proton nuclear magnetic resonance (1H-NMR) spectra were recorded on a Bruker 300, 31P-NMR were recorded on a Bruker 600. Mass spectra were recorded on Agilent Technologies 6130 Quadrupole LC/MS spectrometer with ESI as ion source in positive or negative modes or on a Finnigan MAT-95 spectrometer with FD as ion source. TLC analyses were carried out with silica on aluminium foil (Merck). Radio-TLC analysis of labelled compounds were performed also with silica on aluminium foil (Merck) and a Canberra Packard Instant Imager. For HPLC and radio-HPLC a Waters-system 1525 with an UV- and a radio-detector (Berthold Technologies, Germany) was used. Radioactivity of samples were measured with an Aktivimeter Isomed 2010, MED (Nuklear-Medizintechnik Dresden GmbH). Radioactivity in tissue samples was determined by using a Wallac WIZARD2 automatic gamma counter (Perkin Elmer, Germany). N.c.a. 177Lu was provided by ITG (Garching, Germany) as LuCl3 in 0.05 M HCl. N.c.a. 68Ga was obtained from a 68Ge/68Ga generator system (Eckert & Ziegler). [18F]NaF was purchased from DKFZ (Deutsches Krebsforschungszentrum, Heidelberg, Germany).
Synthesis of DOTAPAM
Pamidronate was synthesized according to a description by (Kieczykowski et al. 1995; Widler et al. 2002). 4.85 g (54.3 mmol) β-alanine was dissolved in 25 mL of methanesulfonic acid. 4.45 g (54.3 mmol) phosphorous acid was added and 10 mL (115 mmol) phosphorus trichloride was added slowly under vigorous stirring at 75 °C. The reaction mixture was kept under Argon atmosphere for 12 h at 75 °C. After cooling to room temperature, 50 g of crushed ice was added under vigorous stirring. The aqueous solution was heated for 12 h under reflux. After cooling to room temperature conc. aquaous NaOH was added until the formation of a white precipitate was observed. The mixture was stored in a fridge for 5 h to complete precipitation and filtered, washed with cold water and recrystallized from boiling water. Precipitation was initiated by adding ethanol. The white solid (5.3 g, 69 %) was filtered, washed with EtOH and dried in an oven at 60 °C. 1H-NMR (D2O/NaOD, 300 MHz): δ 2.30 (m, 2H), 3.34 (t, J H = 6.8 Hz, 2H). 31P-NMR (D2O/NaOD, 162.05 MHz): δ 17.7 (s, 2P). ESI-MS(-) m/z: calculated 234.07 found 234.10 [M-H]+, ESI-MS(+) m/z: calculated 235.07; found 258.0 [M + Na]+.
DOTABP was synthesized according to a describtion by Ogawa et al. (2009). Twenty five (25) mg (0.11 mmol) pamidronate was dissolved in 1 mL 0.1 M Na2CO3 in water. 38 mg (0.05 mmol) DOTA-NHS-ester was dissolved in 0.5 mL deionized water and added to the pamidronate solution. The reaction solution was kept at 40 °C on a thermo shaker for 24 h. The pH was checked periodically and small amounts of aqueous Na2CO3 were used to keep the pH between 8 and 9. The crude mixture was purified initially by preparative HPLC (Phenomenex Synergy Hydro-RP 80, 10 μ, 250 × 30 mm, H2O + 0.1 % TFA). In the second step the crude product was purified further to remove DOTA impurities by solid phase extraction (SPE). An aqueous solution of the compound was passed over a silica NH2-phase (Merck LiChroprep NH2). After washing with water/methanol/water the product was eluted with H2O +2 % TFA. After lyophilisation 4.5 mg (14.5 %) of a white solid was obtained. 1H-NMR (D2O/NaOD, 300 MHz): δ 2.0–2.3 (b, 8H, cyclen-CH 2 ), 2.0–2.33 (b, 2H,-CH 2 -), 2.9–3.5 (b, 8H, cyclen-CH 2 ), 2.9–3.5 (b, 2H,-CH 2 -), 3.74 (bs, 8H, -CH 2 -CO). 31P-NMR (D2O/NaOD, 162.05 MHz): δ 18.3 (s, 2P). ESI-MS(+) m/z: calculated 621.18 found 1243.3 [2 M + H]+, 622.1 [M + H+], 311.6 [M + 2H]2+.
Synthesis of DOTAZOL
1-(benzyl acetate) 4-(ethyl acetamide)-imidazole (2)
One (1) g (6.53 mmol) ω-N-acetylhistamine (1) was dissolved in 50 mL dry DMF. 4.4 g (13 mmol) ceasium carbonate was added. The solution was stirred under argon atmosphere and ice cooling. Benzyl bromoacetate (2.2 g; 13 mmol) was dissolved in 50 mL dry DMF and added slowly to the imidazole solution. The mixture was allowed to warm to room temperature and stirred for 12 h. Charcoal was added and the solids were filtered off. After removing the solvents under reduced pressure, the crude red residue was recrystallized from ethyl acetate to obtain 1.14 g (58 %) pale yellow crystals. 1H-NMR (CDCl3, 300 MHz): δ 1.97 (s, 3H, CH 3 -CO), 2.76 (t, J H = 6.3 Hz, 2H, CH 2 -CH2), 3.53 (q, J H = 6.0 Hz, 2H, CH 2 -CH2), 4.70 (s, 2H, N-CH 2 -CO), 5.22 (s, 2H, Bn-CH 2 -CO), 6.53 (bs, 1H, NH), 6.75 (d, J H = 1.3 Hz, imidazole-H), 7.39 (m, 5H, benzyl), 7.44 (d, J H = 1.3 Hz, 1H, imidazole-H). ). ESI-MS(+) m/z: calculated 301.14 found 302.3 [M + H]+, 603.2 [2 M + H]+.
1-(1-hydroxy-ethane-1,1-bis(phosphonic acid)) 4-(ethyl amine)-imidazole (4)
Three hundred (300) mg (1 mmol) of (2) was dissolved in 20 mL of dry MeOH. Pd/C (10%w) was added and stirred under hydrogen atmosphere (5 bar) for 12 h. After removing the solvents the hydrogenated product (3) was weighted to determine yield (208 mg, 98 %). The acid (3) was used without further purification in the next step. One (1) mL methanesulfonic acid and 164 mg (2 eq.) phosphorous acid was added. The mixture was stirred at 75 °C and 300 mg (2.2 eq.) phosphorus trichloride was added slowly under argon atmosphere. After 12 h the reaction mixture was cooled down to room temperature and 2 mL of iced water was added. The aqueous solution was heated for 24 h under reflux. Charcoal was added and the solution was filtered. After cooling to room temperature concentrated aquaous NaOH was added until the formation of a white precipitate was observed. The mixture was stored in a fridge overnight to complete precipitation and filtered, washed with cold water and recrystallized from boiling water to obtain 88.6 mg (28 %) of a white solid. 1H-NMR (D2O/NaOD, 300 MHz): δ 2.46 (m, 2H, CH 2 -CH2), 2.66 (m, 2H, CH 2 -CH2), 4.28 (m, 2H, N-CH 2 -phosponate), 6.89 (s, 1H, imidazole-H), 7.54 (s, 1H, imidazole-H). 31P-NMR (D2O/NaOD, 162.05 MHz): δ 14.4. ESI-MS(+) m/z: calculated 315.04 found 316.05 [M + H]+, 338.04 [M + Na]+
DOTAZOL (5)
To 15.75 mg (0.05 mmol) (4) in 1 mL water, triethylamine (TEA) was added drop wise until all solids were dissolved. Thirty-eight (38) mg (0.05 mmol) DOTA-NHS-ester was dissolved in 0.5 mL water and added to the bisphosphonate solution (Ogawa et al. 2009). The reaction solution was kept at 50 °C on a thermo shaker for 24 h. The pH was checked periodically and small amounts of aqueous TEA were used to keep the pH between 8 and 9. The crude mixture was purified initially by preparative HPLC (Phenomenex Synergy Hydro-RP 80, 10 μ, 250 × 30 mm, solvent: H2O + 0.1 % TFA). In the second step the crude product was purified further to remove DOTA impurities by solid phase extraction (SPE). An aqueous solution of the compound was passed over a silica NH2-phase (Merck LiChroprep NH2). After washing with water/methanol/water the product was eluted with H2O + 2 % TFA. After lyophilisation 5.6 mg (15.7 %) of a white solid was obtained. 1H-NMR (D2O/NaOD, 300 MHz): δ 2.42 (m, 2H, CH 2 -CH2), 2.61 (m, 2H, CH 2 -CH2), 2.9–3.5 (b, 16H, cyclen-CH 2 ), 3.75 (bs, 8H, -CH 2 -CO), 4.55 (m, 2H, N-CH 2 -phosponate), 7.28 (s, 1H, imidazole-H), 8.54 (s, 1H, imidazole-H). 31P-NMR (D2O/NaOD, 162.05 MHz): δ 14.3. ESI-MS(+) m/z: calculated 701.2 found 702.5 [M + H]+, 351.1 [M + 2H]2+.
Radiolabelling with n.c.a. 68Ga, 177Lu and quality control
N.c.a. 68Ga was obtained in 400 μL acetone/HCl by cationic post processing from a 68Ge/68Ga generator system eluted with 0.1 M HCl (Brittelli 1985; Zhernosekov et al. 2007). The labelling itself was performed by adding 500 μL sodium acetate buffer (0.5 M, pH = 4) and 25 nmol ligand to the 68Ga containing solution. The mixture was heated at 98 °C under moderate shaking on a thermo shaker for 15 min. Subsequently the solution was diluted to 5 mL with water and passed over a weak anion exchanger cartridge (25 mg, Merck LiChroprep NH2). After washing with 1 mL water the purified product was eluted with 2 mL PBS. Radiochemical yields and purities were determined by radio-TLC (Merck silica, solvent: acetone/acetylacetone/HCl, 10/10/1) and radio-HPLC (Merck Chromolite RP-18e 100 × 4.6 mm, gradient: H2O to acetonitrile). To discriminate between free 68Ga and 68Ga-bisphosphonates a small aliquot of the product solution was incubated for 1 min with a 0.25 M DFO (Desferoxamine) solution. DFO complexes unlabelled 68Ga instantly and shows a longer retention time on RP-18 HPLC (see Additional file 1: Figure S1). 1 GBq n.c.a. 177LuCl3 present in 500 μL of a 0.04 M HCl (obtained from ITG, Germany) was added to 20 nmol of the ligand DOTAZOL in 1 mL of a 0.25 M sodium acetate solution and heated for 30 min at 98 °C. Radiochemical yields were determined by radio-TLC using sodium citrate (0.1 M, pH = 4) as the solvent.
Adsorption experiments on apatite
Hydroxy apatite (HAP) (20 mg, Sigma-Aldrich, reagent grade powder) was incubated in isotonic saline (1 mL) for 24 h. Fifty (50) μL of the 68Ga-labelled bisphosphonate solution was added to the HAP suspension. After vortexing for 10 s, the suspension was incubated for 10 min at room temperature under moderate shaking on a thermo shaker. The supernatant was removed by centrifugation. The HAP fraction was washed with saline (0.5 mL) and the 68Ga radioactivity in the combined liquids and that of the HAP fraction were measured. 68Ga-complex binding to HAP was determined as percentage of 68Ga absorbed to HAP (N = 5) (Fellner et al. 2011).
Animal studies
Male Wistar rats were purchased from Charles River Laboratories Inc weighting 140–220 g. The animal experiments were carried out in consensus to institutional guidelines, the German welfare regulations and the European Convention for the Protection of Vertebrate Animals for Experimental and other Scientific Purposes.
Small animal PET and SPECT
Small animal PET and SPECT imaging was performed under general anesthesia with isoflurane inhalation. The rats were positioned supine in a Siemens Focus 120 μPET (Mediso nanoScan SPECT)and 15 to 18 MBq of the radio tracers (100 MBq for 177Lu-SPECT) were administrated in 0.5 mL isotonic saline intravenously (i.v.) via the tail vein. Images were reconstructed to OSEM 2D and files were processed using PMOD software (TAC) and Amine software (MIP). SPECT images were reconstructed using ROVER software.
Biodistribution
For each compound five animals were injected with 8 to 10 MBq of the radio tracers in 0.5 mL PBS/isotonic saline via the tail vein. The rats were sacrificed at 60 min and 1 d p.i. and organs of interest were excised, weighed and the radioactivity was measured decay corrected on a gamma counter. The amount of organ accumulation is expressed in SUV (standardised uptake value) by the equitation: SUV = (activity per g tissue)/(injected activity) x body weight. Activity in the skeleton was calculated as percentage of injected dose (%ID) by the following equitation: %ID(skeleton) = femur(%ID/g) x skeleton weight (g). The skeleton weight of the rats were calculated using: skeleton weight = 9.66 + 0.0355 x body weight (Vachal et al. 2006).
Statistical analysis
All data were expressed as mean ± SD. Groups were compared using the t-test. All statistical tests were two tailed, with a P-value of less than 0.05 representative for significance.
Change history
26 November 2018
The metadata in the HTML format of these original articles (Eppard et al., 2017; Decristoforo & Patt, 2017; Domnanich et al., 2017; Chan et al., 2017; Jovalekic et al., 2017; Zhang & Villalobos, 2017; Meckel et al., 2017; Li et al., 2017; Colin et al., 2017; Koziorowski et al., 2017; Ooms et al., 2017; Elsinga, 2017; Seemann et al., 2017; Müller et al., 2017; Luurtsema et al., 2017) were published with an incorrect cover date. The correct cover date is December 2016. This does not alter the text of the original articles.
References
Amino Y, Eto H, Eguchi C. Synthesis of 1,5-disubstituted imidazoles including an imidazole analogue of prostaglandine from 4(5)-hydroxymethylimidazole. Chem Pharm Bull. 1989;37:1481–7.
Brittelli DR. A Study of the reaction of 2-haloacyl halides with trialkyl phosphites. Synthesis of (2-substituted acy1)phosphonates. J Org Chem. 1985;50:1845–7.
Evan-Sapir E, Metser U, Mishani E, Lievshitz G, Lerman H, Leibovitch I. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-Fluoride PET, and 18F-Fluoride PET/CT. J Nucl Med. 2006;47:287–97.
Fellner M, Baum RP, Kubicek V, Hermann P, Lukes I, Prasad V, Rösch F. PET/CT imaging of osteoblastic bone metastases with 68Ga-bisphosphonates: First human study. EJNMMI. 2010;37:834.
Fellner M, Riss P, Loktionova N, Zhernosekov K, Thews O, Geraldes CFGC, Kovacs Z, Lukes I, Rösch F. Comparison of different phosphorus-containing ligands complexing 68Ga for PET-imaging of bone metabolism. Radiochim Acta. 2011;99:43–51.
Hamaoka T, Madewell JE, Podoloff DA, Hortobagyi GN, Ueno NT. Bone imaging in metastatic breast cancer. J Clin Oncol. 2004;22:2942–53.
Kieczykowski GR, Robinson RB, Melillo DG, Reinhold DF, Grenda VJ, Shinkai I. Preparation of (4-amino-1-hydroxybutylidene)bisphosphonic acid sodium salt, MK-217 (alendronate sodium). An improved procedure for the preparation of 1-hydroxy-1,1-bisphosphonic acids. J Org Chem. 1995;60:8310–2.
Li C, Winnard PT, Takagi T, Artemov D, Bhujawalla ZM. Multimodal image-guided enzyme/prodrug cancer therapy. J Am Chem Soc. 2006;128:15072–3.
Meckel M, Fellner M, Thieme N, Bergmann R, Kubicek V, Rösch F. In vivo comparison of DOTA based 68Ga-labelled bisphosphonates for bone imaging in non-tumour models. Nucl Med Biol. 2013;40:823–30.
Mitterhauser M, Toegel S, Wadsak W, Lanzenberger RR, Mien LK, Kuntner C, Wanek T, Eidherr H, Ettlinger DE, Viernstein H, Kluger R, Dudczak R, Kletter K. Pre vivo, ex vivo and in vivo evaluations of [68Ga]-EDTMP. Nucl Med Biol. 2007;34:391–7.
Ogawa K, Saji H. Advances in drug design of radiometal-based imaging agents for bone disorders. Int J Mol Imag. 2011;2011:537687.
Ogawa K, Kawashima H, Shiba K, Washiyama K, Yoshimoto M, Kiyono Y, Ueda M, Mori H, Saji H. Development of [90Y]DOTA-conjugated bisphosphonate for treatment of painful bone metastases. Nucl Med Biol. 2009;36:129–35.
Passah A, Tripathi M, Kumar R, Das CJ, Goyal A, Bal CS. Brain metastasis in carcinoma breast demonstrated on (68)Ga NOTA-bisphosphonate PET/CT. Clin Nucl Med. 2014;39:653–4.
N. Pfannkuchen, M. Meckel, V. Kubicek, P. Hermann, R. Bergmann, J. Steinbach, J. Pietzsch, C. S. Bal, H. R. Kulkarni, W. Mohnike, R. P. Baum, F. Rösch, 68Ga- und 177Lu-markierte Bisphosphonate als Knochenmetastasen-Theranostika, Der Nuklearmediziner 2015; 38: 138-144
Ruel R, Bouvier JP, Young RN. Single-step preparation of 1-hydroxy bisphosphonates via addition of dialkyl phosphite potassium anions to acid chlorides. J Org Chem. 1995;60:5209–13.
Russell RGG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008;19:733–59.
Suzuki K, Satake M, Suwada J, Oshikiri S, Ashino H, Dozono H, Hino A, Kasahara H, Minamizawa T. Synthesis and evaluation of a novel 68Ga-chelate-conjugated bisphosphonate as a bone-seeking agent for PET imaging. Nucl Med Biol. 2011;38:1011–8.
Vachal P, Hale JJ, Lu Z, Streckfuss EC, Mills SG, MacCross M, Yin DH, Algayer K, Manser K, Kesisoglou F, Ghosh S, Alani LL. Synthesis and study of alendronate derivatives as potential prodrugs of alendronate sodium for the treatment of low bone density and osteoporosis. J Med Chem. 2006;49:3060–3.
Vitha T, Kubicek V, Hermann P, Kolar ZI, Wolterbeek HT, Peters JA, Lukes I. Complexes of DOTA-bisphosphonate conjugates: Probes for determination of adsorption capacity and affinity constants of hydroxyapatite. Langmuir. 2008;24:1952–8.
Widler L, Jaeggi KA, Glatt M, Müller K, Bachmann R, Bisping M, Born AR, Cortesi R, Guiglia G, Jeker H, Klein H, Ramseier U, Schmid J, Schreiber G, Seltenmeyer Y, Green JR. Highly potent geminal bisphosphonates. from pamidronate disodium (aredia) to zoledronic acid (Zometa). J Med Chem. 2002;45:3721–38.
Yousefnia H, Zolghadri S, Sadeghi HR, Naderi M, Jalilian AR, Shanehsazzadeh S. Preparation and biological assessment of 177Lu-BPAMD as a high potential agent for bone pain palliation therapy: comparison with 177Lu-EDTMP. J Radioanal Nucl Chem. 2016;2:1243–51.
Zhernosekov K, Filosofov DV, Baum RP, Aschoff P, Bihl H, Razbah AA, Jahn M, Jennewein M, Rösch F. Processing of generator-produced 68Ga for medical application. J Nucl Med. 2007;48:1741.
Acknowledgment
The authors are grateful to Dr. V. Kubicek and Prof. P. Hermann (both Charles University Prague) for providing the compound BPAPD. The animal handling and processing of the biodistribution data was done by the help of Andrea Suhr (HZDR), Dr. Nicole Bausbacher and Barbara Biesalski (University Hospital, Mainz), who are gratefully acknowledged. We thank the company ITG Munich for the donation of n.c.a. 177Lu. This work was supported by the grant of the Max Planck Graduate Center, Mainz.
Authors’ contributions
MM wrote the manuscript and synthesized the labelled and non-labelled compounds. RB and MM performed the animal experiments. MM and FR were involved in data interpretation and in experimental design. All authors supported the writing of this manuscript. All authors read and approved the final manuscript.
Competing interests
All authors declare to have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1:
Figure S1. Quality control of [68Ga]DOTAZOL by means of cross checked radio-HPLC and radio-TLC, as a representative of 68Ga-labelled bisphosphonates. A: After 15 min reaction time. B: After SPE purification. A(HPLC): Rt([68Ga]DOTAZOL) = 0.6 min. (88 %), Rt([68Ga]DFO) = 4.1 min. (12 %). B(HPLC): Rt([68Ga]DOTAZOL) = 0.6 min. (98 %). A(TLC): Rf([68Ga]DOTAZOL) = 0.1 (85 %), Rf([68Ga]acetylacetonate) = 0.9 (15 %). B(TLC): Rf([68Ga]DOTAZOL) = 0.1 (99 %). Control(TLC): Rf([68Ga] acetylacetonate) = 0.9 (99 %). Figure S2. Radio-HPLC of [177Lu]DOTAZOL on a Zorbax 300SB-C18 9,4 × 250 mm 5 μ, A = 100 mM TEAP pH = 2,24, isocratic flow: 1 ml/min. Figure S3. Radio-HPLC of urine and blood samples after administration of [177Lu]DOTAZOL. The HPLC method used for analysing the Lu-177 complexes is also suitable for the Ga-68 complexes of DOTA-Bisphosphonates. The Ga-68 complexes showed a different retention time based on the different character of the DOTA complex. This is also known for other DOTA-Compounds like DOTATOC or DOTATATE. Figure S4. Radio-HPLC of [68Ga]DOTAZOL on a Zorbax 300SB-C18 9,4 × 250 mm 5 μ, A = 100 mM TEAP pH = 2,24, isocratic flow: 1 ml/min. (DOCX 742 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Meckel, M., Bergmann, R., Miederer, M. et al. Bone targeting compounds for radiotherapy and imaging: *Me(III)-DOTA conjugates of bisphosphonic acid, pamidronic acid and zoledronic acid. EJNMMI radiopharm. chem. 1, 14 (2017). https://doi.org/10.1186/s41181-016-0017-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41181-016-0017-1