Abstract
Seafood lipids encompass important healthy nutrients, such as n-3 polyunsaturated fatty acids (n-3 PUFAs), which may have a significant effect on human cardiovascular health and needs to be supplied by the human diet. Particularly, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are the most abundant n-3 PUFA present in seafood and have an impact on the cardiovascular health. DHA and EPA are deemed to display anti-inflammatory, cell membrane modulation, and biophysical properties, thereby offsetting the pro-inflammatory effects of n-6 PUFA, and to reduce the risk of cardiovascular disease. Consumption of large amounts of n-3 PUFA exerts a positive effect on a wide array of cardiovascular health concerns ranging from hypertension and atherosclerosis to myocardial infarction and stroke. In fact, animal studies indicate that n-3 PUFAs play a bioactive cardiovascular protective role. Therefore, it is recommended up to two servings of fatty fish per week or up to 500 mg/day of EPA and DHA (World Health Organization).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Seafood products are a rich source of lipids, including some that are not found in other foods, such as n-3 polyunsaturated fatty acids (n-3 PUFAs), particularly, the very important n-3 PUFA, eicosapentaenoic (EPA) and docosahexaenoic (DHA) acids [1, 2]. The abundance of the fatty acids (FAs) in seafood products (fish, crustaceans, cephalopods, bivalves, etc.) varies widely. It is well known that seafood composition depends on many factors, such as species, sex, sexual maturity degree, size, location, water temperature, type of feeding, and season [3]. The variation in the FA composition is explained by fluctuations in the quality and amount of available food, especially phytoplankton [4]. Seafood FAs are typically part of larger molecules, such as triacylglycerols (TAGs). However, these molecules have to be hydrolysed into FAs in the human gut in order to be absorbed. The efficiency of this hydrolysis as well as other factors (chemical affinity, protein-lipid interactions) is crucial for the level of FA bioaccessibility and bioavailability, which is important for a proper evaluation of the effects of seafood lipids on health.
On the other hand, evidence has been accumulating of the importance of n-3 PUFA in the prevention and treatment of cardiovascular disease (CVD), since the first cross-cultural epidemiologic studies conducted in the 1970s [5]. Evidence from observational studies, randomized controlled trials (RCTs), and clinical, animal, and in vitro studies suggests that higher intake of very long-chain n-3 PUFA found in fatty fish or fish oil supplements, namely, EPA and DHA, may reduce CVD risk [2]. EPA and DHA can be synthesized from α-linolenic acid (ALA) in humans, but most studies suggest that humans only convert <5 % of ALA to EPA [6] and <0.05 % of ALA to DHA [7]. This is related to the overall low activity of enzymes essential for this conversion in mammals, namely, elongase, desaturase, and peroxisomal β-oxidation enzymes [8]. It is assumed that through elongation and desaturation, ALA is converted to EPA and 22:5 n-3. Afterwards, this FA is elongated to 24:5 n-3, which is desaturated to 24:6 n-3, and, finally, is metabolized via β-oxidation to DHA. This is a relatively demanding metabolic route that makes a high synthesis of EPA and especially DHA from ALA difficult.
This review will be focused on the effects of seafood lipids on the cardiovascular system and main-related health problems and outcomes.
Main seafood lipids and their contents
Seafood products contain a wide variety of lipid classes, ranging from TAG, free fatty acids (FFAs), and sterols (cholesterol) to phospholipids (PL). These lipids are composed by different components, including a fatty acid (FA) moiety. Among these FAs, n-3 PUFAs represent a large share [9]. Particularly, marine fish and shellfish are rich sources of EPA (20:5 n-3) and DHA (22:6 n-3) [10]. In wild fish, EPA and DHA may represent as much as 90 % of the total PUFA [11]. EPA and DHA are considered to be essential FAs in that they cannot be biosynthesized with a level of efficiency sufficient to meet human needs [12]. In fact, there have been several studies pointing to a very limited extent of α-linolenic (ALA, 18:3 n-3) conversion to EPA and DHA [13, 14]. However, farmed fish have a different FA profile due to the incorporation of vegetable oils and other vegetable materials containing fat in their diets. Indeed, their FA profile shows high levels of oleic acid as well as n-6 PUFA and is less rich in EPA and DHA [15].
Regarding saturated fatty acids (SFAs), palmitic acid (16:0) is the main SFA in fish, being found in all lipid classes (TAG, PL, others). Indeed, according to results attained in herring [16, 17], 16:0 is a key metabolite and its content is not significantly affected by fish feeding. Myristic acid (14:0) and stearic acid (18:0) have lower percentages, and the first of these is preferably in TAGs. The SFAs with longer chain such as 20:0, 22:0, and 24:0 are typically found at levels below 1 % [18]. FAs with an odd number of carbon atoms have also been detected and are probably derived by oxidation of the alcohol present in copepods or microorganisms. [19] Indeed, it has been claimed that these organisms biosynthesize FAs via propionyl-coenzyme A leading to less usual FAs [20].
The most abundant monounsaturated fatty acid (MUFA) in seafood lipids are usually palmitoleic acid (16:1 n-7) and oleic acid (18:1 n-9). It has been shown that, for a given species, the sum of the 16:1 and 18:1 contents was almost constant and this has been justified with the observation that the level of these FAs is controlled by the organism metabolism [21]. With regard to MUFAs with a long chain, such as 20:1 and 22:1, they appear to have an exogenous origin, possibly from copepods [22]. These FAs are not involved in the structure of membranes. There is also a MUFA with 24 carbon atoms whose content does not exceed 1 % [23].
The lipid content and total PUFA, EPA, and DHA contents of representative seafood are presented in Table 1.
The FA profiles of seafood are quite well studied, being available huge masses of FA data in national food composition tables in the internet [24] and in books [25]. Some systematic studies on the FA profile in seafood have been done in Europe [26, 27]. This profile varies considerably with temperature, salinity, and the type and availability of food, factors which, in turn, are affected by geographical area and by season [28]. Studies have shown that the water temperature has an effect on the FA composition of carp [29]. It was reported that a decrease in temperature leads to a higher PUFA content due to an enhanced activity of desaturases, which contribute to the biosynthesis of more unsaturated FAs (with lower melting point) in order to keep their physiological role. Nonetheless, it must be stressed that FA profile in seafood results from a balance between FAs from diet and those formed by biosynthesis [30]. But, whenever food is rich enough in the necessary FAs, fish and other aquatic organisms do not spend energy in FA biosynthesis [31].
The lipid bioaccessibility and bioavailability
Seafood lipids have an important role in human nutrition. However, for an appropriate assessment of their importance and role in health, it is fundamental that instead of dealing with the total contents of seafood lipids, the contents of the FAs and other lipid components that are available to intestinal absorption after digestion of cooked seafood in a typical meal be accounted for using appropriate methodologies. Indeed, the level of lipids in a portion of seafood that is eaten may be quite different from the bioaccessible level, that is, the lipid concentration that is released from the seafood matrix into the intestinal lumen after digestion and is available for absorption [32, 33]. On the other hand, bioavailability is usually defined as the fraction of an oral dose of a substance that reaches the systemic circulation [34]. The bioaccessible content is always equal or higher than the bioavailable content [32]. Bioaccessibility is determined by in vitro simulations of human digestion [32, 35]. For bioavailability, according to the definition given above, cell lines and transwell assays are used for the simulation of the intestinal lining barrier [36] and cell cultures mimicking the relevant liver tissues may also be used [37].
For better grasping the concepts, lipid digestion has to be duly understood. Indeed, the digestion of the seafood lipids is composed of three distinct phases. Firstly, lipids are physically and chemically modified resulting in simpler molecules, for instance, TAGs generate FAs and glycerol [38, 39]. The gastric and pancreatic lipases catalyze the hydrolysis of FAs, leading to monoacylglycerols (MAGs) type sn-2 and free fatty acids (FFAs). Secondly, digested material is transported into the intestinal mucosa. Finally, the digested molecules are reconstituted. This reconstitution is related to transport of FAs (as FFA or MAG) to the lymph and the blood. For this transport to occur, FAs are re-esterified to TAG (rTAG) in the endoplasmic reticulum of enterocytes [40]. All the aforementioned processes may affect lipid bioavailability.
Bioaccessibility should be studied under realistic conditions, which entails taking into account the effect of culinary treatments upon seafood lipids. Hence, a first step towards a more accurate determination of the lipids effectively provided by any given diet containing seafood entails cooking foods according to the typical culinary methods [41, 42]. Boiling, grilling, roasting, frying, and other culinary treatments can alter lipid content either by leaching it out or decomposing it or concentrating it due to water loss. Moreover, cooking generates several biochemical and physical transformations that bring about relevant matrix changes, which, in turn, affect seafood lipid bioaccessibility [41, 42]. Indeed, it was observed that grilled fish displayed lower FA bioaccessibility than raw fish. For instance, the lipid bioaccessibility in meagre (Argyrosomus regius) was reduced from 89 % in the raw fish to 68 % in the grilled fish [41]. This was probably due to the very harsh thermal treatment (180 °C and direct conductive heat) associated to grilling. Under these extreme heating conditions, protein denaturation is enhanced and digestibility is reduced because covalent bonds between polypeptide chains are established [43]. In fact, there was also a reduction of protein digestibility with grilling. It thence appears that protein aggregates formed during grilling trap a significant portion of the lipids, thereby reducing lipid bioaccessibility [41].
Besides cooking-related changes, other factors affect seafood lipid bioaccessibility deviating it from the ideal 100 %. It is important to stress that the different physical and chemical properties of each lipid compound, such as each FA, influence processes such as digestion, absorption, and transport in blood [44]. Indeed, it has been shown that FA bioaccessibility in salmon (Salmo salar) is reduced as the number of double bonds increases [42]. Hence, n-3 PUFAs with three or more double bonds (a very high level of unsaturation) present low FA bioaccessibility. For instance, while erucic acid (22 carbons and one double bond) exhibited a bioaccessibility of 93.9 % in raw fish, the bioaccessibility of DHA (22 carbons and six double bonds) was only 73.5 % in the same raw fish [42]. This phenomenon may be explained by three main causes: chemical affinity of each FA, digestive lipases favoring the hydrolysis of less unsaturated FAs, [45] and location of n-3 PUFA (namely, EPA and DHA) in the sn-2 position of TAGs, which is less accessible to lipases, particularly the important pancreatic lipase [40].
Bioaccessibility and, as a consequence, bioavailability of FAs may also depend on the chemical binding form (FA bound in ethyl esters, EE, TAG, or PL) [40]. It has been observed in in vivo studies with humans that FAs bound in TAG are more bioavailable than FAs bound in EE form [46, 47]. The latter form is found sometimes in n-3 PUFA supplements. A first explanation for this difference may be found in in vitro studies showing that pancreatic lipases hydrolyze EEs 10 to 50 times less efficiently than glycerol esters in TAGs [48]. Another reason may be differences in the re-esterification of FAs to TAG after absorption. This process requires glycerol and 2-MAG, which are readily available when FAs are bound in TAG, but absent when FAs are bound in the EE form, since there is no release of glycerol during digestion in this case. Accordingly, re-esterification may be delayed due to difficulties in providing the missing glycerol [49].
In krill oil, which is rich in PLs, it has been claimed that its FA bioavailability is higher on the basis of in vivo studies [50–52]. This higher bioavailability may be related to the high share of PLs. Taking into account that FFAs also exhibit a high bioavailability [46, 53] because no chemical bond needs to be broken and that rTAG seem to have a bioavailability higher than natural fish oil (TAGs) and FFAs [46], it can be put forward a possible FA bioavailability order: rTAG>TAG~FFA>EE, with the position of PL above that of TAG. Nonetheless, more scientific research is necessary for proving this order, especially concerning a supposed higher bioavailability of PLs with respect to TAGs and even rTAGs.
Seafood lipids: benefits to cardiovascular system and other health effects
Studies’ results in the recent decades show that morbidity and mortality related to chronic diseases in the general population have a multifactorial origin, resulting mostly from the interaction between genetic and dietary factors. The results from current epidemiological, clinical, and experimental studies allow concluding that dietary patterns have a profound influence on health outcomes and well-being of populations. Many studies also indicate that the major groups of FAs are associated with different health effects and confirm that modulation of dietary lipid composition affects lipid concentrations and composition in the blood. Thence, Public Health Institutes and Health Associations as well as many western country authorities have advised independently daily amounts for each group of SFA, PUFA, and long-chain PUFA (LC-PUFA) [54–56].
SFAs have been cited as responsible for a minor increase of HDL-cholesterol; however, such positive effect does not avoid the harmful increase of low-density lipoprotein (LDL) cholesterol and plasma TAG [57]. For the other lipid groups, there is a large body of epidemiological evidence about the different health aspects.
Although the available information about the health benefits of MUFA is not as broad and convincing as for PUFA, there is a considerable level and strength of evidence. MUFAs are considered more stable than PUFA and more resistant to oxidation [58]. Namely, oleic acid may prevent the development of atheromas and subsequent thrombi due to its impact on MUFA/SFA ratios, oxidation resistance, and induction of large hydrolysable chylomicrons (CM). Oleic acid and MUFA consumption decreases platelet sensitivity and aggregation and increases fibrinolysis. However, a full mechanistic explanation of the ability of dietary MUFA to decrease platelet aggregation has yet to be determined [59]. Most noticeable effects of MUFA come from studies where the substitution of SFA by oleic acid has been tested. The consumption of MUFA, especially oleic acid, has been shown to decrease plasma TAG and cholesterol concentrations, without affecting plasma high-density lipoproteins (HDL) levels in healthy subjects [60]. Based on the data of nine intervention studies on the cardiovascular (CV) effects of milks containing EPA and DHA and/or oleic acid, it was concluded that these types of enriched milks in the context of a balanced diet and healthy lifestyle lead to desirable CV effects. Regarding the relationship between oleic acid intake and cancer risk, this MUFA may have a potential role in lowering the risk of breast and stomach cancer, as well as in ovary, colon, and endometrium cancer [61, 62]. It has been hypothesized that the anticancer effect may relate mainly to the ability of oleic acid to regulate oncogenes [63]. However, both SFA and MUFA are FA classes that may be attained from many different food sources and not specific to seafood.
The benefits from the ingestion of n-3 PUFA were demonstrated some years ago with studies in Inuit populations of Greenland Eskimos of Alaska fishermen and coastal areas of Japan. Studies revealed that the risk of CVD was higher in Caucasian populations, compared with the Inuit populations studied. These same studies showed that the Eskimos had a lower level of plasma cholesterol, TAG and very low-density lipoprotein (VLDL) and a higher level of HDL, despite a higher fat intake from mammals and fish. The major cause was ascribed to the high n-3 PUFA intake in the traditional Inuit diet compared with the typical Caucasian population diet [64, 65]. In addition, the two most important n-3 PUFAs, DHA and EPA, are deemed to display some anti-inflammatory properties, thereby offsetting the pro-inflammatory effects of n-6 PUFA [66].
Moreover, studies on humans and experimental animal studies indicate that consumption of LC-PUFA fosters calcium absorption, increasing bone density [67]. A strong correlation between dietary intake of DHA and EPA and the consequent increase in bone calcium and reduced urinary deoxypyridinoline [67] was also observed. Possible mechanisms underlying these effects have been related with the renal calcium metabolism, the inhibition of the production of inflammatory cytokines that act as stimulants of osteoclastic bone resorption, and the regulation of the normal balance between bone and ectopic calcification.
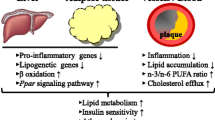
Several epidemiological studies also pointed out a beneficial effect of ingesting daily doses of EPA/DHA on the risk of certain cancers such as breast cancer, prostate cancer, and colon cancer [68]. DHA is selectively concentrated in the synaptic and retinal membranes, and it is thought to be related to visual function, brain development, behavior, and learning. Indeed, some studies have shown that DHA concentration in blood plasma in pregnant women is related with properties in visual and cognitive development of children [69]. Other studies indicate the beneficial effect of n-3 PUFA at the neurological level as well as regarding antidepressive and mental health [69, 70]. Indirectly, the intake of EPA/DHA can be linked to the prevention of the development of type 2 diabetes due to decreased activation of transcription factor NF-kB (nuclear factor kappa-light-chain-enhancer of activated B cells) associated with increases in the activity of PPAR (peroxisome proliferator-activated receptors) from peroxisome (Fig. 1). Specifically, EPA/DHA either directly or through their prostaglandin and leukotriene derivatives activate PPARs, which heterodimerize with the retinoid X receptor (RXR) and operate together with coactivators (Coact), and these sets PPAR-RXR-Coact bind to specific regions of some genes altering their transcription and leading to an increase of FA storage in adipocytes, thereby reducing the FAs in circulation. This, in turn, fosters carbohydrate oxidation as an energy source for cell metabolism. On the other hand, the binding of PPAR to Coacts seems to reduce the levels of Coacts available for binding to NF-kB. As a result, there is a reduction of NF-kB activation that may decrease the expression of the transmembrane receptor for advanced glycosylation end products, which is associated to atherosclerosis and diabetes [71]. It seems that n-3 PUFA intake has a therapeutic effect in lowering levels of total TAG and preventing type 2 diabetes, playing a protective role against lipid peroxidation [72]. Thus, the importance of seafood EPA and DHA to health is quite evident.
n-3 PUFA and the cardiovascular system: evidence
Among seafood lipids, the n-3 PUFAs are the most relevant concerning cardiovascular health. Indeed, the n-3 PUFAs have been linked to benefits for the cardiovascular system [64, 65]. Epidemiological studies have confirmed that the fat consumed by the Inuit population was rich in the n-3 PUFAs EPA and DHA, and this fact was correlated with a cardioprotective effect on the CVD risk [64, 73]. Further studies in this area have been published. Namely, two important intervention studies have found a cardioprotective effect of EPA and DHA consumption:
-
(1)
The DART—Diet and reinfarction trial—was a study of secondary prevention, in which male individuals, who had previously suffered a myocardial infarction (MI), ingested over 2-year oil capsules with 900 mg/day of EPA and DHA or 200–400 g/week of fatty fish containing 500–800 mg/day of n-3 PUFA. The study showed a 29 % reduction in overall mortality as a result of fatty fish intake [74].
-
(2)
The GISSI, “Gruppo Italiano per Studio della Sopravvivenza nell miocardio Infarction,” a secondary prevention study with 11,324 patients surviving a myocardial infarction, which for 42 months received capsules with 850 mg/day of EPA + DHA with or without vitamin E. In both cases, there was a 15 % reduction in deaths due to heart attack or stroke. The overall mortality decreased by 21 % and sudden death caused by heart disease fell by 45 % [75].
On the other hand, there have also been meta-analysis studies that draw on intervention and/or epidemiological studies and try to assess the level of evidence. Namely, a comprehensive analysis of 11 studies, comprising 222,364 people and an average follow-up of 11.8 years, showed that the relative risk of cardiovascular disease was 0.85 for eating fish once a week, 0.77 for two to four times per week and 0.62 for ≥5 times per week compared to people with an intake of less than a monthly portion of fish. Furthermore, for each increase in fish consumption of 20 g/day, a decrease of 7 % in mortality from CVD was determined [76].
In most studies, risk reduction has been observed for fatal coronary heart disease (CHD) [76] and less consistently for non-fatal coronary events [77]. In addition to benefit from moderate fish intake, it was found in a Japanese cohort that the risk of CHD events has been lower in individuals consuming fish, at least, eight times per week than in those consuming only once a week [77]. However, this was not observed in another Japanese cohort study [78]. In general, studies provide evidence for beneficial effects on the risk of fatal CHD events in individuals consuming fish in moderate amounts, but the extra health benefit from higher consumption is still disputed. Moreover, there are specific groups deserving special attention. Namely, in diabetic patients with a high risk of CHD, beneficial associations between fish intake and CHD risk have been reported [79].
The omega-3 index (the sum of the content of EPA and DHA in erythrocyte membrane) was proposed as a new risk factor for CHD evaluation [80]. From the analysis of the relationship between omega-3 index and risk of death from CHD, these authors concluded that this risk decreased by 90 % when the omega-3 index increased 4 % to greater than 8 %. These authors also defined a scale for assessing risk of death from CHD using this index of omega-3: 0–4 % area of highest risk; 4–8 % zone of intermediate risk; and more than 8 %, low risk area [80].
All the aforementioned studies show that there is a significant amount of evidence associating cardiovascular health benefits and fatty fish consumption with particular importance of EPA and DHA contents. It should also be noted that the fat level of fish and its culinary preparation methods are possible confounders in studying the relationship between fish intake and CHD risk. Therefore, further study is still required for consolidating evidence, ascertaining the most adequate levels of EPA and DHA intake and the special needs of particular population groups.
n-3 PUFA and the cardiovascular system: mechanisms and particular effects
The relationship between n-3 PUFA, namely, EPA and DHA, and the cardiovascular system has elicited many studies concerning the mechanisms underlying this connection. Such studies may shed light into the subject and provide help in modulating fish consumption and EPA + DHA intake for a maximal cardiovascular health benefit.
Originally, the benefits associated to the consumption of n-3 PUFA were ascribed to antithrombotic effects. However, more recent studies point to the predominance of the antiarrhythmic benefits of n-3 PUFA intake. It was found that supplementation of n-3 PUFA in patients who suffered myocardial infarction was correlated with decreased risk of mortality associated with cardiac arrhythmia [2]. Permeability modulation of the plasma membrane in the stabilization of the ion channels suggests that EPA/DHA may have protective properties in the cells of the heart muscle tissue. This effect is achieved through the enrichment of cardiac membranes in EPA and DHA [2, 81].
Another route for the cardiovascular benefits of n-3 PUFA may be the lowering of blood pressure. Indeed, consumption of oily fish high in DHA such as anchovy, herring, mackerel, and salmon has been suggested to decrease blood pressure in some individuals [82, 83]. DHA appears to have a more important role than EPA in lowering blood pressure. This decrease could be related to inhibition of the renin-angiotensin system (RAS), including angiotensin converting enzyme (ACE), thereby inhibiting the release of the hormone aldosterone, which is responsible for the increase in blood pressure [84, 85]. More recently, it has been argued that n-3 PUFA lower blood pressure by directly activating large-conductance Ca2+-dependent K+ channels [86].
Intake of daily doses of n-3 PUFA is also associated with improvements in the reduction of atherosclerotic platelet aggregation as well as decreased production of clotting factors and fibrinolytic factors [80, 87]. For atherosclerosis, a study [88] has indicated that the dietary intake of long-chain n-3 PUFAs or non-fried fish can be brought together with a lower prevalence of subclinical atherosclerosis classified by common carotid intima-media thickness (cCIMT), although significant changes in internal CIMT (iCIMT), coronary artery calcium (CAC) score, and ankle-brachial index (ABI) were not observed. These findings also suggested that the association of fish and atherosclerosis may vary depending on the type of fish meal consumed and the ways used to measure atherosclerosis. Regarding the precise underlying mechanisms for the n-3 PUFA effects on atherosclerosis, different hypotheses have been put forward over the years. Namely, platelet-derived growth factor (PDGF) has been proposed to play a key role in the development of advanced atherosclerotic lesions by stimulating the migration and proliferation of vascular smooth muscle cells [89], and n-3 PUFA has been reported to significantly inhibit PDGF-induced migration [90]. It has also been shown that atherosclerotic plaques readily incorporate EPA and a higher EPA plaque content is associated with a reduced number of foam cells and T cells, less inflammation, and increased plaque stability [91]. Hence, an alternative n-3 PUFA protective mechanism of action could be the stabilization of atherosclerotic plaques through the anti-inflammatory actions of some particular n-3 PUFA.
Among the possible mechanisms for the intake of n-3 PUFA to reduce CVD and platelet stability, the effect on decreasing the total lipids in plasma, TAG (approximately 25–30 %), and VLDL production [64, 92] is described. Studies also indicate that consumption of n-3 PUFA is related to the biophysical properties of cell membranes (namely, membrane fluidity enhancement due to the highly unsaturated degree of DHA) as well as to the relaxation of the endothelium, the path involving vasodilatation, enhanced by increased production of NO (nitric oxide), and/or by suppression of the influx of Ca2+ through the channel activated by transmembrane potential differences in smooth muscle cells and endothelium. Inhibition of these channels also contributes to the reduction of the large and rapid fluctuations in the concentration of free Ca2+, which, in turn, favors the reduction of ventricular arrhythmias—seen with supplementation with fish oil capsules [44, 93].
This last aspect is very important, since both fatal coronary heart disease (CHD) and sudden cardiac death (SCD) most often share fatal ventricular arrhythmia as a final common pathway [94]. Observational studies, randomized clinical trials, and experimental studies provide concordant evidence that not very high consumption of fish or fish oil (about 250 mg of EPA + DHA/day) decreases the risk of CHD death and SCD. The magnitude of the effect is also important, with pooled analysis indicating 36 % lower risk of CHD death with modest consumption compared with no consumption [94].
A beneficial effect of n-3 PUFA on the prevention of stroke [95] through the reduction of blood cholesterol [96] and blood pressure [86] has also been reported. Furthermore, in an experimental study with mice, it has been claimed that n-3 PUFA supplementation is a potential angiogenic treatment capable of augmenting brain repair and improving long-term functional recovery after ischemic stroke [97]. Mechanistically, n-3 PUFAs were able to induce upregulation of angiopoietin 2 (Ang 2) in astrocytes after transient focal cerebral ischemia and stimulated extracellular Ang 2 release from cultured astrocytes after oxygen and glucose deprivation. Ang 2 facilitated endothelial proliferation and barrier formation in vitro by potentiating the effects of vascular endothelial growth factor, for instance, on phospholipase Cγ1 [97].
Therefore, CVD—an entity that includes arteriosclerosis, atherosclerosis, thrombosis, myocardial infarction, and stroke, provided that the occurrence of stroke is related to the physiological condition of a blood vessel [98] and that has high blood pressure, hyperlipidemia, and hyperglycemia as risk factors—may be prevented and mitigated by n-3 PUFA intake through reduction of, at least, two major factors: hypertension and hyperlipidemia.
Seafood lipids and cardiovascular health: dietary recommendations
In general, dietary advices acknowledge the importance of seafood lipids, thereby prescribing a weekly consumption of one to two portions of fatty fish [99]. Particularly, there are currently numerous recommendations for the intake of n-3 PUFA for the prevention of deficiency in essential FAs and to decrease the risk of developing cardiovascular disease. The World Health Organization (WHO) recommends for the prevention of CVD and ischemic stroke, the intake of n-3 PUFA in the diet should represent 6 to 10 % of total energy intake (5–8 % n-6 PUFA and 1–2 % n-3 PUFA), and a regular intake of one to two servings of fatty fish per week, equivalent to 200 to 500 mg/day of EPA and DHA [56]. Moreover, the EPA + DHA recommendation daily intake (RDI) by the European Food Safety Authority (EFSA) based on cardiovascular risk considerations for European adults is between 250 and 500 mg/day1, and the American Heart Association (AHA) recommends a daily intake of 400–500 mg of EPA + DHA [100].
Conclusions
Seafood lipids are a quite vast set of molecules comprising TAG, FFA, PL, sterols, and others. The molecules mainly yield FA molecules after digestion in the human gut. The bioaccessibility and bioavailability of these FAs depend on diverse factors, namely, degree of saturation and chemical binding form. The bioavailable FAs are the fraction of the seafood lipids that has the largest effect on human health. Different classes of FAs (SFA, MUFA, n-3 PUFA) exert an influence upon health outcomes. Among these, cardiovascular health is much improved by the n-3 PUFA and, particularly, by EPA and DHA. It has been found that n-3 PUFA has antiarrhythmic benefits, reduces atherosclerotic platelet aggregation, diminishes the prevalence of subclinical atherosclerosis, and lowers blood pressure. Nevertheless, the underlying mechanisms are not completely understood and require further experimental studies for a deeper knowledge. The mentioned beneficial effects help to explain the positive impact of n-3 PUFA on CHD and SCD as well as in other CVDs, such as stroke. Accordingly, it is recommended up to two servings of fatty fish per week or up to 1000 mg/day of EPA and DHA.
Abbreviations
- ABI:
-
Ankle-brachial index
- ACE:
-
Angiotensin converting enzyme
- AHA:
-
American Heart Association
- ALA:
-
α-Linolenic acid
- Ang 2:
-
Angiopoietin 2
- CAC:
-
Coronary artery calcium
- cCIMT:
-
Common carotid intima-media thickness
- CHD:
-
Coronary heart disease
- CM:
-
Chylomicron
- Coact:
-
Coactivator
- CVD:
-
Cardiovascular disease
- DAG:
-
Diacylglycerol
- DART:
-
Diet and reinfarction trial
- DHA:
-
Docosahexaenoic acid
- EE:
-
Ethyl esters
- EFSA:
-
European Food Safety Authority
- EPA:
-
Eicosapentaenoic acid
- FA:
-
Fatty acid
- FFA:
-
Free fatty acid
- GISSI:
-
Gruppo italiano per studio della sopravvivenza nell miocardio infarction
- HDL:
-
High-density lipoprotein
- iCIMT:
-
Internal carotid intima-media thickness
- LC-PUFA:
-
Long-chain polyunsaturated fatty acid
- LDL:
-
Low-density lipoprotein
- MAG:
-
Monoacylglycerol
- MUFA:
-
Monounsaturated fatty acid
- NF-kB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- n-3 PUFA:
-
n-3 Polyunsaturated fatty acid
- n-6 PUFA:
-
n-6 Polyunsaturated fatty acid
- PDGF:
-
Platelet-derived growth factor
- PL:
-
Phospholipid
- PPAR:
-
Peroxisome proliferator-activated receptors
- RAS:
-
Renin-angiotensin system
- RCT:
-
Randomized controlled trial
- RDI:
-
Recommendation daily intake
- rTAG:
-
Re-esterified triacylglycerol
- RXR:
-
Retinoid X receptor
- SCD:
-
Sudden cardiac death
- SFA:
-
Saturated fatty acid
- TAG:
-
Triacylglycerol
- VLDL:
-
Very low-density lipoprotein
- WHO:
-
World Health Organization
References
EFSA (2012). EFSA panel on dietetic products, nutrition and allergies (NDA); scientific opinion related to the tolerable upper intake level of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA). Accessed on 23rd July 2013. (URL: http://www.efsa.europa.eu/en/efsajournal/doc/2815.pdf).
Wang C, Harris WS, Chung M, Lichtenstein AH, Balk EM, Kupelnick B, Jordan HS, Lau J. n-3 Fatty acids from fish or fish-oil supplements, but not α-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am J Clin Nutr. 2006;84:5–17.
Botta JR, Kennedy K, Squires BE. Effect of method of catching and time of season on the composition of Atlantic cod (Gadus morhua). J Food Sci. 1986;52(922–924):927.
Soccol MCH, Oetterer M. Seafood as functional food. Braz Arch Biol Technol. 2003;46(3):443–54.
Bang HO, Dyerberg J, Sinclair HM. The composition of the Eskimo food in northwestern Greenland. Am J Clin Nutr. 1980;33:2657–61.
Brenna JT. Efficiency of conversion of alpha-linolenic acid to long chain n-3 fatty acids in man. Curr Opin Clin Nutr Metab Care. 2002;5:127–32.
Burdge GC, Finnegan YE, Minihane AM, Williams CM, Wootton SA. Effect of altered dietary n-3 fatty aid intake upon plasma lipid fatty acid composition, conversion of [13C]α-linolenic acid to longer-chain fatty acids and partitioning towards β-oxidation in older men. Br J Nutr. 2003;90:311–21.
Voss A, Reinhart M, Sankarappa S, Sprecher H. The metabolism of 7,10,13,16,19-docosapentaenoic acid to 4,7,10,13,16,19-docosahexaenoic acid in rat liver is independent of a 4-desaturase. J Biol Chem. 1991;266(30):19995–20000.
Médale F, Lefèvre F, Corraze G. Qualité nutritionnelle et diététique des poissons, constituants de la chair et facteurs de variations. Cahiers de Nutrition et de Diététique. 2003;38(1):37–44.
Ackman RG, Ratnayake WMN, Olsson B. The “basic” fatty acid composition of Atlantic fish oils: potential similarities useful for enrichment of polyunsaturated fatty acids by urea complexation. J Am Oil Chem Soc. 1988;65:136–8.
Ackman RG, Ratnayake WMN. (1992). Non-enzymatic oxidation of seafood lipids. In: Advances in Seafood Biochemistry. Composition and Quality, G. J. Flick, R. E. Martin (Eds), pp. 245-267. Technomic publishing Co., Inc.: Basel, Switzerland.
Arts MT, Ackman RG, Holub BJ. “Essential fatty acids” in aquatic ecosystems: a crucial link between diet and human health and evolution. Can J Fish Aquat Sci. 2001;58:122–37.
Pawlosky RJ, Hibbeln JR, Novotny JA, Salem N. Physiological compartmental analysis of alpha-linolenic acid metabolism in adult humans. J Lipid Res. 2001;42:1257–65.
Plourde M, Cunnane SC. Extremely limited synthesis of long chain polyunsaturates in adults: implications for their dietary essentiality and use as supplements. Appl Physiol Nutr Metab. 2007;32(4):619–34.
Rincón L, Castro PL, Álvarez B, Hernández MD, Álvarez A, Claret A, Guerrero L, Ginés R. Differences in proximal and fatty acid profiles, sensory characteristics, texture, colour and muscle cellularity between wild and farmed blackspot seabream (Pagellus bogaraveo). Aquaculture. 2016;451:195–204.
Ackman RG. Structural homogeneity in unsaturated fatty acids of marine lipids. A review. J Fish Res Board Can. 1964;21:247–54.
Ackman RG, Eaton CA. Some commercial Atlantic herring oils; Fatty acid composition. J Fish Res Board Can. 1966;23:991–1006.
Sargent JR, Whittle KJ. (1981). Lipids and hydrocarbons in the marine food web. In: Analysis of Marine Ecosystems, A. R. Longhurst (Ed.), pp. 491-533. Academic Press, Inc.: London, UK.
Ackman RG. (1979). Fish lipids. Part 1. In: Advances in Fish Science and Technology, J. J. Connell (Ed.), pp. 86-103. Fishing News Books Ltd.: Farham, UK.
Christie WW. (1989). Fatty acids and lipids: structures, extraction and fractionation into classes. In: Gas chromatography and lipids, W. W. Christie (Ed), pp. 11-62. The Oily Press: Ayr, UK.
Eaton CA, Ackman RG, Tocher CS, Spencer KD. Canadian capelin 1972-1973. Fat and moisture compositions, and fatty acids of some oils and lipid extract triglycerides. J Fish Res Board Can. 1975;32:507–13.
Ackman RG. Simplification of analyses of fatty acids in fish lipids and related lipid samples. Acta Medica Scandinavica. 1987;222:99–103.
Shanta NC, Ackman RG. Fish oil tetracosenoic acid isomers and GLC analyses of polyunsaturated fatty acids. Lipids. 1991;26:237–9.
US Department of Agriculture, Agricultural Research Service, USDA-ARS (2005). USDA Nutrient Database for Standard Reference, Release 18. Accessed 2013-01-21 from the Nutrient Data Laboratory (URL: http://www.ars.usda.gov).
Souci SW, Fachmann W, Kraut H. Food composition and nutrition tables (6th edition revised and completed). Boca Raton: Medpharm Scientific Publishers, CRC Press; 2000.
Bandarra NM, Calhau MA, Oliveira L, Ramos M, Dias MG, Bartolo H, Faria MR, Fonseca MC, Gonçalves J, Batista I, Nunes ML. Composição e valor nutricional dos produtos da pesca mais consumidos em Portugal. Publicações Avulsas IPIMAR. 2004;11:1–103.
Sirot V, Oseredczuk M, Bemrah-Aouachria N, Volatier JL, Leblanc JC. Lipid and fatty acid composition of fish and seafood consumed in France: CALIPSO study. J Food Compos Anal. 2008;21:8–16.
Hayashi K, Takagi T. Seasonal variation in lipids and fatty acids of Japanese anchovy Engraulis japonica. Bull Fac Fish Hokkaido University. 1978;29:38–47.
Kayama M, Hirata M, Hisai T. Effect of water temperature on the desaturation of fatty acids in carp. Bull Jpn Soc Sci Fish. 1986;52:853–7.
Cowey CB, Sargent JR. Fish nutrition. Adv Mar Biol. 1972;10:383–492.
Worthington RE, Lovell RT. Fatty acids of channel catfish (Ictalurus punctatus): variance components related to diet, replications within diets, and variability among fish. J Fish Res Board Can. 1973;30:1604–8.
Cardoso C, Afonso C, Lourenço H, Costa S, Nunes ML. Bioaccessibility assessment methodologies and their consequences for the risk-benefit evaluation of food. Trends Food Sci Technol. 2015;41:5–23.
Paustenbach DJ. The practice of exposure assessment: a state-of-the-art review (reprinted from Principles and Methods of Toxicology, 4th edition, 2001). J Toxicol Environ Health B Crit Rev. 2000;3:179–291.
Schumann K, Classen HG, Hages M, Prinz-Langenhol R, Pietrzik K, Biesalski HK. Bioavailability of oral vitamins, minerals and trace elements in perspective. Drug Res. 1997;47:369–80.
Versantvoort CHM, Oomen AG, Van de Kamp E, Rompelberg CJM, Sips AJAM. Applicability of an in vitro digeston model in assessing the bioaccessibility of mycotoxins from food. Food Chem Toxicol. 2005;43:31–40.
Minoo P, Moyer MP, Jass JR. Role of BRAF-V600E in the serrated pathway of colorectal tumourigenesis. J Pathol. 2007;212(2):124–33.
LeCluyse EL, Witek RP, Andersen ME, Powers MJ. Organotypic liver culture models: meeting current challenges in toxicity testing. Crit Rev Toxicol. 2012;42(6):501–48.
Mu H, Høy C. The digestion of dietary triacylglycerols. Prog Lipid Res. 2004;3:105–33.
Nelson L, Cox M. Lehninger principles of biochemistry. 4th ed. New York: W. H. Freeman and Company; 2005.
Schuchardt JP, Hahn A. Bioavailability of long-chain omega-3 fatty acids. Prostaglandins Leukot Essent Fat Acids. 2013;89:1–8.
Afonso C, Costa S, Cardoso C, Bandarra NM, Batista I, Coelho I, Castanheira I, Nunes ML. Evaluation of the risk/benefit associated to the consumption of raw and cooked farmed meagre based on the bioaccessibility of selenium, eicosapentaenoic acid and docosahexaenoic acid, total mercury, and methylmercury determined by an in vitro digestion model. Food Chem. 2015;170:249–56.
Costa S, Afonso C, Cardoso C, Batista I, Chaveiro N, Nunes ML, Bandarra NM. Fatty acids, mercury, and methylmercury bioaccessibility in salmon (Salmo salar) using an in vitro model: effect of culinary treatment. Food Chem. 2015;185:268–76.
Dadorama, S. (1996). Amino acids, peptides, and proteins. In: Food Chemistry Fennema, O. R. (ed.), pp. 321–429. Marcel Dekker Inc.: New York, USA (ISBN: 978-0824796914).
Doughman S, Krupanidhi S, Sanjeevi CB. Omega-3 fatty acids for nutrition and medicine: considering microalgae oil as a vegetarian source of EPA and DHA. Curr Diabetes Rev. 2007;3:198–203.
Akanbi TO, Sinclair AJ, Barrow CJ. Pancreatic lipase selectively hydrolyses DPA over EPA and DHA due to location of double bonds in the fatty acid rather than regioselectivity. Food Chem. 2014;160:61–6.
Dyerberg J, Madsen P, Møller JM, Aardestrup I, Schmidt EB. Bioavailability of marine n-3 fatty acid formulations. Prostaglandins Leukot Essent Fat Acids. 2010;83:137–41.
Neubronner J, Schuchardt JP, Kressel G, Merkel M, von Schacky C, Hahn A. Enhanced increase of omega-3 index in response to long-term n-3 fatty acid supplementation from triacylglycerides versus ethylesters. Eur J Clin Nutr. 2011;65:247–54.
Yang LY, Kuksis A, Myher JJ. Lipolysis of menhaden oil triacylglycerols and the corresponding fatty acid alkyl esters by pancreatic lipase in vitro: a reexamination. J Lipid Res. 1990;31:137–47.
Yang LY, Kuksis A, Myher JJ. Intestinal absorption of menhaden and rapeseed oils and their fatty acid methyl and ethyl esters in the rat. Biochem Cell Biol. 1990;68:480–91.
Maki KC, Reeves MS, Farmer M, Griinari M, Berge K, Vik H, Hubacher R, Rains TM. Krill oil supplementation increases plasma concentrations of eicosapentaenoic and docosahexaenoic acids in overweight and obese men and women. Nutr Res. 2009;29:609–15.
Schuchardt JP, Schneider I, Meyer H, Neubronner J, von Schacky C, Hahn A. (2011). Incorporation of EPA and DHA into plasma phospholipids in response to different omega-3 fatty acid formulations—a comparative bioavailability study of fish oil vs. krill oil. Lipids in Health and Disease, 10: 145.
Mun S, Decker EA, McClements DJ. Influence of emulsifier type on in vitro digestibility of lipid droplets by pancreatic lipase. Food Res Int. 2007;40:770–81.
Kling DF, Johnson J, Rooney M, Davidson M. Omega-3 free fatty acids demonstrate more than 4-fold greater bioavailability for EPA and DHA compared with omega-3-acid ethyl esters in conjunction with a low-fat diet: the ECLIPSE study. J Clin Lipidol. 2011;5(3):231.
EFSA (2009). Scientific opinion of the panel on dietetic products, nutrition and allergies on a request from the commission related to labelling reference intake values for n-3 and n-6 polyunsaturated fatty acids. Accessed on 10th March 2011. (URL:http://www.efsa.europa.eu/en/efsajournal/doc/1176.pdf).
Kris-Etherton PM, Taylor DS, Yu-Poth S, Huth P, Moriarty K, Fishell V. Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr. 2000;71:179S–88S.
WHO (2003). Diet nutrition and the prevention of chronic diseases: report of the WHO/FAO joint expert consultation. WHO Technical Report Series 916: Geneva, Switzerland.
Grundy SM, Denke MA. Dietary influences on serum lipids and lipoproteins. J Lipid Res. 1990;31:1149–72.
Kratz M, Cullen P, Kannenberg F, Kassner A, Fobker M, Abuja PM, Assmann G, Wahrburg U. Effects of dietary fatty acids on the composition and oxidizability of low-density lipoprotein. Eur J Clin Nutr. 2002;56(1):72–81.
Larsen L, Jespersen J, Marckmann R. Are olive oil diets antithrombotic? Diets enriched with olive, rapeseed, or sunflower oil affect postprandial factor VII differently. Am J Clin Nutr. 1999;70:976–82.
Feldman EB. Assorted monounsaturated fatty acids promote healthy hearts. Am J Clin Nutr. 1999;70:953–4.
Braga C, La Vecchia C, Franceschi S, Negri E, Parpinel M, Decarli A, Giacosa A, Trichopoulos D. Olive oil, other seasoning fats, and the risk of colorectal carcinoma. Cancer. 1998;82:448–53.
Zamora-Ardoy MA, Báñez SF, Báñez SC, Alaminos GP. Olive oil: influence and benefits on some pathologies. An Med Interna. 2004;21:138–42.
Gallus S, Bosetti C, La Vecchia C. Mediterranean diet and cancer risk. Eur J Cancer Prev. 2004;13:447–52.
Barrow CJ, Nolan C, Holub BJ. Bioequivalence of encapsulated and microencapsulated fish-oil supplementation. J Funct Foods. 2009;1:38–43.
Dyerberg J, Bang HO, Hjorn N. Fatty acid composition of the plasma lipids in Greenland Eskimos. Am J Clin Nutr. 1975;28:958–66.
Calder PC. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2015;1851:469–84.
Kruger M, Schollum L. Is docosahexaenoic acid more effective than eicosapentaenoic acid for increasing calcium bioavailability? Prostaglandins Leukot Essent Fat Acids. 2005;73:327–34.
Beelen VA, Spenkelink B, Mooibroek H, Sijtsma L, Bosch ID, Rietjens MCM, Alink GM. An n-3 PUFA-rich microalga oil diet protects to a similar extent as a fish oil-rich diet against AOM-induced colonic aberrant crypt foci in F344 rats. Food Chem Toxicol. 2009;47:316–20.
Dyall SC, Michael-Titus AT. Neurological benefits of omega-3 fatty acids. Neruomol Med. 2008;4:219–35.
Kimura F, Endo Y, Fujimoto K, Doisaki N, Koriyama T. Administration of two oils rich in n-3 long-chain polyunsaturated fatty acids to rat pups of dams fed a diet high in fat and low in n-3 polyunsaturated fatty acids. Fish Sci. 2005;71:431–40.
Bierhaus A, Schiekofer S, Schwaninger M, Andrassy M, Humpert PM, Chen J, Hong M, Luther T, Henle T, Klöting I, Morcos M, Hofmann M, Tritschler H, Weigle B, Kasper M, Smith M, Perry G, Schmidt AM, Stern DM, Häring HU, Schleicher E, Nawroth PP. Diabetes-associated sustained activation of the transcription factor nuclear factor-kB. Diabetes. 2001;50(12):2792–808.
Abuissa H, O'Keefe JH. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers for prevention of type 2 diabetes: a meta-analysis of randomized clinical trials. J Am Coll Cardiol. 2005;46:821–6.
Wijendran V, Hayes C. Dietary n-6 and n-3 fatty acid balance and cardiovascular health. Annu Rev Nutr. 2004;24:597–615.
Burr ML, Fehily M, Rogers S, Welsby E, King S, Sandham S. Diet and reinfarction trial (DART): design, recruitment, and compliance. Eur Heart J. 1989;10:558–67.
Prevenzione Investigators GISSI. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E in 11,324 patients with myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354:447–55.
He K, Song Y, Daviglus ML, Liu K, Van Horn L, Dyer AR, Greenland P. Accumulated evidence on fish consumption and coronary heart disease mortality: a meta-analysis of cohort studies. Circulation. 2004;109(22):2705–11.
Iso H, Kobayashi M, Ishihara J, Sasaki S, Okada K, Kita Y, Kokubo Y, Tsugane S, JPHC Study Group. Intake of fish and n3 fatty acids and risk of coronary heart disease among Japanese: the Japan Public Health Center-based (JPHC) study cohort. Circulation. 2006;113:195–202.
Nakamura Y, Ueshima H, Okamura T, Kadowaki T, Hayakawa T, Kita Y, Tamaki S, Okayama A, NIPPON DATA80 Research Group. Association between fish consumption and all-cause and cause-specific mortality in Japan: NIPPON DATA80, 1980–99. Am J Med. 2005;118(3):239–45.
Hu FB, Cho E, Rexrode KM, Albert CM, Manson JE. Fish and long-chain ω-3 fatty acid intake and risk of coronary heart disease and total mortality in diabetic women. Circulation. 2003;107:1852–7.
Harris W, Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med. 2004;39:212–20.
Mozaffarian D, Psaty BM, Rimm EB. Fish intake and risk of incident atrial fibrillation. Circulation. 2004;110:368–73.
Liu JC, Conklin SM, Manuck SB, Yao JK, Muldoon MF. Long-chain omega-3 fatty acids and blood pressure. Am J Hypertens. 2011;24(10):1121–6.
Saravanan P, Davidson NC, Schmidt EB, Calder PC. Cardiovascular effects of marine omega-3 fatty acids. Lancet. 2010;376(9740):540–50.
Das U. Beneficial effects of n-3 fatty acids in cardiovascular diseases: but, why and how? Prostaglandins Leukot Essent Fat Acids. 2000;63:351–62.
Engler MM, Engler MB, Kroetz DL. The effects of a diet rich in docosahexaenoic acid on organ and vascular fatty acid composition in spontaneously hypertensive rats. Prostaglandins Leukot Essent Fat Acids. 1999;61:289–95.
Hoshi T, Wissuwa B, Tian Y, Tajima N, Xu R, Bauer M, Heinemann SH, Hou S. Omega-3 fatty acids lower blood pressure by directly activating large-conductance Ca2+-dependent K+ channels. Proc Natl Acad Sci U S A. 2013;110(12):4816–21.
Thies F, Garry JM, Yaqoob P, Rerkasem K, Williams J, Shearman CP, Gallagher PJ, Calder PC, Grimble RF. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomized controlled trial. Lancet. 2003;361:477–85.
He K, Liu K, Daviglus ML, Mayer-Davis E, Jenny NS, Jiang R, Ouyang P, Steffen LM, Siscovick D, Wu C, Barr RG, Tsai M, Burke GL. Intakes of long-chain n-3 polyunsaturated fatty acids and fish in relation to measurements of subclinical atherosclerosis. Am J Clin Nutr. 2008;88(4):1111–8.
Shiina T, Terano T, Saito J, Tamura Y, Yoshida S. Eicosapentaenoic acid and docosahexaenoic acid suppress the proliferation of vascular smooth muscle cells. Atherosclerosis. 1993;104(1-2):95–103.
Mizutani M, Asano M, Roy S, Nakajima T, Soma M, Yamashita K, Okuda Y. ω-3 Polyunsaturated fatty acids inhibit migration of human vascular smooth muscle cells in vitro. Pharmacol Lett. 1997;61(19):PL-269–74.
Cawood AL, Ding R, Napper FL, Young RH, Williams JA, Ward MJA, Gudmundsen O, Vige R, Payne SPK, Ye S, Shearman CP, Gallagher PJ, Grimble RF, Calder PC. Eicosapentaenoic acid (EPA) from highly concentrated n-3 fatty acid ethyl esters is incorporated into advanced atherosclerotic plaques and higher plaque EPA is associated with decreased plaque inflammation and increased stability. Atherosclerosis. 2010;212:252–9.
Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. 2002;21:495–505.
Hooper L, Thompson RI, Harrison RA. Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: systematic review. Br Med J. 2006;332:752–5.
Mozaffarian D. Fish and n-3 fatty acids for the prevention of fatal coronary heart disease and sudden cardiac death. Am J Clin Nutr. 2008;87:1991S–6S.
Navab M, Ananthramaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Fonarow GC, Vahabzadeh K, Hama S, Hough G, Kamranpour N, Berliner JA, Lusis AJ, Fogelman AM. The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J Lipid Res. 2004;45(6):993–1007.
Apostolopoulou M, Michalakis K, Miras A, Hatziolios A. Nutrition in the primary and secondary prevention of stroke. Maturitas. 2012;72:29–34.
Wang J, Shi Y, Zhang L, Zhang F, Hu X, Zhang W, Leak RK, Gao Y, Chen L, Chen J. Omega-3 polyunsaturated fatty acids enhance cerebral angiogenesis and provide long-term protection after stroke. Neurobiol Dis. 2014;68:91–103.
Foroughi M, Akhavanzanjani M, Magahsoudi Z, Ghiasvand R, Khorvash F, Askari G. Stroke and nutrition: a review of studies. Int J Prev Med. 2013;4 Suppl 2:165–79.
ISSFAL (International Society for the Study of Fatty Acids & Lipids). Report of the sub-committee on recommendations for intake of polyunsaturated fatty acids in healthy adults. Brighton: ISSFAL; 2004.
Kris-Etherton P, Harris W, Appel L, American Heart Association Nutrition Commitee. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–57.
Acknowledgements
The authors, Cláudia Afonso and Carlos Cardoso, acknowledge the individual Post Doctoral Grant (Ref. SFRH/BPD/64951/2009 and Ref. SFRH/BPD/102689/2014, respectively) funded by the “Fundação para a Ciência e a Tecnologia” (FCT).
Authors’ contributions
CC wrote the three last sections, CA wrote the two previous sections, and NB coordinated and wrote the first two sections. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Cardoso, C., Afonso, C. & Bandarra, N.M. Seafood lipids and cardiovascular health. Nutrire 41, 7 (2016). https://doi.org/10.1186/s41110-016-0008-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41110-016-0008-8