Abstract
Background
Renal transplant recipients with chronic kidney disease (CKD) often develop abnormal glucose metabolism. Although recent studies have reported the protective effects of sodium-glucose transport protein 2 (SGLT2) inhibitors on the heart and kidneys, few have assessed their effect in renal transplant patients. Moreover, to our knowledge, there have been no studies on the effects of SGLT2 inhibitors in renal transplant recipients in Japan.
Case presentation
Case 1 was a 67-year-old male renal transplant recipient with post-transplant diabetes mellitus. He was administered empagliflozin 10 mg once a day for 9 months. Over time, his HbA1c levels decreased from 6.8 to 6.0%. Case 2 was a 56-year-old male renal transplant recipient with fatty liver disease. He was administered empagliflozin 10 mg once a day for 9 months. His ALT, γ-GTP, and LDL-cholesterol levels all decreased. In both patients, body weight and the urine albumin to creatinine ratio (UACR) decreased after empagliflozin administration, but there were no changes in the estimated glomerular filtration rate. No adverse events occurred in either case.
Conclusions
Administration of empagliflozin had favorable outcomes in two patients with stage G3b CKD and abnormal glucose metabolism after renal transplantation. Further studies will be required to clarify the efficacy and safety of SGLT2 inhibitors in a larger population of patients with similar medical conditions.
Similar content being viewed by others
Background
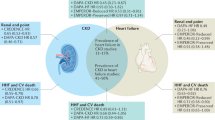
The protective effects of sodium-glucose cotransporter 2 (SGLT2) inhibitors, a class of antidiabetic agents, on the heart and kidneys were recently revealed in three large-scale clinical trials, the EMPA-REG Outcome [1], CANVAS [2], and DECLARE-TIMI 58 [3] trials. Similarly, the CREDENCE trial [4] also showed that renal outcomes were significantly improved with SGLT2 inhibitors, while the DAPA-HF study [5] showed that the SGLT2 inhibitor dapagliflozin was effective in patients with heart failure with decreased left ventricular ejection fraction, with or without type 2 diabetes. Most patients who receive kidney transplantation have chronic kidney disease (CKD) [6]. After renal transplantation, the rapid improvement in nutrition may result in overnutrition. This overnutrition in addition to the steroids and other immunosuppressants administered after kidney transplantation can lead to abnormal glucose metabolism in some patients [7]. Little is currently known about the utility of administering SGLT2 inhibitors to patients after renal transplantation. In one study, Ahmed et al. reported that there is no robust evidence to support the use of SGLT2 inhibitors in post-transplantation diabetes mellitus [8]. Here, however, we report two favorable outcomes with the SGLT2 inhibitor empagliflozin administered to patients with stage G3b CKD2 and impaired glucose metabolism following renal transplantation.
Case presentation
Case 1
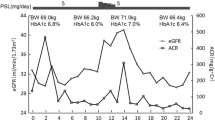
A 67-year-old male was diagnosed with chronic glomerulonephritis in 1972 and started hemodialysis in 1986. He received a living kidney transplant in 1996, with his sister as the donor. His medical history was as follows. His calcineurin inhibitor was changed from tacrolimus (TAC) to cyclosporine (CyA) because he developed diabetes early after kidney transplantation. He suffered from pneumonia seven times from the sixth year after kidney transplantation. Seven months prior to empagliflozin administration, the patient received hydrocortisone for hyponatremia caused by steroid withdrawal syndrome. He had no history of urogenital infection or dehydration. Although his blood sugar was stable, his weight gradually increased, and his glucose metabolism deteriorated. By November 2018, his HbA1c levels had increased to 6.8%, and empagliflozin was administered in addition to sitagliptin in the same month. His physical characteristics at the start of empagliflozin administration were as follows: height, 165.6 cm; weight, 69.0 kg; BMI, 25.2 kg/m2; abdominal circumference, 92 cm; blood pressure, 119/80 mmHg; pulse, 90 beats/min; and body temperature, 36.2 °C. No palpebral conjunctival anemia, ocular conjunctival yellow staining, abnormal breath sounds, heart murmurs, evidence of renal transplant surgery in the right lower abdomen, or lower leg edema were observed. In addition, there were no neurological abnormalities. For immunosuppression, the patient received hydrocortisone 20 mg/day, CyA 50 mg/day, and mizoribine (MZR) 100 mg/day. He also received the following oral medications: sitagliptin 100 mg/day, levothyroxine sodium 50 μg/day, febuxostat 10 mg/day, vonoprazan 10 mg/day, ursodeoxycholic acid 600 mg/day, montelukast sodium 10 mg/day, and pregabalin 50 mg/day. Urine protein was 1 plus. Both urinary occult blood and urinary glucose were negative at the start of the empagliflozin administration. The urine protein to creatinine ratio (UPCR) was 0.40 g/g Cr, and the urine albumin to creatinine ratio (UACR) was 135 mg/g Cr. His creatinine level was 1.69 mg/dL, and his estimated glomerular filtration rate (eGFR) of 32.8 mL/min/1.73 m2, indicating stage G3bA2 CKD. Other laboratory findings were as follows: leukocytes, 9400/μL; erythrocytes, 507 × 104/μL; hemoglobin, 12.8 g/dL; hematocrit, 40.2%; platelets, 36.2 × 104/μL; blood urea nitrogen, 22.7 mg/dL; uric acid, 4.2 mg/dL; total protein, 7.2 g/dL; albumin, 4.2 g/dL; AST, 13 IU/L (normal value, 8–38 IU/L); ALT, 8 IU/L (normal value, 4–40 IU/L); γ-GTP, 12 IU/L (normal value, < 70 IU/L); total bilirubin, 0.47 mg/dL; ALP, 239 IU/L (normal value, 100–335 IU/L); total cholesterol, 180 mg/dL; triglyceride, 121 mg/dL; HDL-cholesterol, 74 mg/dL; and LDL-cholesterol, 82 mg/dL. The area under the curve (AUC0-4) for CyA at the lowest level was 1169 h ng/mL (Table 1). Based on a renal biopsy, the dysfunction of the transplanted kidney was previously diagnosed as CyA arteriolopathy. His body weight and systolic blood pressure decreased by 3.9 kg and 30 mmHg, respectively, after empagliflozin administration.
Figure 1 shows the progress of case 1. His eGFR prior to and 9 months after empagliflozin administration were 32.8 and 32.9 mL/min/1.73 m2, respectively, and was unchanged. His UACR levels transiently increased to 135.7 mg/g Cr but then decreased to 52.8 mg/g Cr. No adverse events related to empagliflozin administration were observed.
Case 2
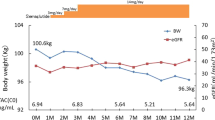
A 56-year-old male was diagnosed with IgA nephropathy in 1983 and started hemodialysis in 1990. He received his first living kidney transplant in 1991, with his father as the donor. He re-started hemodialysis in 2001 due to chronic renal rejection. In 2008, he received a second living kidney transplant, with his sister as the donor. His medical history includes herpes zoster on the right thigh in 1991. In 2013, he became positive for anti-DQ antibodies and began receiving everolimus (EVR). He had no history of urogenital infection or dehydration. In January 2019, he began treatment with empagliflozin due to high HbA1c, ALT, and γ-GTP levels caused by fatty liver disease. His physical characteristics at the start of empagliflozin administration were as follows: height, 164.2 cm; weight, 81.7 kg; BMI, 30.3 kg/m2; abdominal circumference, 104 cm; blood pressure, 129/64 mmHg; pulse, 79 beats/min; and body temperature, 36.0 °C. No palpebral conjunctival anemia, ocular conjunctival yellow staining, abnormal breath sounds, heart murmurs, evidence of renal transplant surgery in the bilateral lower abdomen, or lower leg edema were observed. There were also no neurological abnormalities. For immunosuppression, he received prednisolone (PSL) 5 mg/day, CyA 50 mg/day, MZR 500 mg/day, and EVR 0.75 mg/day. He also received the following oral medications: valsartan 80 mg/day, amlodipine 10 mg/day, fluvastatin 30 mg/day, ezetimibe 10 mg/day, febuxostat 40 mg/day, vonoprazan 10 mg/day, and alfacalcidol 0.5 μg/day. Urinary protein, occult blood, and glucose were all negative at the start of empagliflozin administration. However, the UPCR was 0.40 g/g Cr, and the UACR was 135 mg/g Cr, which was slightly increased. His creatinine level was 1.40 mg/dL, and his eGFR was 39.5 mL/min/1.73 m2, indicating stage G3bA2 CKD. Other laboratory data were as follows: leukocytes, 8700/μL; erythrocytes, 501 × 104/ μL; hemoglobin, 14.1 g/dL; hematocrit, 42.9%; platelets, 24.1 × 104/μL; blood urea nitrogen, 22.7 mg/dL; uric acid, 4.2 mg/dL; total protein, 7.2 g/dL; albumin, 4.2 g/dL; AST, 78 IU/L (normal value, 8–38 IU/L); ALT, 116 IU/L (normal value, 4–40 IU/L); γ-GTP, 112 IU/L (normal value, < 70 IU/L); total bilirubin, 0.78 mg/dL; ALP, 420 IU/L (normal value, 100–335 IU/L); total cholesterol, 203 mg/dL; triglyceride, 188 mg/dL; HDL-cholesterol, 73 mg/dL; and LDL-cholesterol, 92 mg/dL. The AUC0-4 for CyA was 1732 h ng/mL, and the trough value for EVR was 6.3 ng/mL, both of which were reasonable value for post-transplant patients (Table 2). His body weight decreased by 1.2 kg after empagliflozin administration; however, no other significant changes were observed. Figure 2 shows the progress of case 2. His eGFR prior to and 9 months after empagliflozin administration were 39.5 mL/min/1.73 m2 and 40.8 mL/min/1.73 m2, respectively, and thus unchanged. His UACR decreased from 35.8 to 18.4 mg/g Cr, which was close to normal levels. There were no adverse events related to empagliflozin administration.
Discussion
Most renal transplant patients have CKD, with 59.7% having stage 3 CKD [9]. However, thanks to the progress with immunosuppressive therapy, the survival prognosis and graft survival of renal transplant patients have improved. Between 2010 and 2017, the survival and engraftment rates were 97.1% and 94.1%, respectively, 5 years after living kidney transplantation. In addition, the 5-year survival and engraftment rate for renal donor transplants are also favorable at 93.1% and 87.9%, respectively [8]. Notably, Yagisawa et al. reported that the cause of death for 16% of Japanese transplant recipients was heart disease [9]. According to the Japan Society for Clinical Renal Transplantation, among functional graft-related deaths, heart disease was the most common cause at a rate of 11.2–14.3% for all ages. Prior to transplantation, many renal transplant patients are undernourished due to pre-transplant diet therapy, renal insufficiency, CAPD, and hemodialysis [8, 10]. After renal transplantation, the administration of steroids and immunosuppressants can lead to abnormalities in glucose metabolism. In recent years, the incidence of diabetes as a primary disease has increased among kidney transplantation patients [9]. This is especially noteworthy because blood glucose control after transplantation is particularly important [8]. Following transplantation, transplant recipients often lead to overnutrition and weight gain, which becomes a risk for kidney graft loss [8, 10]. In addition, weight gain is an exacerbating factor contributing to abnormal glucose metabolism. In a study of 3899 renal transplant patients by Chang and McDonald, patient body weight increased from 73.4 ± 15.3 kg prior to transplantation to 77.8 ± 16.5 kg after transplantation [11].
Glinide drugs such as biguanides, sulfonylureas, thiazolidines, and nateglinide are contraindicated for patients with renal impairment whose eGFR is less than 30 mL/min/1.73 m2. Dipeptidyl peptidase-4 inhibitors are safer, as they rarely cause hypoglycemia when administered alone, and administration of alogliptin and sitagliptin requires weight loss in patients with impaired renal function. Among glucagon-like peptide-1 (GLP-1) receptor agonists, exenatide is contraindicated for patients with moderate or higher renal impairment, and there are few reports of other GLP-1 receptor agonists administered after renal transplantation [12]. The SGLT2 inhibitor empagliflozin was first shown to offer cardiorenal protection in a large-scale study (EMPA-REG Outcome study [1]) of non-transplant patients. Similar benefits were observed in the CANVAS [2] and DECLARE-TIMI58 trials [3]. The CREDENCE trial [4], which had renal protection as an outcome, also demonstrated the efficacy of empagliflozin. In the DAPA-HF study [5], the efficacy of another SGLT2 inhibitor, dapagliflozin, was observed in patients with heart failure with reduced left ventricular ejection fraction, with and without diabetes.
Despite their efficacy in non-transplant patients, SGLT2 inhibitors are rarely administered to renal transplant patients because of concern about the potential for deterioration of renal function due to dehydration. There are also concerns regarding uro-genital infections [12], and the efficacy of SGLT2 inhibitors is thought to be reduced when there is moderately advanced CKD, which is common in kidney transplant recipients [13].. Recently, however, studies have addressed the issue of SGLT2 inhibitor administration to renal transplant patients [14]. For example, Schwaiger et al. [15] assessed the impact of replacing insulin with empagliflozin 10 mg/day in 14 stable kidney transplantation patients with diabetes mellitus. Although the administration of empagliflozin alone decreased glucose and HbA1c levels, the patients lost an average of 1.6 kg over 4 weeks. Five of the patients had urinary tract infections, though that rate was similar to the controls. Four other groups also reported good results for SGLT-2 inhibitors in diabetic kidney transplant patients [16,17,18,19]. Common features noted in these five reports are that administration of SGLT2 inhibitors led to weight loss, and there were no significant changes in eGFR and no serious adverse events. Although these five studies explored the effects of SGLT-2 inhibitors in renal transplant recipients with stage 1–3b CKD, where renal function was relatively preserved, there are an increasing number of reports showing the usefulness of SGLT2 inhibitors in patients with but with more severe renal damage (Table 3), stage 3–4 CKD [20,21,22,23,24,25].
Although SGLT inhibitors were previously thought to be ineffective for advanced CKD patients [13], there is increasing evidence showing their efficacy. Yamout et al. [20] compared 338 patients receiving canagliflozin at 100 mg/day and 365 patients receiving 300 mg/day with 382 patients receiving placebo. In each group, the eGFR averaged about 40 mL/min/1.73 m2, and both doses significantly decreased HbA1c, body weight, and systolic blood pressure as compared to placebo. In five other studies [21,22,23,24,25], SGLT2 inhibitors were reported to have similar effects in patients with stage 3–4 CKD.
Another concern about new drug administration to renal transplant recipients is drug interactions between the new drug and immunosuppressants. CyA, TAC, and EVR are mainly metabolized by CYP3A4. SGLT2 inhibitors do not induce or inhibit CYP3A4, or their effects are slight. To date, there have been no reports of changes in immunosuppressant doses in cases where an SGLT2 inhibitor was given to renal transplant patients. According to Halden et al. [16], blood trough levels of TAC, CyA, and EVR measured before empagliflozin were unchanged after 4 weeks of empagliflozin administration.
In case 1 in the present study, weight loss and decreased HbA1c and UACR were observed after empagliflozin administration. The mechanism is thought to be osmotic diuresis and caloric loss after empagliflozin administration, similar to its effects in patients with normal renal function [20]. It is unknown why, despite his renal impairment, this patient experienced the same metabolic improvement as patients with normal renal function. In case 2, weight loss and decreases in UACR, ALT, and γ-GTP were observed after empagliflozin administration, but HbA1c remained unchanged. Why HbA1c was not decreased, despite the weight loss, is unknown, but previous studies reported similar findings in patients with renal impairment [20,21,22,23,24,25]. We also suggest that the decrease in ALT and γ-GTP was due to improvement in this patient’s fatty liver disease [26]. In both cases, renal function did not decrease and UACR decreased after administration of empagliflozin. Regarding the decrease in UACR, it is possible that the improvement of tubuloglomerular feedback associated with natriuresis by empagliflozin was involved in the case of also CKD stage3a [15, 20, 22, 25]. The decrease in UACR in both cases may lead to renal protection.
Conclusion
In this report, we describe the efficacy and adverse effects of empagliflozin administered to two Japanese patients with stage G3b CKD and abnormal glucose metabolism after renal transplantation. Urinary albumin levels and body weight decreased in both patients, whereas HbA1c levels and blood pressure decreased in case 1, ALT, γ-GTP, and LDL-cholesterol levels decreased in case 2. No adverse events, including deterioration of renal function, were observed in either patient. Further studies involving a larger patient population will be required to clarify the efficacy and safety of SGLT2 inhibitors in patients with abnormal glucose metabolism after renal transplantation.
Availability of data and materials
We wish to share our data.
Abbreviations
- AUC:
-
Area under the curve
- eGFR:
-
Estimated glomerular filtration rate
- CyA:
-
Cyclosporine
- EVR:
-
Everolimus
- GLP-1:
-
Glucagon-like peptide-1
- MZR:
-
Mizoribine
- PSL:
-
Prednisolone
- SGLT2:
-
Sodium-glucose transport protein 2
- TAC:
-
Tacrolimus
- UACR:
-
Urine albumin to creatinine ratio
- UPCR:
-
Urine protein to creatinine ratio
References
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28.
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57.
Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and Ccardiovascular Ooutcomes in Ttype 2 Ddiabetes. N Engl J Med. 2019;380:347–57.
Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and Rrenal Ooutcomes in Ttype 2 Ddiabetes and Nnephropathy. N Engl J Med. 2019;380:2295–306.
McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in Ppatients with Hheart Ffailure and Rreduced Eejection Ffraction. N Engl J Med. 2019;381:1995–2008.
Karthikeyan V, Karpinski J, Nair RC, Knoll G. The burden of chronic kidney disease in renal transplant recipients. Am J Transplant. 2004;4:262–9.
Pham PT, Pham PM, Pham SV, Pham PA, Pham PC. New onset diabetes after transplantation (NODAT): an overview. Diabetes Metab Syndr Obes. 2011;4:175–86.
Ahmed SH, Biddle K, Augustine T, Azmi S. Post-Ttransplantation Ddiabetes Mmellitus. Diabetes Ther. 2020;11:779–801.
Yagisawa T, Mieno M, Ichimaru N, Morita K, Nakamura M, Kiyohiko Hotta K, et al. Trends of kidney transplantation in Japan in 2018: data from the kidney transplant registry. Renal Replace Ther. 2019;5:3.
Clunk JM, Lin CY, Curtis JJ. Variables affecting weight gain in renal transplant recipients. Am J Kid Dis. 2001;38:349–53.
Chang SH, McDonald SP. Post-kidney transplant weight change as marker of poor survival outcomes. Transplantation. 2008;85:1443–8.
Shivaswamy V, Boerner B, Larsen J. Post-Ttransplant Ddiabetes Mmellitus: Ccauses, Ttreatment, and Iimpact on Ooutcomes. Endocr Rev. 2016;37:37–61.
Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weightand blood pressure but does not improve glycemic control. Kidney Int. 2014;85:962–71.
Hecking M, Jenssen T. Considerations for SGLT2 inhibitor use in post-transplantation diabetes. Nat Rev Nephrol. 2019;15:525–6.
Schwaiger E, Burghart L, Signorini L, Ristl R, Kopecky C, Tura A, et al. Empagliflozin in posttransplantation diabetes mellitus: Aa prospective, interventional pilot study on glucose metabolism, fluid volume, and patient safety. Am J Transplant. 2019;19:907–19.
Halden TAS, Kvitne KE, Midtvedt K, Rajakumar L, Robertsen I, Brox J, et al. Efficacy and safety of empagliflozin in renal transplant recipients with posttransplant diabetes mellitus. Diabetes Care. 2019;42:1067–74.
Rajasekeran H, Kim SJ, Cardella CJ, Schiff J, Cattral M, Cherney DZI, et al. Use of Ccanagliflozin in Kkidney Ttransplant Rrecipients for the Ttreatment of Ttype 2 Ddiabetes: Aa Ccase Sseries. Diabetes Care. 2017;40:e75–6.
Shah M, Virani Z, Rajput P, Shah B. Efficacy and Ssafety of Ccanagliflozin in Kkidney Ttransplant Ppatients. Indian J Nephrol. 2019;29:278–81.
AlKindi F, Al-Omary HL, Hussain Q, Al Hakim M, Chaaban A, Boobes Y. Outcomes of SGLT2 Iinhibitors Uuse in Ddiabetic Rrenal Ttransplant Ppatients. Transplant Proc. 2020;52:175–8.
Yamout H, Perkovic V, Davies M, Woo V, de Zeeuw D, Mayer C, et al. Efficacy and Ssafety of Ccanagliflozin in Ppatients with Ttype 2 Ddiabetes and Sstage 3 Nnephropathy. Am J Nephrol. 2014;40:64–74.
Fioretto P, Del Prato S, Buse JB, Goldenberg R, Giorgino F, Reyner D, et al. Efficacy and safety of dapagliflozin in patients with type 2 diabetes and moderate renal impairment (chronic kidney disease stage 3A): tThe DERIVE Study. Diabetes Obes Metab. 2018;20:2532–40.
Dekkers CCJ, Wheeler DC, Sjöström CD, Stefansson BV, Cain V, Heerspink HJL. Effects of the sodium–glucose co-transporter 2 inhibitor dapagliflozin in patients with type 2 diabetes and Stages 3b–4 chronic kidney disease. Nephrol Dial Transplant. 2018;33:2005–11.
Sugiyama S, Jinnouchi H, Yoshida A, Hieshima K, Kurinami N, Jinnouchi K, et al. Renoprotective Eeffects of aAdditional SGLT2 inhibitor Ttherapy in Ppatients Wwith Ttype 2 Ddiabetes Mmellitus and Cchronic Kkidney Ddisease sStages 3b-4: Aa Rreal Wworld Rreport Ffrom Aa Japanese Sspecialized Ddiabetes Ccare Ccenter. J Clin Med Res. 2019;11:267–74.
Allegretti AS, Zhang W, Zhou W, Thurber TK, Rigby SP, Bowman-Stroud C, et al. Safety and Eeffectiveness of Bbexagliflozin in Ppatients Wwith Ttype 2 Ddiabetes Mmellitus and Sstage 3a/3b CKD. Am J Kidney Dis. 2019;74:328–37.
Grunberger G, Camp S, Johnson J, Huyck S, Terra SG, Mancuso JP, et al. Ertugliflozin in Ppatients with Sstage 3 Cchronic Kkidney Ddisease and Ttype 2 Ddiabetes Mmellitus: Tthe VERTIS RENAL Rrandomized Sstudy. Diabetes Ther. 2018;9:49–66.
Kuchay MS, Krishan S, Mishra SK, Farooqui KJ, Singh MK, Wasir JS, et al. Effect of Eempagliflozin on Lliver Ffat in Ppatients Wwith Ttype 2 Ddiabetes and Nnonalcoholic Ffatty Lliver Ddisease: Aa Rrandomized Ccontrolled Ttrial (E-LIFT Trial). Diabetes Care. 2018;41:1801–8.
Funding
None.
Author information
Authors and Affiliations
Contributions
RM, KM, and MY took care of the patients and participated in the decision of treatment. RM prepared the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
After local ethics committee approval, we obtained written informed consent from both patients.
Consent for publication
We obtained written informed consent for publication from both patients.
Competing interests
All authors have no conflicts to report.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Miyazaki, R., Miyagi, K. & Yoshida, M. Two Japanese patients with stage G3b chronic kidney disease and impaired glucose metabolism after renal transplantation successfully treated with empagliflozin. Ren Replace Ther 6, 55 (2020). https://doi.org/10.1186/s41100-020-00303-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-020-00303-x