Abstract
Background
The reported prevalence rate of depressive symptoms in hemodialysis patients is 40%. Although appropriate management of these symptoms is important, they remain under-recognized and under-treated in hemodialysis patients. Here, we systematically reviewed relevant randomized controlled trials (RCTs) investigating the effects of supervised exercise training on depressive symptoms in hemodialysis patients.
Methods
MEDLINE, Embase, the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, CINAHL, Web of Science, PsycINFO, and PEDro databases were searched from the start until June 2016 for RCTs published in English evaluating the effects of supervised exercise training in hemodialysis patients. The main outcome measures were depressive symptoms.
Results
From a total of 10,923 screened references, five trials were included in the analysis. Exercise training was shown to significantly improve depressive symptoms in comparison with controls (standardized mean difference, SMD = − 1.19; P < 0.001) under a random effects model. Subgroup analyses indicated that aerobic exercise and interventions lasting ≥ 6 months significantly reduced depressive symptoms in hemodialysis patients (P = 0.016, P < 0.001, respectively).
Conclusions
The meta-analysis found that supervised exercise training tends to alleviate depressive symptoms in hemodialysis patients. As our database search identified only a small number of studies on the association between exercise and depressive symptoms, we would surmise that additional high-quality studies are required to explore further this association.
Trial registration
PROSPERO, CRD42015020701.
Similar content being viewed by others
Background
With the increasing prevalence of lifestyle-related diseases, such as diabetes, hypertension, and arteriosclerosis, there are more than 2 million patients undergoing hemodialysis worldwide [1]. Depressive symptoms are common among hemodialysis patients, with a prevalence rate of 40% according to the Dialysis Outcomes and Practice Patterns Study (DOPPS) [2]. Depression is one of the most serious comorbidities among hemodialysis patients [2,3,4] and is associated with elevated mortality risk [2, 5, 6] and reduced quality of life (QOL) [7, 8]. Although the appropriate management of depressive symptoms as a patient-reported outcome (PRO) is known to be clinically important, these symptoms remain under-recognized and under-treated in dialysis patients [9,10,11]. Exercise training is an effective non-pharmacological means of reducing depressive symptoms among people dwelling in the community [12, 13], cancer survivors [14, 15], multiple sclerosis patients [16], stroke patients [17], and patients with chronic illness [18].
Although supervised exercise training has been suggested to improve exercise capacity, muscular strength, and QOL in hemodialysis patients [19,20,22], it remains unclear whether such exercise regimes can ameliorate depressive symptoms in these patients. Systematic reviews with meta-analyses are generally considered good means of determining the efficacy and effectiveness of treatments on selected outcomes.
This study was performed to systematically review relevant randomized controlled trials (RCTs) investigating the effects of supervised exercise training on depressive symptoms in hemodialysis patients. In addition, we performed subgroup analyses to examine the differences in efficacy related to the training program.
Methods
This review is reported in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Additional file 1) and is one of a series of systematic reviews regarding the effects of exercise on depressive symptoms in hemodialysis patients. The protocol used for the systematic review and meta-analysis was registered with the International Prospective Register of Systematic Reviews (PROSPERO) (registration number: PROSPERO 2015: CRD42015020701), and our protocol has already been published (http://bmjopen.bmj.com/content/6/5/e010990.long) [23]. No ethical approval was required because this study did not include confidential personal data and did not involve patient intervention.
Study selection and data management
An electronic database search was performed in MEDLINE, Embase, the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, CINAHL, Web of Science, PsycINFO, and PEDro using the following terms: “dialysis,” “renal replacement therapy,” “exercise,” “physical fitness,” “cycling,” “walking,” and “physical therapy.” The full strategy is described in Additional file 2. To identify any articles missed by the initial search, the reference lists of previously reported systematic reviews were also evaluated in addition to our electronic database search. EndNote X7 for Windows (Thompson Reuters, Philadelphia, PA) was used to manage literature records and data. Reviewers screened all titles, abstracts, and the full texts of the selected publications. In cases where required data were not available, the study authors were contacted by email.
Inclusion and exclusion criteria
Only RCTs published in English that evaluated the effects of supervised exercise training on at least depressive symptoms were included. Supervised exercise included resistance training, aerobic exercise, or combined exercise. Only RCTs treating patients at least 18 years of age and on hemodialysis were included in this meta-analysis. Patients affected by acute kidney failure were also excluded. The main outcome of the study was depressive symptoms.
Risk of bias
The methodological quality of trials included in the review was assessed independently using the Cochrane Collaboration tool [24] by three reviewers to determine the risk of bias. Studies were graded as having a “low risk,” “high risk,” or “unclear risk” of bias across the seven specified domains: random sequence generation, allocation concealment, participant and personnel blinding, outcome assessment blinding, incomplete outcome data, selective reporting, and other sources of bias. Furthermore, the risk of bias of references was assessed using the Tool for the assEssment of Study qualiTy and reporting in EXercise (TESTEX) [25], which consists of 15 different items and shows reliable performance for comprehensive review of exercise 1 training trials.
Data analysis and statistical methods
The effect sizes obtained from the RCTs are reported as mean change scores (Cohen’s d). Although some of the included studies reported change scores and the standard deviations (SDs), we calculated change scores for those that did not by subtracting the mean baseline score from the mean follow-up score and calculated the change score SD. A random effects model was used to compute the overall or mean effect size (ES), as this model assumes that the samples are from populations with different ESs and that the true effect differs between studies. We used fixed effect models in cases in which the degree of statistical heterogeneity was low, while random effect models were used in all other cases. The 95% confidence interval (CI) around the mean ES was further calculated. To test for homogeneity of variance among ESs, we calculated the overall I 2 values, which represent the magnitude of heterogeneity where a larger number indicates greater heterogeneity; I 2 values of 25, 50, and 75% are related to low, moderate, and high degrees of heterogeneity, respectively.
Subgroup analyses were performed based on the categorical variables of exercise mode (i.e., Aerobic vs. Other), exercise duration (≥6 months vs. < 6 months), and type of exercise intervention (intradialytic exercise vs. non-intradialytic exercise). These were identified based on clinical relevance and experience with the characteristics of exercise training interventions. The analyses were performed using R version 3.3.0 (R Foundation for Statistical Computing, Vienna, Austria). In all analyses, P < 0.05 was taken to indicate statistical significance [26].
Results
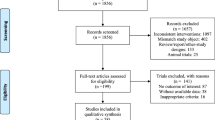
A total of 10,923 references were initially screened, of which 7640 had no duplicates and 7307 were rejected at the title and abstract stage. We then identified 333 studies for potential inclusion and full-text review, and five trials were finally entered into the analysis [27,28,29,30,31] (Fig. 1).
Participants and interventions
The trials included in the analysis are summarized in Table 1. The studies assessed depressive symptoms using the Center for Epidemiologic Studies Depression Questionnaire [27], Self-rating Depression Scale [28, 29], or the Beck Depression Inventory [30, 31]. Three of the studies used an intradialytic exercise program with interventions ranging in duration from 10 weeks to 6 months. Four studies used aerobic training, and one study used a combined exercise program that included calisthenics, steps, flexibility, and low weight resistance training. The interventions were performed two to four times per week in five studies.
Depressive symptoms
Comparison of exercise intervention groups and control groups indicated a small but significant overall standardized mean difference (SMD) = – 0.67 (CI, − 0.97 to − 0.36; P < 0.001) under a fixed effects model (Fig. 2). The mean ES was slightly smaller, but still statistically significant, under a random effects model (SMD = − 1.19; CI, − 2.17 to − 0.22; P < 0.017).
Subgroup analyses indicated significant reductions in depressive symptoms among hemodialysis patients associated with aerobic exercise and interventions lasting ≥ 6 months (P = 0.016, P < 0.001, respectively). However, no significant difference was seen in the remedial effects on depressive symptom between intradialytic and pre- or post-dialysis exercise programs (Figs. 3, 4, 5).
Assessment of bias risks
In the studies included in the analysis, the risks of bias were frequently high or unclear (Table 2). The methods used for random sequence generation, patient allocation, and assessor blinding to patient allocation were unclear in all studies. All trials clearly documented no blinding of participants and personnel. The outcome data were incomplete in one study and were reported only selectively in another study. The total TESTEX score, study quality score, and study reporting score of 5 studies were 7.40 ± 0.89, 1.80 ± 0.48, and 5.60 ± 1.14, respectively.
Discussion
The present meta-analysis was performed to determine the efficacy of supervised exercise training for reducing depressive symptoms in hemodialysis patients. The overall analysis tends to that exercise contributed to a reduction in depressive symptoms, and subgroup analyses showed that aerobic exercise and interventions lasting ≥ 6 months had greater probabilities of reducing the depressive symptoms in these patients. However, the results of the present study and other high-quality studies are required in order to clarify how exercise affects depressive symptoms in hemodialysis patients. To our knowledge, this is the first systematic review and meta-analysis regarding the efficacy of supervised exercise training for depression in hemodialysis patients taking the forms of exercise used and intervention durations into consideration.
The results presented here were consistent with previous meta-analyses regarding the effects of exercise on depression and depressive symptoms in other populations [32, 33]. A previous meta-analysis of 90 RCTs indicated that exercise reduces depressive symptoms among patients with various chronic illnesses, including chronic obstructive pulmonary disease, cardiovascular, fibromyalgia, multiple sclerosis, cancer, and chronic pain disorder [18]. However, it was unclear whether supervised exercise training could reduce depressive symptoms in hemodialysis patients due to major differences from those in populations including cancer survivors, stroke survivors, those with multiple sclerosis, those with other chronic illnesses, and the population in general. There are obvious differences with respect to age, prevalence of comorbidities, the presence of dialysis-related symptoms, and the overlap between symptoms of advanced kidney disease and those of depression. Therefore, the present study was performed using data from trials conducted only in hemodialysis patients, and our results indicated that, consistent with those in other populations, supervised exercise has a positive effect on depressive symptoms in these patients.
Observations regarding the release of monoamine neurotransmitters (i.e., serotonin, dopamine, and norepinephrine) and endorphins during aerobic exercise provided preliminary mechanistic support for the use of aerobic exercise to reduce and manage depressive symptoms [34, 35], and thus avoiding the common side effects associated with antidepressant medications [36]. Physical activity is associated with improved neurological function, with increased levels of neurotropic factors in the brain and improvements in mood [37]. However, these hypotheses cannot fully explain the complex physiological and psychosocial etiologies of depressive symptoms, because the studies included in our meta-analysis rarely reported physiological measures. Further studies are therefore needed to examine the mechanisms underlying the exercise-induced reduction of depressive symptoms.
Based on the results of this study, we may be possible to recommend a structured, supervised aerobic exercise program for at least 6 months to manage or reduce depressive symptoms in hemodialysis patients. Exercise programs of 10–16 weeks produced greater effects in the general population than those lasting < 9 weeks [38]. In addition, Craft and Landers reported that interventions of longer duration resulted in greater decreases in depressive scores [39]. Therefore, further studies are required to examine not only the various effects of exercise on outcomes, but also how best to improve adherence to participation in exercise programs and which types of intervention have the greatest efficacy in hemodialysis patients with depressive symptoms.
Many Cochrane reviews have included cases that analyzed low-quality studies. The analysis of the present study ultimately included five studies with high inconsistency, imprecision, and high risk for bias. Implication for practice, we rated the quality of the body of evidence concerning the effects of exercise on depressive symptoms as low. However, this study helped to confirm that further investigation is necessary, as it clarified that the evidence is poor. It will be important for future studies to calculate sample size according to optimal information size and to report the risk of bias with regard to random sequence generation, allocation concealment, incomplete outcome data, selective reporting, and other sources of bias. Finally, we would suggest that many additional studies are required to examine different variables such as exercise mode, exercise duration, and type of exercise intervention.
This study had a number of limitations due to the original studies and the paucity of data. First, the number of eligible studies investigating the associations between exercise and depressive symptoms was small. And we could not assess publish bias. Second, the studies included in the analyses used a number of different methods to evaluate depressive symptoms. Third, the included studies had high degrees of heterogeneity with regard to the exercise interventions (i.e., differences in modality, duration, volume, and intensity). Therefore, additional RCTs are required to establish adequate evidence. Fourth, the studies eligible for the meta-analysis examined the effects only of exercise therapy. Further randomized control trials and meta-analyses are required to evaluate the effects of exercise in hemodialysis patients with high depressive scores in comparison to other treatment modalities, including cognitive-behavioral therapy and antidepressant medication. Bridle et al. suggested that new RCTs should stratify randomization by severity of depression, receipt of antidepressant medications, and/or level of regular exercise [40]. In fact, appropriate antidepressant treatment may be necessary in chronic hemodialysis patients [41, 42].
Conclusions
The meta-analysis found that supervised exercise training tends to alleviate depressive symptoms in hemodialysis patients. As our database search identified only a small number of studies on the association between exercise and depressive symptoms, we would surmise that additional high-quality studies are required to explore further this association.
Abbreviations
- CI:
-
Confidence interval
- DOPPS:
-
Dialysis Outcomes and Practice Patterns Study
- ES:
-
Effect size
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROSPERO:
-
International Prospective Register of Systematic Reviews
- PRO:
-
Patient-reported outcome
- QOL:
-
Quality of life
- RCTs:
-
Randomized controlled trials
- SDs:
-
Standard deviations
- SMD:
-
Standardized mean difference
- TESTEX:
-
Tool for the assEssment of Study qualiTy and reporting in EXercise
References
Liyanage T, Ninomiya T, Jha V, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet (London, England). 2015;385:1975–82.
Lopes AA, Albert JM, Young EW, et al. Screening for depression in hemodialysis patients: associations with diagnosis, treatment, and outcomes in the DOPPS. Kidney Int. 2004;66:2047–53.
Kimmel PL. Depression in patients with chronic renal disease: what we know and what we need to know. J Psychosom Res. 2002;53:951–6.
Kimmel PL, Peterson RA. Depression in end-stage renal disease patients treated with hemodialysis: tools, correlates, outcomes, and needs. Semin Dial. 2005;18:91–7.
Farrokhi F, Abedi N, Beyene J, et al. Association between depression and mortality in patients receiving long-term dialysis: a systematic review and meta-analysis. Am J Kidney Dis. 2014;63:623–35.
Fan L, Sarnak MJ, Tighiouart H, et al. Depression and all-cause mortality in hemodialysis patients. Am J Nephrol. 2014;40:12–8.
Lopes GB, Matos CM, Leite EB, et al. Depression as a potential explanation for gender differences in health-related quality of life among patients on maintenance hemodialysis. Nephron Clin Pract. 2010;115:c35–40.
Weisbord SD, Fried LF, Arnold RM, et al. Prevalence, severity, and importance of physical and emotional symptoms in chronic hemodialysis patients. J Am Soc Nephrol. 2005;16:2487–94.
Ma TK, Li PK. Depression in dialysis patients. Nephrology (Carlton, Vic). 2016;21:639–46.
Hedayati SS, Yalamanchili V, Finkelstein FO. A practical approach to the treatment of depression in patients with chronic kidney disease and end-stage renal disease. Kidney Int. 2012;81:247–55.
Ohtake Y. Psychonephrology in Japan. Ren Replace Ther. 2017;3:25.
Catalan-Matamoros D, Gomez-Conesa A, Stubbs B, et al. Exercise improves depressive symptoms in older adults: an umbrella review of systematic reviews and meta-analyses. Psychiatry Res. 2016;244:202–9.
Radovic S, Gordon MS, Melvin GA. Should we recommend exercise to adolescents with depressive symptoms? A meta-analysis. J Paediatr Child Health. 2017;53:214–20.
Craft LL, Vaniterson EH, Helenowski IB, et al. Exercise effects on depressive symptoms in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21:3–19.
Brown JC, Huedo-Medina TB, Pescatello LS, et al. The efficacy of exercise in reducing depressive symptoms among cancer survivors: a meta-analysis. PLoS One. 2012;7:e30955.
Ensari I, Motl RW, Pilutti LA. Exercise training improves depressive symptoms in people with multiple sclerosis: results of a meta-analysis. J Psychosom Res. 2014;76:465–71.
Eng JJ, Reime B. Exercise for depressive symptoms in stroke patients: a systematic review and meta-analysis. Clin Rehabil. 2014;28:731–9.
Herring MP, Puetz TW, O'Connor PJ, et al. Effect of exercise training on depressive symptoms among patients with a chronic illness: a systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172:101–11.
Groussard C, Rouchon-Isnard M, Coutard C, et al. Beneficial effects of an intradialytic cycling training program in patients with end-stage kidney disease. Appl Physiol Nutr Metab. 2015;40:550–56.
Wu Y, He Q, Yin X, et al. Effect of individualized exercise during maintenance haemodialysis on exercise capacity and health-related quality of life in patients with uraemia. J Int Med Res. 2014;42:718–27.
DePaul V, Moreland J, Eager T, et al. The effectiveness of aerobic and muscle strength training in patients receiving hemodialysis and EPO: a randomized controlled trial. Am J Kidney Dis. 2002;40:1219–29.
Chen JL, Godfrey S, Ng TT, et al. Effect of intra-dialytic, low-intensity strength training on functional capacity in adult haemodialysis patients: a randomized pilot trial. Nephrol Dial Transplant. 2010;25:1936–43.
Matsuzawa R, Hoshi K, Yoneki K, et al. Evaluating the effectiveness of exercise training on elderly patients who require haemodialysis: study protocol for a systematic review and meta-analysis. BMJ Open. 2016;6:e010990.
Savovic J, Weeks L, Sterne JA, et al. Evaluation of the Cochrane Collaboration’s tool for assessing the risk of bias in randomized trials: focus groups, online survey, proposed recommendations and their implementation. Syst Rev. 2014;3:37.
Smart NA, Waldron M, Ismail H, et al. Validation of a new tool for the assessment of study quality and reporting in exercise training studies: TESTEX. Int J Evid Based Healthc. 2015;13:9–18.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Carmack CL, Amaral-Melendez M, Boudreaux E, et al. Exercise as a component in the physical and psychological rehabilitation of hemodialysis patients. Int J Rehabilitation Health. 1995;1:13–23.
Giannaki CD, Sakkas GK, Karatzaferi C, et al. Effect of exercise training and dopamine agonists in patients with uremic restless legs syndrome: a six-month randomized, partially double-blind, placebo-controlled comparative study. BMC Nephrol. 2013;14 Epub
van Vilsteren M, de Greef MHG, Huisman RM. The effects of a low-to-moderate intensity pre-conditioning exercise programme linked with exercise counselling for sedentary haemodialysis patients in The Netherlands: results of a randomized clinical trial. Nephrology Dialysis Transplantation. 2005;20:141–6.
Kouidi E, Iacovides A, Iordanidis P, et al. Exercise renal rehabilitation program: psychosocial effects. Nephron. 1997;77:152–8.
Ouzouni S, Kouidi E, Sioulis A, et al. Effects of intradialytic exercise training on health-related quality of life indices in haemodialysis patients. Clin Rehabil. 2009;23:53–63.
Conn VS. Depressive symptom outcomes of physical activity interventions: meta-analysis findings. Ann Behav Med. 2010;39:128–38.
Dalgas U, Stenager E, Sloth M, et al. The effect of exercise on depressive symptoms in multiple sclerosis based on a meta-analysis and critical review of the literature. Eur J Neurol. 2015;22:443–e434.
Brosse AL, Sheets ES, Lett HS, et al. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Med. 2002;32:741–60.
Thoren P, Floras JS, Hoffmann P, et al. Endorphins and exercise: physiological mechanisms and clinical implications. Med Sci Sports Exerc. 1990;22:417–28.
Papakostas GI. Tolerability of modern antidepressants. J Clin Psychiatry. 2008;69(Suppl E1):8–13.
Greenwood BN, Fleshner M. Exercise, stress resistance, and central serotonergic systems. Exerc Sport Sci Rev. 2011;39:140–9.
Rethorst CD, Wipfli BM, Landers DM. The antidepressive effects of exercise: a meta-analysis of randomized trials. Sports Med. 2009;39:491–511.
Craft L, Landers D. The effect of exercise on clinical depression and depression resulting from mental illness: a meta-analysis. J Sport Exerc Psychol. 1998;20:339–57.
Bridle C, Spanjers K, Patel S, et al. Effect of exercise on depression severity in older people: systematic review and meta-analysis of randomised controlled trials. Br J Psychiatry. 2012;201:180–5.
Finkelstein FO, Finkelstein SH. Depression in chronic dialysis patients: assessment and treatment. Nephrol Dial Transplant. 2000;15:1911–3.
Koo JR, Yoon JY, Joo MH, et al. Treatment of depression and effect of antidepression treatment on nutritional status in chronic hemodialysis patients. Am J Med Sci. 2005;329:1–5.
Acknowledgements
We thank all of the investigators and contributors to our study.
Funding
Funding for this study was provided by Kitasato University Research Grant.
Availability of data and materials
We decided not to share the data in our study because all data are thoroughly described and reflected in the accompanying tables and figures (all relevant data are within the paper).
Author information
Authors and Affiliations
Contributions
TS, RM, KH, and AM contributed to the research idea and study design; TS, RM, KY, MH, and TW contributed to the data acquisition; RM, MH, and TW contributed to the quality assessment of a risk of bias; TS, RM, KH, and AM contributed to the data analysis/interpretation; TS, RM, and KH contributed to the statistical analysis; AM contributed to the supervision or mentorship. Each author contributed important intellectual content during manuscript drafting or revision. All authors read an approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
PRISMA 2009 Checklist. (DOC 69 kb)
Additional file 2:
Search strategy. (DOCX 24.3 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Shimoda, T., Matsuzawa, R., Hoshi, K. et al. Effects of supervised exercise on depressive symptoms in hemodialysis patients: a systematic review and meta-analysis of randomized controlled trials. Ren Replace Ther 3, 56 (2017). https://doi.org/10.1186/s41100-017-0136-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-017-0136-5