Abstract
Background
Iron-based phosphate binders are widely used in hemodialysis, to avoid the increased mortality associated with high serum phosphate in dialysis patients. However, comparative studies on the effects of phosphate binders are currently limited. In the present study, a comparative analysis of ferric citrate (FC), sucroferric oxyhydroxide (SF), and lanthanum carbonate (LC) was performed to assess their primary phosphate-binding and secondary iron uptake capacities.
Methods
Patients on maintenance hemodialysis visit our group clinics regularly. The FC, SF, and LC groups comprised 101, 82, and 126 patients, respectively. Subjects were observed from December 2015 to April 2016 (5 months).
Serum phosphate levels and other markers were measured in the three medication groups, and changes in phosphate levels and other clinical markers were compared. A drug treatment was considered to be effective if the serum phosphate levels of the patient decreased from baseline in each of the drugs. We evaluated the phosphate-binding capacity compared with each drug using a mixed effect model, for adjusted repeated measured analysis.
Results
SF showed higher phosphate-binding capacity than FC and LC. FC, SF, and LC showed no significant difference in phosphate-binding capacity in the adjusted mixed effect model. However, patients in the FC group exhibited iron accumulation.
Conclusion
Sucroferric oxyhydroxide possesses better phosphate-binding efficacies than ferric citrate and lanthanum carbonate. In addition, ferric citrate showed a strong iron-cumulative effect.
Similar content being viewed by others
Background
High serum phosphate levels in hemodialysis patients are associated with higher mortality, necessitating the use of iron-based phosphate binders with hemodialysis. Ferric citrate (FC) exerts its phosphate-binding effect through ionic interactions and is removed from the body as FePiPO4. Sucroferric oxyhydroxide (SF) binds phosphate as a polymer and causes the excretion of the phosphate. Iron-based phosphate binders are expected to exhibit better phosphate-binding capacity compared to other molecules. In addition, iron is biocompatible and thus possesses high therapeutic potential. However, metals vary in their phosphate-binding capacities and extent of iron release. In this study, we investigated the phosphate-binding capacities and iron absorption effects of SF, FC, and lanthanum carbonate (LC).
Methods
Study subjects
We identified patients who used phosphate binders and visited the Zenjinkai group hospital thrice a week from December 2015 to April 2016. Patients with available electronic medical records were selected for the study. Subjects were categorized into FC, SF, and LC medication groups, which comprised 101, 82, and 126 cases, respectively. SF and FC were administered to circumvent the difficulty in maintaining serum phosphate (Pi) levels below 6.0 mg/dL or unfavorable drug compliance and occurrence of digestion-related symptoms. SF and FC were administered with the starting dose at the baseline week. LC was prescribed continuously and persistently; however, the dosage was increased or decreased to maintain serum Pi under 6.0 mg/dL.
Prescription dose
The Japan Ministry of Health, Labor, and Welfare and the Pharmaceuticals and Medical Devices Agency (PMDA) defined the starting doses of these drugs as SF 750 mg/day (three tablets), FC 1500 mg/day (six tablets), and LC 750 mg/day (three tablets). We prescribed each drug according to these guidelines, and the prescription doses were increased or decreased to regulate serum phosphate levels between 3.5 and 6.0 mg/dL, according to the guidelines of the Japanese Society for Dialysis Therapy and the Japanese Society of Nephrology [1]. The phosphate change rate was defined by the following formula: (Pi_week_n − Pi_week_0) × 100/Pi_week_0. The total prescription dose was defined as the total dose in milligrams during the 5-month observation period, during which each patient visited the hospital 22 times.
Collection of clinical data
For data collection, the STEPII® data system was used to identify patient laboratory data, drug usage, and demographic data. In addition to phosphate, we also analyzed the serum levels of hemoglobin (Hb), STAT (%), and ferritin, as well as the dose of erythropoiesis-stimulating agent (ESA), during each patient visit. The ESA dose for each visit was converted based on the epoetin beta pegol:darbepoetin:epoetin beta ratio of 1:1:200, which is generally used in clinical studies. This study was approved by the facility ethics committee.
Patient characteristics
Three hundred nine patients on maintenance hemodialysis regularly visited our group clinics. The FC, SF, and LC medication groups comprised 101, 82, and 126 cases, respectively (Table 1).
The mean age at the onset of the study was 62.9 years, mean body mass index was 23.2, hemodialysis (HD) vintage was 67.3 months, mean serum Hb was 10.9 g/dL, Pi was 6.1 mg/dL, and percentage of combined phosphate binders (sevelamer and calcium carbonate) was 43.4% (Table 1).
Statistical analysis
We calculated the mean and standard deviation for each parameter and drug dosage and the least square mean for phosphate change rate. We used the least square mean in the phosphate change rate to adjust for the systematic error in the relatively small number of cases analyzed in this study. To evaluate the effect of phosphate binders on phosphate change rate, we fitted the data to the mixed effect model for adjusted repeated measured analysis. The dependent variable was the phosphate change rate, whereas the fixed effect was a combination of drug, age, gender, and phosphate binders. Patients constituted the random effect. The phosphate change rate was calculated and repeatedly measured in this mixed effect model. The estimated differences of the phosphate change rate between FC versus SF, SF versus LC, and FC versus LC were calculated. All statistical analysis were performed using SAS® 9.3 software.
Results
The total usage dose in the LC group was significantly higher than those in the SF and FC groups (Table 2).
The total phosphate change rates for FC, SF, and LC were 2.9, −10.8, and −3.2% (not adjusted), respectively. The time taken to reach serum ferritin levels ≥300 ng/mL was significantly longer in the SF group. The total dose of the ESA was 387.5 IU, and the SF group significantly showed the lowest ESA value among the three medication groups (Table 2).
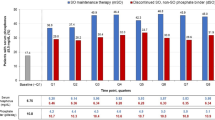
The least square means of phosphate change rate and the difference from the baseline of the three drugs are described in Table 3. The total least square means of the phosphate change rates are also provided (see Table 3). Figure 1 shows that the mean serum phosphate level decreased, especially with SF and LC.
Table 3 shows that in the SF group, the phosphate change rate significantly decreased after 11 weeks described by a decrease of the least square mean. However, the change was not significant except on week 12 for the FC group.
The mixed effect model analysis indicated that there were no significant differences in phosphate change rates in FC versus SF, SF versus LC, and FC versus LC.
Changes in serum phosphate levels in 5 months
Figure 1 shows the mean phosphate level of each phosphate binder with standard error. Phosphate levels decreased in the FC group in the first 3 months but returned to initial levels in the latter half period. In the SF group, phosphate levels decreased significantly from the baseline in each week. In the LC group, phosphate levels were relatively low throughout the observation period. Table 3 provides the least square mean and standard error of the phosphate change rate for the three drugs. There was a reduction from the baseline after 12 weeks in the case of the FC treatment. In the SF group, weeks 6 and 7 increased but week 11 to last decreased significantly. In the LC group, the phosphate change rates decreased significantly across several weeks, but the change rates were relatively smaller than those of FC and SF.
Phosphate change rate per cumulative prescription dose (5 months)
Figure 2 shows the relationship between the cumulative prescription dose of each phosphate binder and the phosphate change rates. The cumulative prescription dose was calculated at each visit by adding the prescription dose from the baseline to the observation visit and dividing by the starting dose of each phosphate binder. The phosphate change rate decreased when the values obtained after dividing the prescription dose by the starting dose increased. The phosphate change rate decreased rapidly, especially in the SF group.
Phosphate change rate per cumulative prescription dose. The relationship between cumulative prescription dose and phosphate change rates for a FC, b SF, and c LC. The cumulative prescription dose was calculated at each visit by adding the prescription dose from the baseline to the observation visit and dividing by the starting dose of each phosphate binder. The phosphate change rate decreased when the values obtained after dividing the prescription dose by the starting dose increased
Changes in serum Hb levels in 5 months
Figure 3 presents the changes in mean serum Hb in 5 months for each prescription group. Hb levels increased in the FC and SF groups, but not in the LC group.
Changes in serum ferritin levels in 5 months
Figure 4 presents the mean ferritin change in 5 months for each prescription group. FC and SF were administered in the low and moderate serum ferritin groups, respectively, after which the serum ferritin levels increased in both the groups. Ferritin levels did not increase in the LC group.
Changes in transferrin saturation in 5 months
Figure 5 presents the changes in transferrin saturation (TSAT) in 5 months for the three prescription groups. TSAT increased considerably in the FC group and negligibly in the SF group and remained unchanged in the LC group.
Table 4 shows the effectiveness of FC versus SF, FC versus LC, and SF versus LC treatments. In the mixed effect model analysis, the phosphate-lowering effect adjusted to the fixed and random effects of the phosphate change rate were not significantly different within each group.
Discussion
High serum phosphate levels are associated with higher mortality [2]. After adjustment with nutritional markers, hemodialysis patients with phosphate levels ≥5.6 mg/dL had a higher risk of mortality; this risk was reduced with the use of phosphate binders [2].
Based on data from a dialysis cohort, Cannata-Andia et al. [3] reported that the use of phosphate-binding agents was associated with lower mortality. A pronounced HR-lowering effect was observed by sevelamer plus lanthanum treatment compared to those of other drug combinations containing phosphate-binding agents. A meta-analysis indicated that the use of Ca-containing phosphate binders resulted in elevated risk of all-cause mortality and cardiovascular mortality [4, 5]. However, arterial media calcification was observed in patients undergoing hemodialysis who consumed Ca-containing phosphate binders [6]. Randomized controlled trials on sevelamer and lanthanum in Japanese patients indicated identical levels of phosphate-binding efficacy [7]. Isakova et al. also reported that treatment with phosphorus binders independently decreased mortality in patients [8]. Recently, three randomized controlled trials were performed using FC [9,10,11] on patients with chronic kidney disease [12]. Iida et al. reported that FC prevented vascular calcification in rats and demonstrated the dose-dependent efficacy of FC [13]. Rodby et al. reported that FC treatment reduced hospitalization costs by an estimated 3002 USD per patient per year [14]. Iron-based phosphate binders that are currently used include Velphoro, Ca-containing phosphate binders, Zerenex, FC, Alpharen Fermagate SBR-759, and PT20 [15]. In particular, PA21 was administered to patients undergoing hemodialysis in 2016. A phase III study was performed to compare PA21 with sevelamer [16], and results showed that PA21 had a lower pill burden and better adherence.
We investigated two iron-based phosphate binders, namely FC and SF, and compared their effectiveness with lanthanum (LC), another metal (lanthanum)-based phosphate binder. However, FC and SF have different phosphate-binding mechanisms—FC acts via ionic binding, whereas SF causes polymer adsorption effects. Thus, FC and SF cannot be compared based on their iron content.
Analysis of the phosphate-binding efficiency using the difference of least square mean showed significantly lower values exclusively in week 12 for the FC group. Meanwhile, the SF group showed a significant lowering effect in the latter half of the observation period, whereas LC showed a relatively significant effect throughout the period (Table 4, Fig. 1). We assumed that the reduction in effect of FC in the latter half of the observation period could be caused by unfavorable gastrointestinal symptoms and occurrence of colored feces. Secondly, elevated serum ferritin level (Fig. 4) and increased percentage of TSAT% (Fig. 5) might also reduce the dosage of FC. In contrast, SF showed a relatively weak iron-cumulative effect, especially indicated in TSAT% (Fig. 5), which could result from a carryover of FC to SF in certain cases. In the SF group, serum Hb was maintained in the latter half of the observation period (Fig. 3), ferritin was elevated from the average level (Fig. 4), and TSAT was slightly increased in the observation period (Fig. 5). The ferritin content of the FC group was ≤60 ng/mL at the baseline (Fig. 4), and therefore, FC might be administrated in cases of iron deficiency anemia. Comparison of the first and last phosphate levels during estimation of the phosphate-binding effect of the three phosphate binders was insufficient because original phosphate levels were regained, which was further confounded with combined drug treatment and other demographic data. To partially avoid these confounders and estimate the repeated measured value, we fitted the date to the mixed effect model [17, 18]. Repeated measured serum phosphate levels were adjusted with combined phosphate binder, age, or gender. Patients were defined as the random effect in the multilevel structure of this mixed effect model. The result of the mixed effect model analysis indicated that SF had relatively strong phosphate-binding effect in these newly developed drugs, but there were no significant differences in the phosphate-binding effect among the three drugs, which might be reflected in the actual medical care response during dialysis therapy.
A limitation of this study is that except for LC, the prescription data of FC and SF were flawed. The LC group was prescribed consistently; however, certain patients in the SF group were prescribed FC before SF. Nevertheless, phosphate change rates and fluctuations and changes of other markers could be evaluated and compared.
Conclusion
Among the newly developed drugs, sucroferric oxyhydroxide exhibited a stronger phosphate-lowering effect than ferric citrate. In addition, ferric citrate showed a stronger iron-cumulative effect than sucroferric oxyhydroxide and thus required adjustment based on iron administration in these iron-based phosphate binders.
Abbreviations
- FC:
-
Ferric citrate
- LC:
-
Lanthanum carbonate
- SF:
-
Sucroferric oxyhydroxide
References
Fukagawa M, Yokoyama K, Koiwa F, Taniguchi M, Shoji T, Kazama JJ, et al. Clinical practice guideline for the management of chronic kidney disease-mineral and bone disorder. Ther Apher Dial. 2013;17:247–88.
Lopes AA, Tong L, Thumma J, Li Y, Fuller DS, Morgenstern H, et al. Phosphate binder use and mortality among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS): evaluation of possible confounding by nutritional status. Am J Kidney Dis. 2012;60:90–101.
Cannata-Andia JB, Fernandez-Martin JL, Locatelli F, London G, Gorriz JL, Floege J, et al. Use of phosphate-binding agents is associated with a lower risk of mortality. Kidney Int. 2013;84:998–1008.
Jamal SA, Vandermerr B, Raggi P, Mendelssohn DC, Chatterley T, Dorgan M, et al. Effect of calcium-based versus non-calcium-based phosphate binders on mortality in patients with chronic kidney disease: an updated systematic review and meta-analysis. Lancet. 2013;38:1268–77.
Jamal SA, Fitchett D, Lok CE, Mendelssohn DC, Tsuyuki RT. The effects of calcium-based versus non-calcium-based phosphate binders on mortality among patients with chronic kidney disease: a meta-analysis. Nephrol Dial Transplant. 2009;24:3168–74.
London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–40.
Kasai S, Sato K, Murata Y, Kinoshita Y. Randomized crossover study of the efficacy and safety of sevelamer hydrochloride and lanthanum carbonate in Japanese patients undergoing hemodialysis. Ther Apher Dial. 2012;16:341–9.
Isakova T, Gutierrez OM, Chang Y, Shar A, Tamez H, Smith K, et al. Phosphorus binders and survival on hemodialysis. J Am Soc Nephrol. 2009;20:388–96.
Yokoyama K, Hirakata H, Akiba T, Sawada K, Kumagai Y. Effect of Rora JTT-751(ferric citrate) on hyperphosphatemia in hemodialysis patients: results of a randomized, double-blind, placebo-controlled trial. Nephrology. 2012;36:478–87.
Dwyer JP, Sika M, Schulman G, Chang IJ, Anger M, Smith M, et al. Dose-response and efficacy of ferric citrate to treat hyperphosphatemia in hemodialysis patients: a short-term randomized trial. Am J Kidney Dis. 2013;61:759–66.
Yokoyama K, Akiba T, Fukagawa M, Nakayama M, Sawada K, Kumagai Y, et al. A randomized trial of JTT-751 versus sevelamer hydrochloride in patients on hemodialysis. Am J Nephrol Dial Transplant. 2014;29:1053–60.
Block GA, Fishbane S, Rodriguez M, Smits G, Shemesh S, Pergola PE, et al. A 12-week, double-blind, placebo-controlled trial of ferric citrate for the treatment of iron deficiency anemia and reduction of serum phosphate in patients with CKD stages 3-5. Am J Kidney Dis. 2015;65:728–36.
Iida A, Kemmochi Y, Kakimoto K, Tanimoto M, Mimura T, Shinozaki Y, et al. Ferric citrate hydrate, a new phosphate binder, prevents the complications of secondary hyperparathyroidism and vascular calcification. Am J Nephrol. 2013;37:346–58.
Rodby R, Umanath K, Niecestro R, Jackson JH, Sika M, Lewis JB, et al. Phosphorus binding with ferric citrate is associated with fewer hospitalizations and reduced hospitalization costs. Expert Rev Pharmacoecon Outcomes Res. 2015;15:545–50.
Negri AL, Urena-Torres PA. Iron-based phosphate binders: do they offer advantages over currently available phosphate binders? Clin Kidney J. 2015;8:161–7.
Floege J, Covic AC, Ketteler M, Mann JFE, Rastogi A, Spinowitz B, et al. Long-term effect of the iron-based phosphate binder, sucroferric oxyhydroxide, in dialysis patients. Nephrol Dial Transplant. 2015;30:1037–46.
Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–74.
Breslow NE, Clayton DG. Approximate inference in generalized linear mixed models. JASA. 1993;88:9–25.
Acknowledgements
The authors thank all the staff members working at Zenjinkai Clinics and data collecting staff.
Funding
No funding.
Availability of data and materials
Not applicable.
Authors’ contributions
TI participated in the design of this study, drafted the manuscript, and performed the statistical analysis. YN contributed on data collection. KO kindly reviewed and revised this manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The Zenjinkai Yokohama Daiichi Hospital ethics committee provided approval.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ishii, T., Nakajima, Y. & Oyama, K. Comparative analysis of the phosphate-binding effects of sucroferric oxyhydroxide, ferric citrate, and lanthanum carbonate. Ren Replace Ther 3, 38 (2017). https://doi.org/10.1186/s41100-017-0119-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41100-017-0119-6