Abstract
Background
The triglyceride glucose (TyG) and triglyceride-to-high-density lipoprotein cholesterol ratio (TG/HDL-c) are the indices that can predict the progression of pre-diabetes to type 2 diabetes mellitus (T2DM). This study aimed to examine the relationship between TyG and TG/HDL-c indices with the incidence of T2DM in pre-diabetes patients.
Methods
A total of 758 pre-diabetic patients aged 35–70 years who were enrolled in a prospective Fasa Persian Adult Cohort were followed up for 60 months. TyG and TG/HDL-C indices were obtained at baseline data and divided into quartiles. The 5-year cumulative incidence of T2DM was analyzed by Cox proportional hazards regression analysis while controlling for baseline covariates.
Results
During 5 years of follow-up, there were 95 incident cases of T2DM, with an overall incidence rate of 12.53%. After adjusting for age, sex, smoking, marital status, socioeconomic status, body mass index, waist circumference, hip circumference, hypertension, total cholesterol, and dyslipidemia, the multivariate-adjusted hazard ratios (HRs) demonstrated that patients with the highest TyG and TG/HDL-C indices quartile were at higher risk of T2DM (HR = 4.42, 95%CI 1.75–11.21) and (HR = 2.15, 95%CI 1.04–4.47), respectively, compared to participants in the lowest quartile. As the quantiles of these indices increase, the HR value shows a significant increment (P < 0.05).
Conclusion
The results of our study showed that the TyG and TG/HDL-C indices can be important independent predictors for the progression of pre-diabetes to T2DM. Therefore, controlling the components of these indicators in pre-diabetes patients can prevent developing T2DM or delay its occurrence.
Similar content being viewed by others
Introduction
Type 2 diabetes mellitus (T2DM) is a serious health concern for health systems and health providers around the world due to its high prevalence, rapid growth, and life-threatening complications [1]. According to the latest diabetes atlas by the International Diabetes Federation (IDF), 10.5% of adults aged 20–79 years were suffered from diabetes in 2021 globally, and it is expected to rise to 12.2% by 2045 [2]. Reports in 2019 stated that T2DM was the direct cause of death for 1.5 million individuals, and 48% of all diabetes-related deaths occurred before age 70 years [3]. A large number of diabetes-related deaths are caused by macro-vascular complications including stroke and heart disease [4]. Therefore, providing a screening model and strategies is necessary for prevention, diagnosis, and medical treatment in the early stages [5].
Given that insulin resistance is the main pathological mechanism of T2DM [6], alternative indices of insulin resistance such as triglyceride glucose (TyG) index and triglyceride-to-high-density lipoprotein cholesterol ratio (TG/HDL-c) can be helpful in the prediction of T2DM development [7, 8]. The results of several studies, including studies conducted on American, European, and Asian populations, showed that although fat deposition is affected by race and ethnicity, these indicators are significantly related to insulin resistance [9,10,11,12,13]. TyG is observed to reflect insulin resistance and identify individuals with a high risk of developing cardiovascular diseases [14]. TyG index is calculated using the measurement of fasting triglyceride (TG) and fasting blood glucose (FBG) (Ln (TG ∗ FBS)/2) [15]. The TyG index is proposed as a simple and low-cost indicator of insulin resistance [15]. It was seen individuals in higher TyG quartiles are 3.67 times higher in the risk of developing T2DM [5]. Moreover, it was shown that the risk of T2DM for the highest quartile of the TG/HDL-C, after adjustment of confounding variables, is 2.54 times higher in comparison with the lowest quartile [16]. Also, another study indicated individuals in the highest quartile of the TyG index, after adjusting for confounding variables, have a 3.67 higher risk of T2DM development in comparison with the lowest quartile [5].
Despite the observed relationship between TyG and TG/HDL-C indices and diabetes incidence, few studies in Iran have investigated the above-mentioned association. To the best of our knowledge, considering the high incidence rate for T2DM [17], these indices have not been studied in the south region of Iran. Also, the spectrum of TyG and insulin resistance levels vary according to ethnicity [18]. Therefore, the present study aimed to determine the relationship between TyG and TG/HDL-C indices with the risk of T2DM in pre-diabetic patients.
Materials and methods
Study population
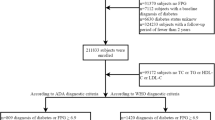
The present prospective cohort study was carried out on the baseline data of the Fasa Persian Adult Cohort Study (FACS) and a 60-month follow-up of participants. Of FACS participants, 35–70-year-old pre-diabetic patients were recognized and their data were extracted from the FACS database. The FACS is a branch of the Persian Cohort Study, which was initiated in 2013 to investigate the risk factors for cardiovascular diseases in the Sheshdeh and Qara-Balagh regions containing 22 villages, Fasa, Fars province, Iran.
Briefly, 10,000 adults aged 35–70 years who were residing in the above-mentioned area formed the initial sample size of the FACS. Phone calls and referrals from health centers located in the region were used to invite individuals to the study center. To record baseline data, an average of 20 people were presented in the center.
A comprehensive questionnaire was designed for data gathering. The questionnaire was filled by the assessments and interviews done by trained personnel. Using the pre-designed questionnaire, FACS’s personnel recorded demographic information, socioeconomic status (SES), medical and clinical information, history of communicable and non-communicable diseases, and nutritional intakes. A separate unique interviewer was considered to complete each part of the questionnaire based on the personnel’s area of expertise. Also, blood samples have been collected, stored, and analyzed. Collected data and blood sample analysis results are stored in an electronically central data server daily. The baseline data collection was completed in 2016, and six follow-up periods have been done so far. Further details of the FACS are available at the FASA cohort study protocol [19].
Variables of the study
Pre-diabetes and T2DM
Participants in the FASA Persian cohort study, whose baseline fasting blood sugar (FBS) was between 100 and 125 mg/dl, were known as pre-diabetic. The pre-diabetic participants were followed up for 60 months for the incidence of T2DM. The incidence of T2DM is confirmed in pre-diabetic individuals by an annual assessment of the fasting blood sugar using the criteria developed by the Diabetes Association Center. Therefore, FBS over 125 mg/dl was considered borderline for diabetes [20].
TyG and TG/HDL-c
Fasting blood sugar (FBS) and triglycerides (TG) were used to calculate the TyG index based on its standard formula: ln[TG (mg/dL) × FBG (mg/dL)/2]. Moreover, TG was divided by high-density lipoprotein (HDL) to calculate TG/HDL-c index [21].
Demographic and socioeconomic status (SES)
Demographic characteristics of participants including gender, age, marital status, and education level were extracted from the FACS’s database. To calculate SES, the principal component analysis (PCA) method based on the lifestyle and household-related variables including the ownership of a house, freezer, washing machine, dishwasher, personal computer (PC), car, motorcycle, color TV, vacuum cleaner, and cell phone, house area, number of rooms in the house, bathroom, family size, Internet access, car price, using a laptop, PC, Internet, car, number of books read, foreign and domestic pilgrim, and foreign and domestic travels were used. According to the results of PCA, individuals were categorized into low, middle, and high SES [22, 23].
Clinical and medical history
Weight, height, body mass index (BMI), waist circumference (WC), hip circumference (HC), lipid profile (including low-density lipoprotein (LDL), HDL, and TG), and cardiovascular disorders including dyslipidemia (dyslipidemia was defined as LDL ≥ 130 mg/dL, or TC ≥ 200 mg/dL, or HDL ≤ 40 mg/dL in men, and 50 mg/dl in women or TG ≥ 150 mg/dL and or use of lipid-lowering medications in the past two weeks) [24], and hypertension (defined as systolic BP ≥ 140 mmHg and/or diastolic BP ≥ 90 mmHg) [25] were obtained from the data center. Moreover, the 5-year incidence rate of T2DM in pre-diabetic individuals was calculated in regard to its quartiles and their 95% confidence intervals (CI).
Statistical analysis
For quantitative variables, the normality of the data was checked by the Shapiro test (p > 0.05) and Q–Q plot. To report the study variables in the TyG and TG/HDL-c quartile, mean and standard deviation (mean ± SD), and frequency and percentage were used for quantitative variables and qualitative variables, respectively. To investigate the differences between the TyG and TG/HDL-c quartile, one-way ANOVA and Chi-square tests were used. The median survival time (T2DM incidence) was calculated using the Kaplan–Meier chart, and the log-rank test was performed to compare the median survival time in the TyG and TG/HDL-c index quartiles.
The Cox proportional hazard model was used as the most common model in survival studies to evaluate the relationship between TyG and TG/HDL-c indices with the incidence of T2DM during 60 months or 5 years of the follow-up period. To control the effect of confounders in this study, other covariates (age, gender, smoking, marital status, SES, BMI, WC, HC, hypertension, total cholesterol, dyslipidemia, HDL) were included in the model. The adjusted hazard ratio with its 95% confidence interval was used to report the association. In all analyses, the first type error (α) was considered 0.05. A p-value lower than 0.05 is considered significant.
Results
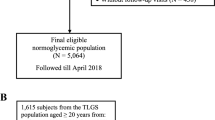
In the present study, a total of 758 participants (59.23% were female), aged 52.57 ± 9.22 years (mean ± SD), with pre-diabetes at baseline assessment of FACS were enrolled. The flow diagram of the study population is shown in Fig. 1.
The baseline characteristics of participants including anthropometric measurements, laboratory examinations, and demographic characteristics according to the quartile of TyG and TG/HDL-c indices are shown in Tables 1 and 2.
Participants were stratified into four groups according to the quartiles of the TG/HDL-c and TyG indices. Age (P = 0.030), BMI (P < 0.001), WC (P < 0.001), HC (P < 0.001), TG (P < 0.001), total cholesterol (P < 0.001), FBS (P < 0.001), HDL-c (P < 0.001), LDL-c (P < 0.001), dyslipidemia (P < 0.001), and hypertension (P = 0.002) were statistically different between TyG quartiles. The results were similar for gender, BMI, WC, HC, total cholesterol, HDL-c, LDL-c, TG, dyslipidemia, and hypertension (P < 0.05) for TG/HDL-c quartiles (Tables 1 and 2).
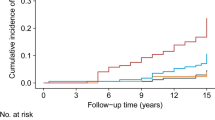
There were 95 incident cases of T2DM, with an overall incidence rate of 12.53%, during the 60-month follow-up. As shown in Table 3 in comparison with individuals in the lowest quartile of TyG and TG/HDL-c indices, participants in higher quartiles showed an increased cumulative incidence. Figures 2 and 3 depict the Kaplan–Meier curves for cumulative incidences of T2DM for TyG and TG/HDL-c indices quartiles. The highest TyG and TG/HDL-c indices quartile was associated with the highest probability of developing T2DM; these probabilities decreased sequentially for lower quartiles (P < 0.05) (Figs. 2 and 3).
Table 3 shows the results of multiple Cox proportional hazards regression analyses. In model 1 and model 2 (adjust for age and sex), a higher TyG and TG/HDL-C had a significant association with incident T2DM in pre-diabetic patients. TyG quartiles compared with Q1, HRs (95%CI, p-value) for the incidence of T2DM in Q2, Q3, and Q4 were 2.21 (1.01–4.80, P = 0.045), 0.48 (1.11–5.53, P = 0.026), and 4.42 (1.75–11.21, P = 0.002), respectively, after adjusting for age, sex, smoking, marital status, socioeconomic status, BMI, WC, HC, hypertension, dyslipidemia, FBS, TG, and HDL-C (Model 3). Also, with an increscent TG/HDL-c quartile compared with the first quartile, the risk of T2DM increases in Q2 (HR: 2.28, 95% CI: 1.11–4.68, P = 0.024) and Q4 (HR: 2.15, 95% CI: 1.04–4.47, P = 0.038), respectively, in model 3.
Discussion
The results of this prospective cohort study showed that TyG and TG/HDL-c indices are independently and positively related to the risk of T2DM development in pre-diabetic patients. Also, an increasing trend of T2DM risk was seen with increasing in TyG and TG/HDL-c indices score after adjustment of the confounding variables of age and sex.
Several previous prospective studies showed that increasing TyG and TG/HDL-c indices increase the risk of T2DM [26,27,28]. A community-based cohort study in China with 687 participants and a 15-year follow-up period showed that an increment in the TG/HDL-c increases the risk of T2DM (OR = 1.34, P = 0.1) [8]. The TyG and TG/HDL-c indices are closely related to insulin resistance, and these indices may be a proxy for insulin resistance to identify individuals with a high risk of T2DM [29]. Also, alternative markers of insulin resistance have been introduced to predict the risk of T2DM such as lipoprotein particles’ concentration and size, glucose, and insulin levels. However, recent studies suggest that TyG and TG/HDL-c indices may be better predictors of insulin resistance and cardiovascular disease risk than single indicators [9, 30].
High levels of TG and low levels of HDL-c are two key metabolic abnormalities [30]. Therefore, high TG/HDL-c may indicate individuals have a metabolic disorder with higher BMI and higher levels of FBS, TG, LDL-c, and very low-density lipoprotein (VLDL-c) and lower levels of HDL-c. Thus, this evidence supports that the above-mentioned index may be a good predictor of T2DM risk [31]. The mechanisms by which the high TG/HDL-c causes T2DM are complicated and unclear. Investigations showed that a higher level of triglycerides can increase the plasma free fatty acid levels, which increases the level of triglycerides and decreases the level of HDL-c in the liver, consequently accelerating insulin resistance and contributing to the development of T2DM [32]. A series of abnormalities related to insulin resistance such as B-cell dysfunction, increased blood glucose, impaired insulin secretion, and B-cell apoptosis can be caused by excessive triglyceride and low HDL-c levels. In addition, increased levels of nitric oxide and ceramide due to increased levels of TG can induce B-cell apoptosis. Accumulation of cholesterol in B cells as a result of low HDL-c can cause cytotoxicity [16]. In addition, low HDL-c levels can directly mediate glucose uptake and thereby lead to T2DM [11].
Central obesity, insulin resistance, dyslipidemia, and hypertension increase the risk of cardiovascular diseases and T2DM. Triglyceride and HDL-c are important risk factors for cardiovascular diseases [33]. Babic et al. [34] showed that this ratio may be a surrogate metabolic variable that predicts the development of T2DM among individuals. In addition, to describe diabetes through insulin resistance, T2DM is also characterized by a decrease in insulin secretion. Therefore, pancreatic B-cell dysfunction can also be one of the possible explanations for the relationship between the TG/HDL-c index and T2DM [16].
TyG index, which is a combination of TG and FBS, is a simple, effective, reproducible, and reliable alternative for insulin resistance [35]. Previous studies showed that fasting blood glucose and TG reflect insulin resistance in the liver and fat cells, respectively. Therefore, the increase in the TyG index over time reflects insulin resistance in both liver and fat cells [36].
The present study faces some limitations. One of the limitations of the study is the identification of T2DM through fasting blood glucose instead of the 2-h oral glucose tolerance test or measuring the level of HbA1c, which may underestimate the incidence of T2DM. It should be noted that the information was obtained from the Iranian population who are at risk of T2DM and the generalizability of the results is limited due to similar genetic characteristics and lifestyle. Finally, a healthy diet can affect fat homeostasis and glucose metabolism, which the dietary data were not evaluated in our study. Studies are needed to determine how a diet affects triglycerides and T2DM risk.
Conclusion
The results from this study showed that a high TyG and TG/HDL-c index is associated with an increased incidence of diabetes in pre-diabetes patients. Therefore, it is necessary to lower the level of TyG and TG/HDL-c through preventive measures such as lifestyle management for middle-aged people.
References
Wang Z, Zhao L, He S. Triglyceride–glucose index as predictor for future type 2 diabetes mellitus in a Chinese population in southwest China: a 15-year prospective study. Endocrine. 2021;72(1):124–31.
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119.
https://www.who.int/news-room/fact-sheets/detail/diabetes: WHO; 2018.
Cusick M, Meleth AD, Agrón E, Fisher MR, Reed GF, Knatterud GL, et al. Associations of mortality and diabetes complications in patients with type 1 and type 2 diabetes: early treatment diabetic retinopathy study report no. 27. Diabetes Care. 2005;28(3):617–25.
Park B, Lee HS, Lee Y-J. Triglyceride glucose (TyG) index as a predictor of incident type 2 diabetes among nonobese adults: a 12-year longitudinal study of the Korean genome and epidemiology study cohort. Transl Res. 2021;228:42–51.
Rhee SY, Woo J-T. The prediabetic period: review of clinical aspects. Diabetes Metab J. 2011;35(2):107–16.
Zheng S, Shi S, Ren X, Han T, Li Y, Chen Y, et al. Triglyceride glucose-waist circumference, a novel and effective predictor of diabetes in first-degree relatives of type 2 diabetes patients: cross-sectional and prospective cohort study. J Transl Med. 2016;14(1):1–10.
He S, Wang S, Chen X, Jiang L, Peng Y, Li L, et al. Higher ratio of triglyceride to high-density lipoprotein cholesterol may predispose to diabetes mellitus: 15-year prospective study in a general population. Metabolism. 2012;61(1):30–6.
Locateli JC, Lopes WA, Simões CF, de Oliveira GH, Oltramari K, Bim RH, et al. Triglyceride/glucose index is a reliable alternative marker for insulin resistance in South American overweight and obese children and adolescents. J Pediatr Endocrinol Metab. 2019;32(10):1163–70.
Calcaterra V, Montalbano C, De Silvestri A, Pelizzo G, Regalbuto C, Paganelli V, et al. Triglyceride glucose index as a surrogate measure of insulin sensitivity in a caucasian pediatric population. J Clin Res Pediatr Endocrinol. 2019;1:1–11.
Cheng C, Liu Y, Sun X, Yin Z, Li H, Zhang M, et al. Dose–response association between the triglycerides: high-density lipoprotein cholesterol ratio and type 2 diabetes mellitus risk: the rural Chinese cohort study and meta-analysis. J Diabetes. 2019;11(3):183–92.
Zheng D, Li H, Ai F, Sun F, Singh M, Cao X, et al. Association between the triglyceride to high-density lipoprotein cholesterol ratio and the risk of type 2 diabetes mellitus among Chinese elderly: the Beijing Longitudinal Study of Aging. BMJ Open Diabetes Res Care. 2020;8(1):e000811.
Chamroonkiadtikun P, Ananchaisarp T, Wanichanon W. The triglyceride-glucose index, a predictor of type 2 diabetes development: a retrospective cohort study. Prim Care Diabetes. 2020;14(2):161–7.
Sánchez-Íñigo L, Navarro-González D, Fernández-Montero A, Pastrana-Delgado J, Martínez JA. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest. 2016;46(2):189–97.
Hong S, Han K, Park C-Y. The triglyceride glucose index is a simple and low-cost marker associated with atherosclerotic cardiovascular disease: a population-based study. BMC Med. 2020;18:1–8.
Lim T-K, Lee HS, Lee Y-J. Triglyceride to HDL-cholesterol ratio and the incidence risk of type 2 diabetes in community dwelling adults: a longitudinal 12-year analysis of the Korean Genome and Epidemiology Study. Diabetes Res Clin Pract. 2020;163:108150.
Sharafi M, Eftekhari MH, Mohsenpour MA, Afrashteh S, Baeradeh N, Fararouei M, et al. Progression of prediabetes to diabetes and its associated factors: The Fasa Adult Cohort Study (FACS). Int J Diabetes Dev Ctries. 2023. https://doi.org/10.1007/s13410-023-01172-w.
Vasques ACJ, Novaes FS, de Oliveira MDS, Souza JRM, Yamanaka A, Pareja JC, et al. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011;93(3):e98–100.
Homayounfar R, Farjam M, Bahramali E, Sharafi M, Poustchi H, Malekzadeh R, et al. Cohort profile: the Fasa Adults Cohort Study (FACS): a prospective study of non-communicable diseases risks. Int J Epidemiol. 2023. https://doi.org/10.1093/ije/dyac241.
American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42:S13–28.
Hong S, Han K, Park C-Y. The triglyceride glucose index is a simple and low-cost marker associated with atherosclerotic cardiovascular disease: a population-based study. BMC Med. 2020;18(1):1–8.
Sharafi M, Bahramali E, Farjam M, Rezaeian S, Afrashteh S, Amiri Z. Socioeconomic inequality in noncommunicable diseases: results from a baseline Persian cohort study. Med J Islam Repub Iran. 2021;35:78.
Farjam M, Sharafi M, Bahramali E, Rezaei S, Hassanzadeh J, Rezaeian S. Socioeconomic inequalities in gastroesophageal reflux disorder: results from an Iranian cohort study. Middle East J Dig Dis. 2018;10(3):180.
Jamali Z, NorooziKarimabad M, Khalili P, Sadeghi T, Sayadi A, MohammadakbariRostamabadi F, et al. Prevalence of dyslipidemia and its association with opium consumption in the Rafsanjan cohort study. Sci Rep. 2022;12(1):11504.
Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16(4):223–37.
Vega GL, Barlow CE, Grundy SM, Leonard D, DeFina LF. Triglyceride–to–high-density-lipoprotein-cholesterol ratio is an index of heart disease mortality and of incidence of type 2 diabetes mellitus in men. J Investig Med. 2014;62(2):345–9.
Lee DY, Lee ES, Kim JH, Park SE, Park C-Y, Oh K-W, et al. Predictive value of triglyceride glucose index for the risk of incident diabetes: a 4-year retrospective longitudinal study. PLoS One. 2016;11(9):e0163465.
Cai Z, Chen Z, Fang W, Li W, Huang Z, Wang X, et al. Triglyceride to high-density lipoprotein cholesterol ratio variability and incident diabetes: a 7-year prospective study in a Chinese population. J Diabetes Investig. 2021;12(10):1864–71.
Chiang J-K, Lai N-S, Chang J-K, Koo M. Predicting insulin resistance using the triglyceride-to-high-density lipoprotein cholesterol ratio in Taiwanese adults. Cardiovasc Diabetol. 2011;10(1):1–6.
Kannel WB, Vasan RS, Keyes MJ, Sullivan LM, Robins SJ. Usefulness of the triglyceride–high-density lipoprotein versus the cholesterol–high-density lipoprotein ratio for predicting insulin resistance and cardiometabolic risk (from the Framingham offspring cohort). Am J Cardiol. 2008;101(4):497–501.
Quispe R, Manalac RJ, Faridi KF, Blaha MJ, Toth PP, Kulkarni KR, et al. Relationship of the triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio to the remainder of the lipid profile: the Very Large Database of Lipids-4 (VLDL-4) study. Atherosclerosis. 2015;242(1):243–50.
Jung UJ, Choi M-S. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15(4):6184–223.
Chen Z, Hu H, Chen M, Luo X, Yao W, Liang Q, et al. Association of Triglyceride to high-density lipoprotein cholesterol ratio and incident of diabetes mellitus: a secondary retrospective analysis based on a Chinese cohort study. Lipids Health Dis. 2020;19(1):1–11.
Babic N, Valjevac A, Zaciragic A, Avdagic N, Zukic S, Hasic S. The triglyceride/HDL ratio and triglyceride glucose index as predictors of glycemic control in patients with diabetes mellitus type 2. Med Arch. 2019;73(3):163.
Khan SH, Sobia F, Niazi NK, Manzoor SM, Fazal N, Ahmad F. Metabolic clustering of risk factors: evaluation of Triglyceride-glucose index (TyG index) for evaluation of insulin resistance. Diabetol Metab Syndr. 2018;10(1):1–8.
Wang A, Tian X, Zuo Y, Chen S, Meng X, Wu S, et al. Change in triglyceride-glucose index predicts the risk of cardiovascular disease in the general population: a prospective cohort study. Cardiovasc Diabetol. 2021;20(1):1–9.
Acknowledgements
We appreciate all participants who participated in the study
Funding
This study was financially supported by Fasa University of Medical Sciences, Fasa, Iran.
Author information
Authors and Affiliations
Contributions
SA, MSH, MAM, and ZA contributed to main idea, data interpretation, and writing the manuscript. BP, MHE, and MSH critically reviewed the manuscript. EH and MKH wrote and edited the manuscript. AF and ZM drafted the article.
Corresponding authors
Ethics declarations
Competing interests
The authors have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sharafi, M., Amiri, Z., Pezeshki, B. et al. Predictive value of triglycerides to high-density lipoprotein cholesterol and triglyceride glycemic index for diabetes incidence in pre-diabetes patients: a prospective cohort study. J Health Popul Nutr 42, 67 (2023). https://doi.org/10.1186/s41043-023-00410-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41043-023-00410-5