Abstract
Background
Spontaneous intracerebral hemorrhage (SICH) has high morbidity and mortality, with no clear standard of treatment available. Compared with the craniotomy approach, neuroendoscopy is a relatively minimally invasive treatment method, and may be an efficient alternative. Therefore, this meta-analysis aimed to assess the clinical efficacy of neuroendoscopy and craniotomy in SICH patients.
Methods
The electronic databases Web of Science, PubMed, EmBase, MEDLINE, and the Cochrane Library were systematically searched. According to the PRISMA template, we finally selected and analyzed 14 eligible studies that evaluated neuroendoscopy versus craniotomy. Primary outcomes included operation time, intraoperative blood loss volume, evacuation rate, residual hematoma, complications, hospital stay duration, clinical outcomes, and other parameters.
Results
A total of 4 randomized controlled trials (RCTs) and 10 retrospective studies (non-RCTs) involving 1652 patients were included in the final analysis. In the neuroendoscopy (NE) group, operation time (p < 0.00001), intraoperative blood loss volume (p < 0.0001), hematoma evacuation rate (p = 0.0002), complications (p < 0.00001), hospitalization days (p = 0.004), and mortality (p < 0.0001) were significantly different from those of the craniotomy (C) group, with a higher rate of good recovery compared with the craniotomy group (P < 0.00001).
Conclusions
These findings suggest that patients with SICH and physicians may benefit more from neuroendoscopic surgery than craniotomy.
Similar content being viewed by others
Background
Intracerebral hemorrhage (ICH) accounts for 10–15% of all strokes in the USA, Europe and Australia, and 20–30% of Asian cases, with a 30-day mortality rate of 35% to 52%; half of the related deaths occur in the first 2 days [1,2,3,4]. Its overall incidence is 24.6 per 100,000 person-years, indicating that it represents the most fatal type of stroke around the world [5]. It is worth noting that most of individuals living with ICH have varying degrees of long-term disability. Only 20% of ICH patients survive independently within 6 months [3]. Its main risk factors include age, a history of hypertension, East and Southeast Asian ethnicity, smoking, drug, and alcohol abuse, inherited or acquired coagulopathies, anticoagulant use, past stroke history, vascular abnormalities (arteriovenous malformations, developmental venous abnormalities, amyloid angiopathy) and potential tumors [6]. Gender may also be a risk factor, although not statistically significant, but ICH incidence in women is 15% lower than in men [7]. Such high mortality and disability rates undoubtedly impose great mental and economic burden upon patients and their families.
Currently, ICH treatment options mainly include endoscopic evacuation, stereotactic aspiration, conventional craniotomy, and conservative treatment. Indeed, treatment of patients with ICH encounters two major problems. The first problem is treatment selection, i.e., conservative or surgical treatment; the second is the selection of the operation, i.e., endoscopic surgery or craniotomy. In general, patients with small hematomas and no neurological deficits tend to opt for conservative treatment, while surgery tends to be performed in those with massive hemorrhage and progressive neurological deterioration [8, 9]. In recent years, neuroendoscopic surgery for ICH has attracted much attention, because it is more minimally invasive than craniotomy, and can reduce the characteristic peripheral brain injury. An increasing number of physicians now select endoscopy for the treatment of ICH patients, but its long-term efficacy and complications remain controversial. Therefore, clarifying which surgical method between neuroendoscopy and craniotomy is more efficient for ICH patients is critical.
Methods
Search strategy
A literature search was performed by two independent investigators (Du and Wang) in various electronic databases, including PubMed, EmBase, MEDLINE, Cochrane Controlled Trials Register and Web of Science, from inception to July 2018, with the following keywords: “(Endoscope OR Endoscopy OR endoscopic surgery OR neuroendoscopic surgery) AND (Intracranial Hemorrhage OR Intracranial Hemorrhage, Hypertensive OR cerebral Hemorrhage OR brain Hemorrhage OR putaminal Hemorrhage OR basal ganglia Hemorrhage OR thalamic Hemorrhage OR subcortex Hemorrhage)”. After sequentially reviewing the titles, abstracts, and full texts of the retrieved reports according to the PRISM statement, studies clearly irrelevant were excluded. Any disagreement between the two investigators was resolved by consensus or a third investigator if required. The study authors were contacted for clarifications and further information when necessary. The search was limited to studies published in English.
Inclusion and exclusion criteria
Inclusion criteria were (1) diagnosis of intracranial hemorrhage by computed tomography; (2) treatment methods included endoscopic surgery and craniotomy, with or without intralesional thrombolysis; (3) randomized controlled trials (RCTs) or prospective controlled studies (non-RCTs). Studies were excluded if they included or were (1) infratentorial intracerebral hemorrhage; (2) brain injury, bleeding due to brain tumor, and bleeding tendencies caused by uremia, liver cirrhosis, or anticoagulation therapy, intracranial aneurysm, cerebral arteriovenous malformation, subdural hemorrhage, extradural hemorrhage subarachnoid hemorrhage or pituitary apoplexy; (3) incomplete data or non-English publication; (4) meta-analyses, editorials, letters, errata, case reports, reviews, and animal experiments.
Data extraction
The data were extracted by two investigators independently according to eligibility criteria, and included basic information (author name, year, and type of documents) and basic patient characteristics (gender and age, number of cases, hematoma location, hematoma evacuation rate, hematoma residual volume, intraoperative blood loss, operation time, hospitalization duration, postoperative complications, mortality, and good recovery). All data were recorded using an Excel sheet. Good functional outcome (GFO) was defined as a patient being able to care for him/herself, corresponding to a modified Rankin Scale (mRS) score of 0, 1, 2, or 3, a Glasgow Outcome Scale (GOS) score of 4 or 5, or activities of daily living (ADL) score of 1, 2, or 3, or a Barthel index (BI) > 60. If more than 1 scale was used to evaluate the patients’ functional outcomes within an article, we first selected the GOS as the assessment scale, and then the modified Rankin Scale, the BI, and ADL score. Any discrepancies were solved by discussion and consensus.
Statistical analysis
Statistical analyses were performed with the Review Manager 5.3 software and forest plots were generated, with statistical significance defined as p < 0.05. Between studies heterogeneity was assessed by the standard chi-square test and I2 statistic; heterogeneity was pre-specified at p ≤ 0.10 or I2 ≥ 50% in this study. In case of low-moderate heterogeneity, a fixed-effects model was used for data analysis. Otherwise, a random-effects model was used to analyze the pooled data. Dichotomous variables were expressed as relative risk ratio (RR) with a 95% confidence interval (CI). Continuous variables were assessed using standard mean difference (SMD). All tests were two-tailed, and publication bias was assessed using funnel plots.
Results
Study selection
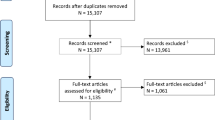
The electronic search yielded 2526 hits in all databases; of which, 1419 were included after duplicates were removed. Title and abstract review were performed for the remaining 1419 articles, and 1257 hits were excluded. The full text of the remaining 62 articles were retrieved, and 48 were excluded for the following reasons: (1) lack of control group (n = 27); (2) meta-analysis (n = 8); (3) review papers (n = 4); (4) bleeding located in the cerebellum (3); (5) Clinical protocol models (n = 2); (6) full texts not available (n = 2);( 7) erratum (n = 1); (8) letter to editors (n = 1). Finally, 14 studies were included in the final analysis, comprising 4 RCTs [10,11,12,13] and 10 non-RCTs [14,15,16,17,18,19,20,21,22,23]) (Fig. 1).
Main characteristics
The main characteristics of studies included study basic information and patient basic characteristics (Tables 1 and 2). Each trial described the baseline characteristics of the enrolled participants, with no significant differences between the neuroendoscopy(NE) and craniotomy(C) groups.
Fourteen studies (4 RCTs and 10 non-RCTs) with a total of 1652 patients (598 and 1054 patients in the endoscopy and craniotomy groups, respectively) were included in the current meta-analysis of ICH treatment,among whom 9(4 and 5 patients in the endoscopy and craniotomy groups, respectively) were lost to follow-up [23]. Therefore, 1643 patients were finally being analyzed. The rate of patients lost to follow-up was within the permissible range. It should be noted that the endoscopic group included patients who underwent endoscopic surgery alone or in combination with stereotactic aspiration, e.g., Cho’s study [10]. The craniotomy group included large and small bone flap craniotomies. Large and small bone flap craniotomies in Chi’s article [17] were compared respectively, and were grouped into the craniotomy group. Three articles were excluded, including one [24] for subtentorial hematoma assessment; in the other two articles [25, 26], some of the patients were cerebellar hemorrhage cases, which would not significantly affect the outcomes of good recovery and mortality (data not shown).
Quality assessment of the selected articles
The Cochrane criteria were used to assess the quality of RCTs: low risk indicated low risk bias of bias; high risk reflected high risk of bias; unclear risk indicated that the report did not provide sufficient or uncertain information for bias assessment. The Newcastle-Ottawa scale was used to assess the quality of non-TRCTs: a total score of < 5 reflected low-quality. Table 2 summarizes the risk of bias of all included studies. In addition, to test whether publication bias was present among trails included in this meta-analysis, we used funnel plots (Figs. 2 and 3). Although the total number of studies in this meta-analysis is small, distribution in the funnel plots is symmetrical. This result suggests that there is no publication bias.
Pooled results
Rebleeding in the NE and C groups
In seven studies [10, 11, 16, 18, 21,22,23] including 453 participants, the rate of rebleeding was statistically significant in the non-RCT group and the overall effect, with RR = 0.40 (95%CI 0.17–0.98; p = 0.04) and RR = 0.40 (95%CI 0.19–0.87; p = 0.02), respectively; meanwhile, there was no significant difference in the RCT group, with RR = 0.40 (95%CI 0.08–1.87; p = 0.24). There was no evidence of statistically significant heterogeneity (RCTs, p = 0.82 and I2 = 0%; non-RCTs, p = 0.62 and I2 = 0%; overall, p = 0.85 and I2 = 0%), and a fixed-effects model was used for analysis. This finding was discordant with a previous publication [27]. In a forest plot, one article was excluded [20] because there was no case of rebleeding in the endoscopy and craniotomy groups (Fig. 4).
Evacuation rates in the NE and craniotomy groups
Nine trials [10,11,12,13, 16, 19, 21,22,23] (4 RCTs and 5 non-RCTs) evaluating 744 patients (378 and 366 patients in the experimental and control arms, respectively) assessed evacuation rates. There were significant differences in evacuation rate for the RCT and non-RCTs groups, with SMD = 1.08 (95%CI 0.31–1.86, P = 0.006) and SMD = 1.08 (95%CI 0.15–2.01, P = 0.007), respectively,indicating that the patients who administered NE had a higher evacuation rate than those who underwent craniotomy in the RCT and non-RCT group. However, heterogeneity was significant among articles, with I2 = 92% (p < 0.00001) and I2 = 92% (p < 0.00001) in the RCT and non-RCT groups, respectively, and a random-effects model was used (Fig. 5).
Duration of operation in the NE and craniotomy groups
A total of 11 trials [10,11,12,13, 15, 16, 19,20,21,22,23] (4 RCTs and 7 non-RCTs) comprising 832 patients (423 and 409 patients in the experimental and control arms, respectively) assessed operation time. There were significant differences in operation time for the RCT and non-RCT groups, with SMD = − 3.29(95%CI − 4.56 to − 2.02, p < 0.00001) and SMD = − 3.82 (95%CI − 4.91 to − 2.72, p < 0.00001), respectively, indicating that the patients administered NE had reduced operation time compared with the craniotomy group in RCTs and non-RCTs, with I2 = 94% (p < 0.00001) and I2 = 90% (p < 0.00001), respectively; a random-effects model was used for analysis (Fig. 6). Sensitivity analyses also indicated a statistical difference between the two groups, and the funnel plot was visually symmetric (Fig. 3).
Good recovery in the NE and craniotomy groups
Eight trials [11, 17, 23] assessing 1383 patients (460 and 923 patients in the experimental and control arms, respectively) were included in the meta-analysis of good recovery. There were significant differences in good recovery for the RCT and non-RCTs groups, with RR = 1.61 (95%CI 1.35–1.92, P < 0.00001) and RR = 1.55 (95%CI 1.38–1.75, P < 0.00001), respectively. Eight studies documented good recovery, and the overall effect showed more patients with good recovery in the NE than C group (RR = 1.57, 95%CI 1.42− 1.73, p < 0.00001). There was no evidence of statistically significant heterogeneity (p = 0.75 and I2 = 0%), and a fixed-effects model was used (Fig. 7).
Mortality in NE and craniotomy groups
A total of 11 trials [10,11,12, 16,17,18,19,20,21,22,23] evaluating 1454 patients (498 and 956 patients in the experimental and control arms, respectively) found mortality rates of 9.8% (49/498) and 20.4% (195/956) in the NE and craniotomy groups, respectively, after NE or craniotomy. The effect of NE or craniotomy on death at the end of follow-up was available in each included study. The pooled RRs of death at the end of follow-up using NE compared to craniotomy for the RCT and the non-RCT groups showed values of 0.45 (95%CI 0.19–1.08, P = 0.08) and 0.53 (95% CI 0.38–0.74, P = 0.0002), respectively, indicating that patients who underwent NE had a lower mortality rate than craniotomy cases in non-RCTs, while there was no significant difference in the RCTs group. There was no evidence of statistically significant heterogeneity (p = 0.73 and I2 = 0%), and a fixed-effects model was used (Fig. 8). Although the total number of studies in this meta-analysis was small, a symmetrical distribution of funnel plots was observed, suggesting no publication bias (Fig. 2).
Additional analyses
Table 3 gives pooled RRs and corresponding 95% CI for the association between neuroendoscopy and craniotomy in ICH patients, according to selected subgroups. There were significant differences between NE group and craniotomy (C) group regarding epilepsy (p = 0.01), pneumonia (p < 0.00001), hypoproteinemia (p = 0.01), tracheotomy (p = 0.003), length of ICU stays (p=0.002), length of hospital stays (p = 0.02), hospital expenses (p < 0.0001), intraoperative blood loss volume (p < 0.00001), with NE group having a higher rate of good recovery than craniotomy group (postoperative BI, mRS, and GOS score). In addition, we also evaluated the incidence of intracranial or wound infections (p = 0.27), digestive diseases (p = 0.72), shunt surgery (p = 0.37), postoperative residual hematoma volume (p = 0.11) of NE and craniotomy groups, and no significant differences were found between these two groups.
Discussion
Intracerebral hemorrhage is a common and devastating disease, which requires improved treatment. Surgical treatment of supratentorial intracranial hematoma has the advantages [28] of reducing intracranial pressure, preventing herniation, eliminating the source of hemorrhage, reducing the source of localized mass lesions, and mitigating secondary neuro-inflammatory cascades. Although clinical guidelines [29] for intracranial hemorrhage are widely used, accompanying factors [30, 31] such as patient age, the Glasgow Coma Scale (GCS) score at admission, and hematoma volume, depth and location, usually influence the neurosurgeon’s decision regarding surgical treatment. Currently, there are many surgical procedures for treating intracranial hematoma, including traditional craniotomy, stereotactic aspiration, and endoscopic surgery. Compared with craniotomy, endoscopic surgery has direct vision and less damage to the surrounding normal brain tissue, and is highly recommended by many neurosurgeons [32]. However, indiscriminate restriction of the ICH indication based solely on dominance criteria could reduce the odds of patient survival in some cases.
The main complications of ICH are re-bleeding, intracranial infection, and pulmonary infection. In this study, the observation group’s incidence of postoperative complications was noticeably lower than that of the control group. The main reasons were analyzed as follows: (1) the traditional craniotomy is more traumatic and causes irreversible damage to the brain tissue and blood vessels in a fistula. Neuroendoscopic surgery is more consistent with the concept of minimally invasive surgery and can effectively avoid important brain functional areas. (2) Craniotomy adopts exterior lighting that is not bright enough for deep hematoma, while neuroendoscopy uses internal lighting, which allows for close observation. The brightness remains unchanged even with the changes in the hematoma depth, which improves the procedure’s accuracy by clearly displaying the intraoperative condition. (3) The infection risk is reduced due to the short operation time, small incision, and minor brain tissue damage.
A systematic review published in 2017 noted [27] that patients with ICH may benefit more from endoscopic surgery than from craniotomy, which supports the current study. In comparison to craniotomy, neuroendoscopic surgery has the advantages of higher hematoma evacuation rate, shorter operation time, better prognosis, and lower mortality.
However, some data included in this analysis were incorrect, such as data regarding patients with rebleeding (NE vs. C, 1 vs. 3) in Cho’s study [10] and death in Feng’s study [12] (NE vs. C, 6 vs. 8), although this did not affect the overall results. Furthermore, this study provided a different point of view in terms of re-bleeding and hospital stay duration; in addition, we also assessed the incidence of shunt surgery and the improvement of postoperative BI, GOS and GCS score, hospital costs, etc., for patients who administered neuroendoscopic surgery or craniotomy, adding new findings into this study. Endoscopic craniotomy with small bone window does not require the use of artificial materials (such as artificial dura mater) that are necessary for an operation and is not subject to secondary cranioplasty, hospitalization therefore cost less. In conclusion, this may serve as a constructive guideline for neurosurgeons in selecting the surgical procedure for treating intracranial hemorrhage. We found that endoscopic surgery, as opposed to craniotomy, can improve patient prognosis.
In this review, statistical heterogeneity was found between endoscopic surgery and craniotomy, in terms of operation time, hematoma residual volume, intraoperative blood loss, and hematoma evacuation, so a random-effects model and the jack-knife method were used to analyze pooled data with high heterogeneity.
In this review, statistical heterogeneity was found between endoscopic surgery and craniotomy in terms of operation time, hematoma residual volume, intraoperative blood loss, and hematoma evacuation, so a random-effect model and the jack-knife method were used to analyze pooled data with high heterogeneity.
For operative time analysis, when the studies by Cho and Feng [10, 12] in RCTs were excluded, further analysis showed that there was no heterogeneity (P = 0.85; I2 = 0%). But heterogeneity remained in the non-RCT group and the overall group; similar results were obtained for the overall effect (data not shown). When compared to the traditional craniotomy, neuroendoscopic surgery has the advantages of small incision and simple operation with the endoscopic working channel, thus reducing the operation time.
Neuroendoscopic minimally invasive surgery with small incision and small opening of bone window can effectively reduce traumatic injuries to patients. A clear surgical field helps prevent damage to normal tissues surrounding a lesion, shorten the operation time, avoid brain tissue being massively exposed for a long period of time, reduce stress reactions, and lower the risk of cerebral edema. The hematoma can be precisely located and effectively removed using neuroendoscopic observation in conjunction with CT positioning, and the removal process is regulated and safer with constant speed, which is beneficial to lower the risk of reperfusion injury and protect cerebral vessels and cranial nerve tissue.
The more the residual hematoma during operation, the worse the operative outcome. We found that there was no statistical heterogeneity for hematoma residual volume in non-RCTs (p = 0.61; I2 = 0%) after excluding Li Y’s study [21] and different results were observed for the overall effect. NE had a higher evacuation rate compared with the craniotomy groups, with SMD = − 0.59 (95%CI − 0.92 to − 0.26, p = 0.0005) (data not shown).
Theoretically, massive intraoperative blood loss may lead to hypoproteinemia or anemia after surgery. We found that the rate of hypoproteinemia after NE was lower than upon craniotomy,with no statistical heterogeneity for intraoperative blood loss volume in non-RCTs (p = 0.65; I2 = 0%) after excluding Xu’s study [23]. However, heterogeneity remained in the overall population; similar results were found for the overall effect, with SMD = − 3.00 (95%CI − 4.20 to − 1.80, p < 0.00001) (data not shown).
Enlargement of intracerebral hemorrhage is the main cause of early clinical deterioration. About 20–40% of the patients show hematoma re-expansion within the first 24h after hemorrhage [33]. Large amounts of hematoma are one of the causes of poor prognosis and high mortality. Previous findings indicate that NE has a high evacuation rate, from 79.2 to 99%, with significant difference compared with craniotomy [12, 26, 34, 35]. Theoretically, surgical hematoma evacuation would benefit patients. We used the jack-knife method to perform sensitivity analysis in the hematoma evacuation group. Therefore, the meta-analysis was repeated four and five times, respectively, each omitting a different study; finally, there was no statistical significance after excluding Zhang J’s article [13] in the RCT group and Zhu or Eroglu’s article [16, 22] in the non-RCT group. However,the same results were obtained for the overall effect (data not shown).
A possible reason for heterogeneity is that this study included multicenter trials, with differences in surgical procedures and treatments in many countries or different hospitals in the same country potentially leading to heterogeneity. We performed two subgroup analyses according to country and publication year, for preoperative Glasgow Coma Scale score and hematoma volume, and similar results were obtained in this work (data not shown).
In this study,the total rebleeding rate was significantly lower in the endoscopy group (3.5%; 8/227) compared with the craniotomy group (9.3%; 21/226), in disagreement with a previous publication [27] (2017). In addition, we assessed complications, including the rates of rebleeding, wound and intracranial infection, epilepsy, pneumonia, digestive tract disease, tracheotomy, hypoproteinemia, and shunt surgery respectively, as well as the incidence of total complications. There was no significant heterogeneity among articles, with I2 = 0% (p = 1.0) in total complications. In the NE group, 8.0% (84/1051) of patients had complications, while 18.7% (199/1066) was found in the craniotomy group. Pooled analysis showed that occurrence of total complications between the NE and craniotomy groups showed a significant difference (p < 0.00001, data not shown). The higher complications in the craniotomy group may be due to longer operation time, larger damage and elevated blood loss.
The cost for treating ICH was reported to be high, up to more than $44,000 in the first year of treatment alone [36]. In the current trials, NE incurred less hospital expenses compared with craniotomy due to shorter hospital stay, lower rate of complications, shorter operation time and better recovery in the latter procedure.
Zhang [13] mentioned that SP (serum substance P) and IL-2 levels in the NE group are significantly higher than control values four weeks after the operation, while IL-6, hs-CRP (high sensitive C-reactive protein), TNF-α (tumor necrosis factor-α) and SF (serum ferritin) levels are significantly lower compared with the craniotomy group. These results showed that endoscopic surgery effectively promotes the recovery of damaged glial cells and is helpful for the prognostic rehabilitation of patients.
The findings show that minimally invasive neuroendoscopic surgery can effectively lower the risk of complications, promote the recovery of neurological function, and improve patients’ life quality. This may be attributed to the minor harm that minimally invasive neuroendoscopic surgery causes to brain tissue. Brain tissue can avoid being massively exposed for a long period of time due to small incision, tiny bone foramen, and short operation time, thus reducing the chance of intracranial and pulmonary infections, intracranial re-bleeding, upper gastrointestinal hemorrhage, and other complications, effectively relieving brain tissue damage caused by cerebral hemorrhage and cerebral edema as well as decreasing the risk of death. Moreover, minimally invasive surgery can effectively reduce the stress stimulation of surgical operation on a body, lessen the pathological damage to brain tissue, relieve the pain of patients, shorten the ICU stay length and speed up the recovery of the patient's neurological function, thus improving the patient’s life quality [37,38,39].
In addition, different surgical approaches may improve the outcome of patients with ICH [40]. Based on previous reports and our own experience, we believe that a single surgical procedure cannot be fully adapted to all patients, and the procedure should be selected dialectically. Endoscopic surgery combined with stereotactic navigation, 3D reconstruction, intraoperative CT imaging, B-ultrasound or other techniques may cause more patients to benefit from this operation [41,42,43].
Limitations of this meta-analysis must be pointed out. Firstly, some of the included trials were non-RCTs, and most studies did not report random sequence generation and allocation concealment. Secondly, the duration of follow-up differed in these studies. Therefore, more studies addressing complications, good recovery, and mortality with uniform follow-up times of at least 6 months are required. Thirdly, the number of included patients was relatively limited in this review, which may affect the obtained results. Furthermore, heterogeneity was found in the pooled data for operation time,evacuation rate, residual hematoma volume and intraoperative blood loss volume, and a random-effects model was used to estimate the overall effects more conservatively.
Conclusion
Neuroendoscopic surgery is associated with significantly reduced complication and death rates after surgical evacuation of ICH. There was also a statistically significant reduction in the risk of poor functional outcome after neuroendoscopy. These findings clearly demonstrate the advantages of neuroendoscopic surgery for ICH treatment. This study could guide clinicians in selecting treatment options and appropriate patients for neuroendoscopic surgery in ICH. However, further randomized controlled trials are required to control all confounding factors and confirm this conclusion. Meanwhile, neurosurgeons should also improve their surgical skills to reduce the impact of human factors in surgical procedures.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ADL:
-
Activities of daily living
- BI:
-
Barthel index
- C:
-
Craniotomy
- CI:
-
Confidence interval
- GCS:
-
Glasgow Coma Scale
- GFO:
-
Good functional outcome
- GOS:
-
Glasgow Outcome Scale
- RCT:
-
Randomized controlled trial
- RR:
-
Relative risk ratio
- mRS:
-
Modified Rankin Scale
- NE:
-
Neuroendoscopy
- SICH:
-
Spontaneous intracerebral hemorrhage
- SMD:
-
Standard mean difference
References
Adeoye O, Broderick JP. Advances in the management of intracerebral hemorrhage. Nat Rev Neurol. 2010;6:593–601.
Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines forthe early management of patients with acute ischemic stroke:2019 update to the 2018 guidelines for the early management of a-cute ischemic stroke: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2019;50(12):e344–418.
Godoy DA, Nunez-Patino RA, Zorrilla-Vaca A, et al. Intracranial hypertension after spontaneous intracerebral hemorrhage: a systematic review and meta-analysis of prevalence and mortality rate. Neurocrit Care. 2019;31(1):176–87.
Ren J, Wu X, Huang J, et al. Intracranial pressure monitoringaided management associated with favorable outcomes in patients with hypertension-related spontaneous intracerebral hemorrhage. Transl Stroke Res. 2020;11(6):1253–63.
van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–76.
Rennert RC, Signorelli JW, Abraham P, Pannell JS, Khalessi AA. Minimally invasive treatment of intracerebral hemorrhage. Expert Rev Neurother. 2015;15:919–33.
Ikram MA, Wieberdink RG, Koudstaal PJ. International epidemiology of intracerebral hemorrhage. Curr Atheroscler Rep. 2012;14:300–6.
Kelly ML, Sulmasy DP, Weil RJ. Spontaneous intracerebral hemorrhage and the challenge of surgical decision making: a review. Neurosurg Focus. 2013;34:E1.
Rincon F, Mayer SA. Clinical review: Critical care management of spontaneous intracerebral hemorrhage. Crit Care. 2008;12:237.
Cho DY, Chen CC, Chang CS, Lee WY, Tso M. Endoscopic surgery for spontaneous basal ganglia hemorrhage: comparing endoscopic surgery, stereotactic aspiration, and craniotomy in noncomatose patients. Surg Neurol. 2006;65:547–55 discussion 555-546.
Zhang HZ, Li YP, Yan ZC, Wang XD, She L, Wang XD, et al. Endoscopic evacuation of basal ganglia hemorrhage via keyhole approach using an adjustable cannula in comparison with craniotomy. Biomed Res Int. 2014;2014:898762.
Feng Y, He JQ, Liu B, Yang LK, Wang YH. Endoscope-assisted keyhole technique for hypertensive cerebral hemorrhage in elderly patients: a randomized controlled study in 184 patients. Turk Neurosurg. 2016;26:84–9.
Zhang JX, Lu SY, Wang SZ, Zhou NY, Li GL. Comparison and analysis of the efficacy and safety of minimally invasive surgery and craniotomy in the treatment of hypertensive intracerebral hemorrhage. Pak J Med Sci. 2018;34:578–82.
Nakano T, Ohkuma H, Ebina K, Suzuki S. Neuroendoscopic surgery for intracerebral haemorrhage--comparison with traditional therapies. Minim Invasive Neurosurg. 2003;46:278–83.
Qiu Y, Lin Y, Tian X, Luo Q. Hypertensive intracranial hematomas: endoscopic-assisted keyhole evacuation and application of patent viewing dissector. Chin Med J (Engl). 2003;116:195–9.
Zhu H, Wang Z, Shi W. Keyhole endoscopic hematoma evacuation in patients. Turk Neurosurg. 2012;22:294–9.
Chi FL, Lang TC, Sun SJ, Tang XJ, Xu SY, Zheng HB, et al. Relationship between different surgical methods, hemorrhage position, hemorrhage volume, surgical timing, and treatment outcome of hypertensive intracerebral hemorrhage. World J Emerg Med. 2014;5:203–8.
Wang WH, Hung YC, Hsu SP, Lin CF, Chen HH, Shih YH, et al. Endoscopic hematoma evacuation in patients with spontaneous supratentorial intracerebral hemorrhage. J Chin Med Assoc. 2015;78:101–7.
Yamashiro S, Hitoshi Y, Yoshida A, Kuratsu J. Effectiveness of endoscopic surgery for comatose patients with large supratentorial intracerebral hemorrhages. Neurol Med Chir (Tokyo). 2015;55:819–23.
Cai Q, Zhang H, Zhao D, Yang Z, Hu K, Wang L, et al. Analysis of three surgical treatments for spontaneous supratentorial intracerebral hemorrhage. Medicine (Baltimore). 2017;96:e8435.
Li Y, Yang R, Li Z, Yang Y, Tian B, Zhang X, et al. Surgical evacuation of spontaneous supratentorial lobar intracerebral hemorrhage: comparison of safety and efficacy of stereotactic aspiration, endoscopic surgery, and craniotomy. World Neurosurg. 2017;105:332–40.
Xu X, Chen X, Li F, Zheng X, Wang Q, Sun G, et al. Effectiveness of endoscopic surgery for supratentorial hypertensive intracerebral hemorrhage: a comparison with craniotomy. J Neurosurg. 2018;128:553–9.
Eroglu U, Kahilogullari G, Dogan I, Yakar F, Al-Beyati ESM, Ozgural O, et al. Surgical management of supratentorial intracerebral hemorrhages: endoscopic versus open surgery. World Neurosurg. 2018;114:e60–5.
Yamamoto T, Nakao Y, Mori K, Maeda M. Endoscopic hematoma evacuation for hypertensive cerebellar hemorrhage. Minim Invas Neurosur. 2006;49:173–8.
Nagasaka T, Tsugeno M, Ikeda H, Okamoto T, Inao S, Wakabayashi T. Early recovery and better evacuation rate in neuroendoscopic surgery for spontaneous intracerebral hemorrhage using a multifunctional cannula: preliminary study in comparison with craniotomy. J Stroke Cerebrovasc. 2011;20:208–13.
Wang ZF, Liu F, Liao DG, Zhang TY. Endoscopic surgery for hypertensive cerebral hemorrhage. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2005;30:424–6.
Ye Z, Ai X, Hu X, Fang F, You C. Comparison of neuroendoscopic surgery and craniotomy for supratentorial hypertensive intracerebral hemorrhage: a meta-analysis. Medicine (Baltimore). 2017;96:e7876.
Babi MA, James ML. Spontaneous intracerebral hemorrhage: should we operate? Front Neurol. 2017;8:645.
Broderick J, Connolly S, Feldmann E, Hanley D, Kase C, Krieger D, et al. Guidelines for the management of spontaneous intracerebral Hemorrhage in adults - 2007 update - A guideline from the American Heart Association/American Stroke Association Stroke Council, high blood pressure research council, and the quality of care and outcomes in research interdisciplinary working group - The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:2001–23.
Chen CJ, Ding D, Ironside N, et al. Intracranial pressure monitoring in patients with spontaneous intracerebral hemorrhage. J Neurosurg. 2019:1–11.
Garibi J, Bilbao G, Pomposo I, Hostalot C. Prognostic factors in a series of 185 consecutive spontaneous supratentorial intracerebral haematomas. Brit J Neurosurg. 2002;16:355–61.
Zhao YN, Chen XL. Endoscopic treatment of hypertensive intracerebral hemorrhage: a technical review. Chronic Dis Transl Med. 2016;2:140–6.
Keep RF, Hua Y, Xi GH. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurology. 2012;11:720–31.
Angileri FF, Esposito F, Priola SM, Raffa G, Marino D, Abbritti RV, et al. Fully Endoscopic Freehand Evacuation of Spontaneous Supratentorial Intraparenchymal Hemorrhage. World Neurosurgery. 2016;94:268–72.
Dye JA, Dusick JR, Lee DJ, Gonzalez NR, Martin NA. Frontal bur hole through an eyebrow incision for image-guided endoscopic evacuation of spontaneous intracerebral hemorrhage. J Neurosurg. 2012;117:767–73.
Russell MW, Boulanger L, Joshi AV, Neumann PJ, Menzin J. The economic burden of intracerebral hemorrhage: evidence from managed care. Manag Care Interface. 2006;19(24-28):34.
Sun SW,Li YP,Zhang HZ,et al. Neuroendoscopic surgery versus craniotomy for supratentorial hypertensive intracerebral hemorrhage: a systematic review and meta-analysis [J]. World Neurosurg, 2020;134(10): 477-488.
Shah M, Birnbaum L, Rasmussen J, et al. Effect of hyperosmolar therapy on outcome following spontaneous intracerebralhemorrhage: Ethnic/racial variations of intracerebral hemorrhage(erich) study. J Stroke Cerebrovasc Dis. 2018;27(4):1061–7.
Cook AM, Morgan Jones G, Hawryluk GWJ, et al. Guidelines for the acute treatment of cerebral edema in neurocritical care patients [J]. Neurocrit Care. 2020;32(2).
Ding D, Przybylowski CJ, Starke RM, Street RS, Tyree AE, Crowley RW, et al. A minimally invasive anterior skull base approach for evacuation of a basal ganglia hemorrhage. J Clin Neurosci. 2015;22:1816–9.
Lin HL, Lo YC, Liu YF, Cho DY. Endoscopic evacuation of hypertensive putaminal hemorrhage guided by the 3D reconstructed CT scan: A preliminary report. Clin Neurol Neurosur. 2010;112:892–6.
Yang Z, Hong B, Jia Z, Chen J, Ge J, Han J, et al. Treatment of supratentorial spontaneous intracerebral hemorrhage using image-guided minimally invasive surgery: initial experiences of a flat detector CT-based puncture planning and navigation system in the angiographic suite. Am J Neuroradiol. 2014;35:2170–5.
Chartrain AG, Kellner CP, Fargen KM, Spiotta AM, Chesler DA, Fiorella D, et al. A review and comparison of three neuronavigation systems for minimally invasive intracerebral hemorrhage evacuation. J Neurointerv Surg. 2018;10:66–73.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Conception or design of the work: Xiaolin Du and Xinhua Tian. Data collection: Xiaolin Du, Cheng Wang, and Yigong Wei. Data analysis and interpretation: Xiaolin Du and Xiaoning Lin. Drafting the article: Xiaolin Du and Cheng Wang. Critical revision of the article: all authors. Other (study supervision, fundings, materials, etc.): Xinhua Tian and Kun Zhou. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Du, X., Lin, X., Wang, C. et al. Endoscopic surgery versus craniotomy in the treatment of spontaneous intracerebral hematoma: a systematic review and meta-analysis. Chin Neurosurg Jl 8, 36 (2022). https://doi.org/10.1186/s41016-022-00304-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41016-022-00304-1