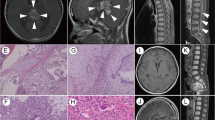
Abstract
Background
Central nervous system germ cell tumors (CNS GCTs) represent a class of rare tumors that exhibit region-specific prevalence in some Asian areas (15.3%), higher than that in North America (3.6%), and age-specific prevalence in children and adolescents. According to the 2016 World Health Organization (WHO) classification, CNS GCTs can be categorized into germinomas and non-germinomatous GCTs (NGGCTs). Owing to the compression of the interventricular foramen by enlarged GCTs in the pineal gland, the resultant obstructive hydrocephalus may result in high intracranial pressure (HIP) at an alarming pace, which urgently requires a ventriculoperitoneal shunt for the relief of severe HIP. Although CNS GCT cells tend to migrate through the cerebrospinal fluid (CSF) starting from the subependymal lining, metastasis along the ventriculoperitoneal shunt tube is extremely rare.
Case presentation
In this study, we reported two cases of iGCTs with intraperitoneal metastasis. Both patients underwent ventriculoperitoneal shunt placement to alleviate HIP, and both received standard radiotherapy and chemotherapy, but they still developed abdominal metastasis, and all the abdominal masses were pathologically confirmed to be iGCTs.
Conclusions
We performed a literature study and found that from 1979 to 2020, a total of 18 cases of iGCTs were metastasized outside the nervous system. We also found a shift of the median of 13.5 months and that the most common primary site was the pineal region (83.3%); moreover, nearly half of the patients (44%) died within 1 year of metastasis, indicating a poor prognosis after celiac metastasis.
Similar content being viewed by others
Background
Central nervous system germ cell tumors (CNS GCTs) represent a class of rare tumors that exhibit region-specific prevalence in some Asian areas (15.3%), higher than that in North America (3.6%), and age-specific prevalence in children and adolescents [1]. According to the 2016 World Health Organization (WHO) classification, CNS GCTs can be categorized into germinomas and non-germinomatous GCTs (NGGCTs) [2]. Owing to the compression of the interventricular foramen by enlarged GCTs in the pineal gland, the resultant obstructive hydrocephalus may result in high intracranial pressure (HIP) at an alarming pace, which urgently requires a ventriculoperitoneal shunt for the relief of severe HIP. Although CNS GCT cells tend to migrate through the cerebrospinal fluid (CSF) starting from the subependymal lining, metastasis along the ventriculoperitoneal shunt tube is extremely rare [3].
Case presentation
Case 1
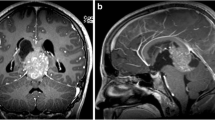
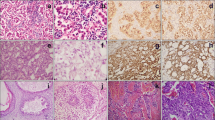
A 13-year-old Asian male presented with unsteady gait for 4 months and complained of headache, nausea, a drooping left eyelid, blurred vision for 11 days, and convulsions for 3 days. The magnetic resonance imaging (MRI) scan (Fig. 1) revealed a mass occupying the pineal gland and HIP. Alpha-fetoprotein (AFP) and chorionic gonadotropin β (β-HCG) levels in the serum were 188.2 ng/ml (0–7 ng/ml) and 716 mIU/ml (0–2.6 IU/ml), respectively, while AFP and β-HCG levels in the CSF were 5.9 ng/ml (0–7 ng/ml) and 542.9 mIU/ml (0–2.6 IU/ml). The child received a ventriculoperitoneal (VP) shunt for the relief of HIP. In accordance with the SIOP CNS GCT 96, all patients with α-AFP > 25 ng/ml or β-hCG > 50 mIU/ml levels in the serum or CSF were diagnosed with non-germinomatous germ cell tumors (NGGCTs) [3, 4]. After establishing the diagnosis, the patient initially received two cycles of platinum-based chemotherapy followed by tumor resection and completed another two courses of chemotherapy using the same regimen. Although the postoperative MRI examination showed that the tumor was completely resected and there was no metastasis, 3 months later, the patient developed spinal cord dissemination and metastasis. Craniospinal irradiation (CSI) was given after chemotherapy at a dose of 30.6 Gy/17 f intensity-modulated radiotherapy (IMRT) and a boost of 23.4 Gy/13 f IMRT. Unexpectedly, the boy reported cough alongside pain in the right costal margin and the right clavicle 8 months after placement of the VP shunt, accompanied by elevated serum AFP and β-HCG levels of 873.3 ng/ml and 742.1 mIU/ml, respectively. Whole-body 18-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) showed multiple intra-abdominal foci of increased metabolic activity measured by a maximum standardized uptake value (SUVmax) of 9.8–23.1 (Fig. 4a). The patient subsequently underwent abdominal mass resection, and rapid freezing pathology during the operation proved the following immunohistochemical results: CK (+), AFP (+), HCG-β (+), Ki-67 (+), Syn (+), SMA (+), and CD30 (+). Combining the immunohistochemical results with the judgment under light microscopy, the pathologist determined that the abdominal mass in the patient was a metastasis of iGCTs (Fig. S2). Based on the combined imaging findings and laboratory results, as well as the patient having no other history except for a brain tumor, extensive intraperitoneal metastasis (EIM) of CNS GCTs was considered.
Case 2
A 17-year-old Asian male presented with a long-standing history of generalized headache, diabetes insipidus, and intermittent fever without obvious inducement. An MRI examination (Fig. 2) revealed hydrocephalus due to space-occupying lesions, so the patient received a VP shunt to reduce HIP. Moreover, serum AFP tests were negative, but the β-HCG level in the cerebrospinal fluid reached 175.4 mIU/ml. Based on the imaging features and his symptoms, we speculated that this young man had iGCTs with intraventricular dissemination. Therefore, he received two cycles of a platinum-based regimen. Tests of the two blood tumor markers were negative, and MRI revealed 95% disappearance of the tumor after chemotherapy. Then, the patient completed irradiation of the whole central nervous system at a dose of 40 Gy/25 f. After 32 months of chemoradiation therapy, he reported intermittent abdominal pain without obvious inducement. Therefore, the abdomen of the young man was examined, and a mass in the right upper abdomen was discovered. Tests of the blood tumor markers were still negative, and MRI of the central nervous system showed no evidence of tumor recurrence (Fig. S1). However, rough-edged masses adjacent to the liver area were detected on abdominal MRI (Fig. 3). The patient then underwent a PET-CT examination, which showed multiple-site SUV elevation (12.1 to 13.6) in the abdominal cavity, so abdominal metastasis of GCTs was considered (Fig. 4 b1, b2). Therefore, urgent tumor resection was performed, and the returned pathological findings were consistent with metastatic iGCTs. IHC analysis of the abdominal lesion showed the following: AE1/AE3 (+), CD117 (+), Ki67 (70%), OCT3/4 (+), and sall-4 (+) (Fig. 5). After four courses of platinum-based chemotherapy, the patient was discharged and upgraded to a fair condition.
There were abnormal metabolic nodules near the inferior vena cava and the diaphragmatic apex, small nodules with slightly increased metabolism at the anterior right costal diaphragmatic angle, and extensive nodules with increased metabolism in the peritoneum (a) (marked in red and black arrows). Multiple-site SUV elevation in the abdominal cavity. The two main metabolic enhancement foci are concentrated in the right upper quadrant (b1, b2)
Discussion and conclusions
While iGCTs in the pineal gland are more prevalent in the pediatric population, they are still relatively rare, with a low incidence rate of less than 1% of all intracranial tumors [5]. Nevertheless, because postoperative metastasis appeared successively in two recently hospitalized patients, we conducted a literature search in PubMed to confirm whether a VP shunt can lead to tumor metastasis (Table 1). As the histological features of intracranial GCTs are comparable to those of gonadal seminoma, we quickly focused on a retrospective study of 1011 cases of germinoma conducted by Haimovic et al. [6]. All the patients in his study were children who were diagnosed with primary CNS tumors and whose longest survival time reached 32 years. Only 10 patients developed EIM, 2 of whom were diagnosed with GCTs, 6 with neural tube cell tumors, 1 with ependymoma, and 1 with an atypical rod-shaped tumor. Another study by Belongia and Jogal [7, 8] reported that only 0.98% of patients with primary intracranial tumors developed EIM. In addition, Kun et al. [9] recently reported 2 cases of pineal tumors. Consistent with the previous cases [5], these authors demonstrated the risk of metastasis caused by endoscopic biopsy. Although endoscopic biopsy was not performed in our patients, similar procedures of inserting a tube and collecting excess CSF during placement of a VP shunt were alarming. The possibility of peritoneal implant metastasis by VP shunts in these cases, though uncertain, cannot be denied [10].
Although abdominal metastasis is a low-probability event, the two patients described above were treated with total central radiotherapy, which poses a challenge for subsequent treatment after abdominal metastasis. We believe that chemotherapy alone is far from sufficient for germ cell tumors. Radiotherapy is an important way to improve prognosis under the premise of inoperability. However, since patients undergo whole-CNS radiotherapy, the spinal cord and bilateral kidneys are irradiated with different doses; therefore, whether there is an opportunity for whole-abdominal radiotherapy needs to be assessed according to the first radiotherapy plan. Given the pathogenetic process, history, and clinical and imaging manifestations, which are entirely in line with the primary diagnosis and standard treatment, their diagnoses were highly likely to be GCTs. Patients receiving the standard regimen (a VP shunt followed by chemoradiotherapy) rarely develop EIM after treatment, but the two patients developed extensive peritoneal metastasis 8 and 32 months after placement of VP shunts. Therefore, we aimed to determine whether tumor cells can be metastasized through a shunt. Unfortunately, cytological analysis of the CSF specimen of the first case was not performed after surgery, but the pathological report of the second case revealed metastatic GCTs. Through previous studies reported in the literature, we found that although the placement of a VP shunt is a classic surgical procedure for the emergency relief of hydrocephalus, it still has a risk of metastasis. Therefore, for tumors with a high risk of dissipating, the third ventricle fistula may be a better choice, which not only relieves hydrocephalus but also avoids the maintenance of the shunt tube and the risk of abdominal dissemination in the later stage. Moreover, if conditions permit, neurosurgeons have an opportunity to perform a biopsy to obtain a clear pathology.
Intracranial GCTs are rare and rarely progress to distant metastases; however, peritoneal implant metastasis occurred in two cases 8 and 32 months after placement of VP shunts. As Devkota et al. [5] stated that GCTs could metastasize along the endoscopic biopsy pathway, we believe that peritoneal metastases are likely to be disseminated from the shunt pathway.
Regarding the metastatic pathway of the two cases reported herein, we believe that GCTs are likely to metastasize through the CSF and have a high possibility of developing distant metastasis along the VP shunt pathway.
Availability of data and materials
Not applicable.
Abbreviations
- CNS GCTs:
-
Central nervous system germ cell tumors
- WHO:
-
World Health Organization
- NGGCTs:
-
Non-germinal GCTs
- HIP:
-
High intracranial pressure
- CSF:
-
Cerebrospinal fluid
- AFP:
-
Alpha-fetoprotein
- β-HCG:
-
Chorionic gonadotropin β
- VP:
-
Ventriculoperitoneal
- IHC:
-
Immunohistochemical
- 18F-FDG PET/CT:
-
18-Fluorodeoxyglucose positron emission tomography/computed tomography
- EIM:
-
Extensive intraperitoneal metastasis
References
Altundag OO, Celik I, Kars A. Pineal germ cell tumor metastasis via ventriculoperitoneal shunt. Am J Clin Oncol. 2002;25(1):104–5. https://doi.org/10.1097/00000421-200202000-00025.
Back MR, Hu B, Rutgers J, French S, Moore TC. Metastasis of an intracranial germinoma through a ventriculoperitoneal shunt: recurrence as a yolk-sac tumor. Pediatr Surg Int. 1997;12(1):24–7. https://doi.org/10.1007/BF01194796.
Calaminus G, Kortmann R, Worch J, Nicholson JC, Alapetite C, Garre ML, et al. SIOP CNS GCT 96: final report of outcome of a prospective, multinational nonrandomized trial for children and adults with intracranial germinoma, comparing craniospinal irradiation alone with chemotherapy followed by focal primary site irradiation for patients with localized disease. Neuro-Oncology. 2013;15(6):788–96. https://doi.org/10.1093/neuonc/not019.
Murray MJ, Bartels U, Nishikawa R, Fangusaro J, Matsutani M, Nicholson JC. Consensus on the management of intracranial germ-cell tumours. Lancet Oncol. 2015;16(9):e470–7. https://doi.org/10.1016/S1470-2045(15)00244-2.
Devkota J, Brooks BS, el Gammal T. Ventriculoperitoneal shunt metastasis of a pineal germinoma. Comput Radiol. 1984;8(3):141–5. https://doi.org/10.1016/0730-4862(84)90051-9.
Haimovic IC, Sharer L, Hyman RA, Beresford HR. Metastasis of intracranial germinoma through a ventriculoperitoneal shunt. Cancer. 1981;48(4):1033–6. https://doi.org/10.1002/1097-0142(19810815)48:4<1033::aid-cncr2820480430>3.0.co;2-r.
Belongia M, Jogal S. Extraneural metastasis of a nongerminomatous germ cell tumor of the central nervous system in a pediatric patient with a ventriculoperitoneal shunt: a case report and review of the literature. J Pediatr Hematol Oncol. 2012;34(1):e12–6. https://doi.org/10.1097/MPH.0b013e31823dd370.
Kim K, Koo BC, Delaflor RR, Shaikh BS. Pineal germinoma with widespread extracranial metastases. Diagn Cytopathol. 1985;1(2):118–22. https://doi.org/10.1002/dc.2840010207.
Kun LE, Tang TT, Sty JR, Camitta BM. Primary cerebral germinoma and ventriculoperitoneal shunt metastasis. Cancer. 1981;48(1):213–6. https://doi.org/10.1002/1097-0142(19810701)48:1<213::aid-cncr2820480134>3.0.co;2-5.
Lesoin F, Cama A, Dhellemmes P, Nuyts JP, Andreussi L, Jomin M, et al. Extraneural metastasis of a pineal tumor. Report of 3 cases and review of the literature. Eur Neurol. 1987;27(1):55–61. https://doi.org/10.1159/000116130.
Murray MJ, Metayer LE, Mallucci CL, Hale JP, Nicholson JC, Kirollos RW, et al. Intra-abdominal metastasis of an intracranial germinoma via ventriculo-peritoneal shunt in a 13-year-old female. Br J Neurosurg. 2011;25(6):747–9. https://doi.org/10.3109/02688697.2011.566383.
Ung AO, Triscott JA, Leditschke JF, Smith JA. Metastasis of pineal germinoma via ventriculoperitoneal shunt. Aust N Z J Surg. 1993;63(5):409–12. https://doi.org/10.1111/j.1445-2197.1993.tb00412.x.
Pallini R, Bozzini V, Scerrati M, Zuppi C, Zappacosta B, Rossi GF. Bone metastasis associated with shunt-related peritoneal deposits from a pineal germinoma. Case report and review of the literature. Acta Neurochir. 1991;109(1-2):78–83. https://doi.org/10.1007/bf01405704.
Triolo PJ, Schulz EE. Metastatic germinoma (pinealoma) via a ventriculoperitoneal shunt. AJR Am J Roentgenol. 1980;135(4):854–5. https://doi.org/10.2214/ajr.135.4.854.
Wood BP, Haller JO, Berdon WE, Lin SR. Shunt metastases of pineal tumors presenting as a pelvic mass. Pediatr Radiol. 1979;8(2):108–9. https://doi.org/10.1007/bf00974001.
Acknowledgements
The authors thank the two patients for their contributions.
Funding
This work was supported by the Beijing Municipal Bureau of Health (to Xiaoguang Qiu, grant number: N/A).
Author information
Authors and Affiliations
Contributions
PW prepared the original draft. YNL analyzed the patients’ information. XGQ supervised the entire research process. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures described in this study were conducted according to the Declaration of Helsinki and approved by the Ethics Committee of Beijing Tiantan Hospital. All participants provided written informed consent before entry into the study.
Consent for publication
Written informed consent for publication of their clinical details and clinical images was obtained from the guardian of the patient. A copy of the consent form is available for review by the editor of this journal.
Competing interests
The authors declare that they have no competing interests.
Supplementary Information
Additional file 1: Fig. S1.
MRI of the central nervous system showed no evidence of tumor recurrence in the second patient.
Additional file 2: Fig. S2.
Intraoperative resection of the lesion confirmed the diagnosis of mixed germ cell tumor.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, P., Li, Y. & Qiu, X. The external metastasis of the central nerve system germ cell tumors: case report and review of the literature. Chin Neurosurg Jl 7, 29 (2021). https://doi.org/10.1186/s41016-021-00246-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41016-021-00246-0