Abstract
Many biologic products have improved the outcomes of cancer patients, but the costs can substantially burden healthcare systems. Biosimilar products can potentially reduce drug costs and increase patient access to beneficial treatments. Approval of a biosimilar product relies on the demonstration of “comparability” or “no clinically meaningful differences” as compared to its reference biologic product. Biosimilar products for erythropoietin, granulocyte colony-stimulating factor, trastuzumab, and rituximab are already available, and the regulatory processes in various countries are constantly evolving. It is important that oncologists be familiar with the potential issues surrounding the clinical use of biosimilar products. In this review article, we provide background information about biosimilar products and their regulatory approval processes, followed by a discussion of individual biosimilar drugs.
Similar content being viewed by others
Background
Biologic therapies, such as the monoclonal antibodies (mAbs) trastuzumab and bevacizumab, have prolonged the survival of cancer patients, but their high costs have also resulted in substantial financial burdens being placed on individual patients and on healthcare systems as a whole [1]. Because the patents of many biologic products in oncology are set to expire (Table 1), many biosimilar products are being developed and will soon become available in global pharmaceutical markets. The lower costs of these biosimilar drugs compared with their reference biologics can help reduce cancer drug costs and potentially allow more patients to gain access to the drugs, thus improving patient outcomes.
“Biosimilar”, as the name implies, is a biologic that is similar to the licensed “reference” drug. Before a biosimilar is incorporated into the clinical management of patients, clinicians should determine whether, based on efficacy and safety, the biosimilar can be used “instead of” or “interchangeably with” the reference biologic. Regulatory authorities play an important role in the development of biosimilars, and medical societies help guide clinicians on the use of biosimilars [2–5]. Currently available and of most interest in the field of oncology are biosimilars of the supportive agents filgrastim and erythropoietin and the mAbs trastuzumab and rituximab. Bevacizumab and cetuximab are innovator drugs in oncology whose patents will expire in the next few years [6]. Recent surveys conducted in America, Europe, and Asia showed that most clinicians are not very familiar with biosimilars [5, 7, 8]. In this review, we provide information that oncologists need to know about this new category of medicine.
What is a biosimilar?
“Biosimilar” [3, 4], “similar biotherapeutic product” [2], “follow-on biologic” [9], and “subsequent entry biologic” [10] are terms for a successor drug that has the same mechanism of action as the original biologic. The World Health Organization (WHO) defines biosimilar as “a biotherapeutic product which is similar in terms of quality, safety, and efficacy to an already licensed reference biotherapeutic product” [2]. Unlike simple generic medicines that are chemically synthesized, biologics are produced by a more complex manufacturing process, are derived from living cells or organisms, and consist of relatively large and complex molecules [11]. Any variations in the manufacturing condition can result in alterations in biological function, causing changes in efficacy; and/or induce an immune response (immunogenicity), leading to a new adverse reaction. There is certain degree of variability in the manufacturing process of biologics that exists even between different batches of the same product. The expiration of a biologic’s patent unveils only the primary amino sequence and the structure of the drug, not precise production information. The attempted replica can be considered only highly similar to the original biologic, not truly “generic.” Therefore, the criteria for obtaining regulatory approval of chemically derived generic drugs are inappropriate for biosimilars. Table 2 summarizes the differences between biosimilars and generic drugs.
Biosimilar regulatory approval
The European Medicines Agency (EMA) is the body responsible for approving biosimilars in the European Union (EU), and it established the first legislative pathway for doing so. In 2005, it published guidelines governing the development of biosimilars; since then, it has developed individual guidelines for the biosimilars of granulocyte colony-stimulating factor (G-CSF), erythropoietin, and various mAbs [3]. The EMA defines biosimilars as “a biological medicinal product that contains a version of the active substance of an already authorized reference medicinal product and similarity to the reference product in terms of quality characteristics, biological activity, safety and efficacy based on a comprehensive comparability exercise needs to be established” [3]. The concept of “comparability” in reference to the original biologic is considered fundamental to the approval of a biosimilar. Comprehensive comparability studies should demonstrate similarity in physiochemical, biological, and immunological characteristics and in efficacy and safety. Immunogenicity has been a safety concern for biosimilars; thus, a robust pharmacovigilance system and risk management procedures should be in place to ensure long-term safety.
In the EU, the first biosimilar product was approved in 2006 [12]. Many countries soon followed the EMA’s lead. Since 2008, Australia has followed the EU guidelines and approved its first biosimilar in 2010 [13]. In 2013, the Australia’s Therapeutic Goods Administration released a guidance document on the evaluation of biosimilars [14]. Canada also follows the EU regulatory process and finalized its guidelines in 2010 [10]. In 2009, the WHO formalized guidelines on the evaluation of similar biotherapeutic products [2]. The United States of America (USA) has lagged behind in the development of biosimilars, having approved its first biosimilar only in March 2015 [9]. The United States Food and Drug Administration (US FDA) issued final guidelines in April 2015 [4]. It defines biosimilar as “a biological product that is highly similar to the reference product notwithstanding minor differences in clinically inactive components and that there are no clinically meaningful differences between the biological product and the reference product in terms of safety, purity and potency.” Emerging drug markets of Asia are typically generics driven; they offer an attractive market for the developers of biosimilars, but regulations vary across countries, and not every country has issued guidelines [15–21] (Table 3). Some Asian countries follow a specific biosimilar pathway that is akin to the EMA model [16–19, 22]. Singapore has approved biosimilar products only if they have been approved by other agencies namely, the EMA, the US FDA, the Australian Therapeutic Goods Administration, or the Health Canada [19]. In India, less stringent regulatory requirements have led to many biosimilars being available in the market since 2000; however, not until 2012 were guidelines issued [21]. In China, specific guidelines to be developed by the China Food and Drug Administration are pending; currently, the approval of biosimilars follows a simplified new product approval pathway [20]. Worldwide, regulations are evolving, and global harmonization of regulatory standards would definitely help manufacturers develop biosimilars in a more cost-effective way [23].
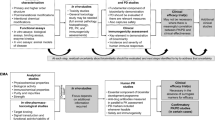
Overall, the USA and the EU have similar basic principles in terms of quality and clinical and non-clinical comparability testing strategies [3, 4]. A single reference product as comparator should be used throughout the studies to enable the generation of coherent data and conclusions. The dosage and the form and route of administration of the biosimilar and the reference biologic should be the same. The totality-of-the-evidence approach is adopted to review the marketing authorization application of biosimilar products, and a stepwise approach for demonstrating biosimilarity is recommended (Fig. 1). At each step, the biosimilar developer should evaluate and justify any identified uncertainties or differences between the biosimilar and the reference biologic and maintain an active dialogue with the approval agency, all of which affect the type and amount of data required to move on to the next step. Demonstrating high molecular similarity between the biosimilar and the reference biologic at the beginning reduces the amount of preclinical and clinical data that are subsequently required. If the reference biologic is licensed for more than one indication, similarity of the biosimilar should be demonstrated separately for each indication. Extrapolation of efficacy and safety data from one therapeutic indication to another is allowed, provided that there is evidence that the mechanisms of action and safety in the subpopulations evaluated are very likely to be equivalent. Currently, the EMA and the US FDA are collaborating to facilitate the global development of biosimilars. Regular meetings are being held between the two agencies to exchange information on the development of biosimilar products and on pharmacovigilance.
Pharmacovigilance of biosimilars
Pharmacovigilance is “the science and activities relating to the detection, assessment, understand and prevention of adverse effects or any other drug related problem” [24]. Pharmacovigilance is particularly important when dealing with biologics because safety data from pre-authorization clinical studies only identify some potential risks and are insufficient to detect rare adverse events. Immunogenicity can be related to the route of administration, dosing regimen, patient-related factors, and disease-related factors, and it is an ongoing safety concern [3]. The case of erythropoietin antibody-mediated pure red cell aplasia (PRCA) is a good example of immunogenicity being identified by post-marketing surveillance, when a small change in the formulation of the biologic led to an adverse immune response [25]. In addition to immunogenicity, other safety concerns, such as the administration of hematopoietic colony-stimulating factors to healthy donors, which led to the development of hematologic malignancies, have been observed with biologics [26]. To ensure long-term safety, biosimilars are required to follow the same pharmacovigilance regulations as their reference counterparts. Required as part of the marketing application are a risk management plan describing the safety profile of the drug as well as proposed pharmacovigilance and risk minimization measures. After biosimilars are approved, companies are required to submit periodic safety reports. Prescribers should report any suspected serious adverse reactions associated with the use of biosimilars, and ensuring traceability of the biosimilars associated with adverse events is essential. To date, no specific safety concerns regarding approved and marketed biosimilars have been identified.
Interchangeability between a biologic and its biosimilar
Generic medications can be used interchangeably with their branded originators since they are considered therapeutically equivalent; often, pharmacists may substitute a prescribed drug for a generic medication without the prior consent of the treating physician (known as “automatic substitution”) [23]. Interchangeability refers to switching back and forth between two medicinal products without any observed changes in efficacy or safety risk. Biosimilarity does not imply interchangeability, and interchangeability does not always imply substitutability.
Some biosimilars might not have all the same indications that the reference biologics are approved for, especially if the reference biologics have multiple mechanisms of action. Even if a biosimilar is approved for the same indication as the reference originator, it is generally recommended that automatic substitution not be allowed because the clinician should be fully aware of which drug is given to the patient. If, during a treatment period, alternation or switching between the biosimilar and the branded biologic cannot be avoided, this must be recorded accurately; maintaining pharmacovigilance by clearly delineating between biosimilars and branded originators is important.
The USA allows an “interchangeable” designation for biosimilars, provided that, in any given patient, the biosimilar can be expected to produce the same clinical results as the reference product and that the safety and efficacy observed when alternating or switching between the two remain the same [4]. Other countries, such as Australia and Canada, do not provide recommendations on whether a biosimilar can be used interchangeably with its reference medicine, and they have officially prohibited the automatic substitution of biologics [10, 14]. The EMA also does not provide an interchangeable recommendation when approving a biosimilar [3]. Individual EU countries must decide on the interchangeability between a biologic and its biosimilar [27].
Biosimilar epoetins
Erythropoiesis-stimulating agents (ESAs), such as epoetin alfa (Eprex, Erypro), epoetin beta (NeoRecormon), and darbepoetin alfa (Aranesp), are approved for cancer patients with chemotherapy-induced anemia [28, 29]. They are considered equivalent in terms of efficacy and safety. In 2010, concerns about an increased risk of venous thromboembolism and an increased mortality risk associated with the use of ESAs by cancer patients led the European Society for Medical Oncology and the American Society of Clinical Oncology/American Society of Hematology to issue revised guidelines [29]. These guidelines recommended against the use of ESAs for the treatment of malignancy-associated anemia in patients not receiving concurrent myelosuppressive chemotherapy; they also recommended against the use of ESAs for patients receiving chemotherapy for curative intent, until further safety data are collected. However, the EMA’s Committee for Medicinal Products for Human Use stated that the benefits of using ESAs for approved indications (hemoglobin target range of 10–12 g/dL in chemotherapy-induced anemia) continue to outweigh the associated risks, including increased risk of tumor progression and venous thromboembolism and reduced survival, except for those cancer patients with a reasonably long life expectancy whose anemia should be treated by blood transfusions [30].
In 2007, two biosimilar epoetins–epoetin alfa, marketed as Binocrit, Abseamed, and Epoetin Alfa Hexal; and epoetin zeta, marketed as Retacrit and Silapo were approved in Europe [31–35] (Table 4). Comparability of these biosimilar epoetins with their reference drugs Eprex and Erypo was demonstrated in the setting of renal anemia [36]. Their use in the treatment of chemotherapy-induced anemia in cancer patients was approved because, based on the extrapolation of data, the mechanisms of action for epoetins are the same for all approved indications. PRCA caused by cross-reacting neutralizing antibodies against erythropoietin is a rare but known serious adverse event that has been observed with the use of erythropoietin in patients with chronic renal failure [37]. Therefore, the post-marketing and risk management plans of biosimilar epoetins particularly focus on PRCA.
Biosimilar G-CSF
G-CSF is widely used in the field of oncology [38]. It is used to decrease the incidence of chemotherapy-related febrile neutropenia, facilitate dose-intensity chemotherapy, and mobilize hematopoietic stem cells for collection. Currently, there are eight EMA-licensed filgrastim biosimilars, the first of which was licensed in Europe in 2008 [39–46]. These G-CSF biosimilars were approved by the EMA for all the registered indications of the reference drug Neupogen, based on their comparable efficacy and safety profiles in the treatment of chemotherapy-induced neutropenia [47–51] (Table 5). In March 2015, the US FDA approved its first biosimilar product Zarxio (filgrastim-sndz), which has all the same indications as the reference drug Neupogen [52]. Key studies showing the comparability in efficacy and safety of the biosimilar filgrastim to its reference drug are summarized in Table 6.
Enhanced by a 20-kDa polyethylene glycol molecule compared with filgrastim, pegfilgrastim has a longer half-life and is administered less frequently [53]. Pegfilgrastim has been approved by the US FDA and the EMA since 2002 and is indicated in non-myeloid cancer patients undergoing chemotherapy to decrease the incidence of febrile neutropenia [54, 55]. Unlike filgrastim, pegfilgrastim is not approved for hematopoietic progenitor cell mobilization. Since 2007, four pegfilgrastim biosimilars have been approved in India [56]. In December 2014, Apotex, in conjunction with Intas Pharmaceuticals, successfully filed its biosimilar pegylated apofilgrastim with the US FDA. Another biosimilar pegfilgrastim, LA-EP2006, developed by Sandoz, is being compared with its reference product Neulasta in three phase III studies; the results will be used to support the company’s registration application in the USA and the EU. The US patent for pegfilgrastim expired in October 2015; the EU patent is expected to expire in August 2017.
Biosimilar trastuzumab
Trastuzumab is a humanized mAb that targets human epidermal growth factor receptor 2 (HER2). For patients with HER2-positive breast cancer, it is the standard of care in the neoadjuvant, adjuvant, and palliative settings [57, 58]. It is also indicated for the palliative treatment of HER2-positive gastric cancer. Biosimilar trastuzumab is the first biosimilar mAb available for the treatment of cancer (Table 7). In November 2013, the trastuzumab biosimilar Hertraz was launched in India after approval was granted by the Drug Controller General of India [59]. In January 2014, the South Korean Ministry of Food and Drug Safety approved the trastuzumab biosimilar CT-P6 (Herzuma) based on the results of global clinical trials involving 558 patients in 18 countries and 115 sites [60]. In January 2016, the Ministry of Health of the Russian Federation approved the trastuzumab biosimilar HERtiCAD based on the results of a randomized, multicenter clinical study (NCT01764022) comparing the pharmacokinetics, immunogenicity, safety, and efficacy of BCD-022 with those of the reference drug Herceptin, both in combination with paclitaxel in treating patients with metastatic breast cancer [61]. The study demonstrated the non-inferiority of BCD-022 compared with Herceptin in terms of efficacy and safety. The primary patent on Herceptin in Europe expired in July 2014. Biosimilar trastuzumab has not yet been approved in the EU, but several are in phase III development.
Biosimilar rituximab
Rituximab is an mAb that acts against CD20 to treat non-Hodgkin’s lymphoma (NHL) and chronic lymphocytic leukemia. Marketed by Roche as Rituxan and MabThera, rituximab was originally approved for use in 1997 in the USA [62] and in 1998 in the EU [63]. In November 2013, the patent for rituximab expired in the EU; in September 2016, it will expire in the USA. In 2007, Dr. Reddy’s Laboratories marketed Reditux in India as a biosimilar of rituximab to treat diffuse large B cell lymphoma [64]. However, Reditux is not a true biosimilar because it has not been studied head to head against its reference product. In May 2014, Biocad’s Acellbia (also known as BCD020), a biosimilar of rituximab, was approved in Russia for the treatment of NHL [65] (Table 6). The approval was based on the results of a randomized, open-labelled study, involving over 30 centers in Ukraine, Russia, and India, which showed that the drug’s pharmacokinetics, pharmacodynamics, efficacy, and safety were similar to rituximab [66].
Conclusions
The rationale for the development of biosimilar products is to improve patient access to biological therapies in a safe and cost-effective way. As the technology advances and evolves, so too do regulations governing the development of biosimilars. Oncologists should be aware that biosimilars are not generics of biological medicines, but they do have comparable efficacy and safety to the originators. Pharmacovigilance is critical to ensuring the long-term quality and safety of biosimilars. With biosimilars, it is important to be mindful of concerns about drug substitutions and extrapolation across indications.
References
Soria JC, Blay JY, Spano JP, Pivot X, Coscas Y, Khayat D. Added value of molecular targeted agents in oncology. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2011;22(8):1703–16.
WHO. WHO expert committee on biological standardization sixtieth report—guidelines on evaluation of similar biotherapeutic products (SBPs). Geneva: World Health Organization; 2013. http://www.who.int/biologicals/publications/trs/areas/biological_therapeutics/TRS_977_Annex_2.pdf. Accessed 29 Jul 2016.
Multidisciplinary: Biosimilar. European Medicines Agency, United Kingdom. http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000408.jsp&mid=WC0b01ac058002958c#Overarchingbiosimilarguidelines. Accessed 29 Jul 2016.
Biosimilars. US Food and Drug Administration, United States http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm290967.htm. Accessed 29 Jul 2016.
Zelenetz AD, Ahmed I, Braud EL, Cross JD, Davenport-Ennis N, Dickinson BD, et al. NCCN biosimilars white paper: regulatory, scientific, and patient safety perspectives. J Natl Compr Cancer Netw JNCCN. 2011;9(Suppl 4):S1–22.
US$54 billion worth of biosimilar patents expiring before 2020. GaBI online—generics and biosimilars initiative. 2011. http://www.gabionline.net/Biosimilars/Research/US-54-billion-worth-of-biosimilar-patents-expiring-before-2020. Accessed 29 Jul 2016.
ASBM presents new European survey findings on biosimilars and the importance of nonproprietary naming. ASBM presents new European survey findings on biosimilars and the importance of nonproprietary naming, Alliance for Safe Biologic Medicines. 2013. https://www.safebiologics.org/2013/11/asbm-presents-new-european-survey-findings-on-biosimilars-and-the-importance-of-nonproprietary-naming/. Accessed 29 Jul 2016.
South Korean oncologists are significantly less familiar with biosimilars than their European counterparts. Decision Resources Group. 2013. https://www.decisionresourcesgroup.com/report/541/. Accessed 29 Jul 2016.
Sarpatwari A, Avorn J, Kesselheim AS. Progress and hurdles for follow-on biologics. New Engl J Med. 2015;372(25):2380–2.
Guidance for sponsors: information and submission requirements for subsequent entry biologics (SEBs). Health Canada, Government of Canada. 2010. http://www.hc-sc.gc.ca/dhp-mps/brgtherap/applic-demande/guides/seb-pbu/seb-pbu_2010-eng.php. Accessed 29 Jul 2016.
Weise M, Bielsky MC, De Smet K, Ehmann F, Ekman N, Giezen TJ, et al. Biosimilars: what clinicians should know. Blood. 2012;120(26):5111–7.
Biosimilars approved in Europe. GaBI online—generics and biosimilars initiative. 2011. http://www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-Europe. Accessed 29 Jul 2016.
Biosimilars approved in Australia. GaBI online—generics and biosimilars initiative. 2014. http://www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-Australia. Accessed 29 Jul 2016.
Evaluation of biosimilars: the evaluation of biosimilars. Therapeutic Goods Administration (TGA), Department of Health, Australian Government. 2013. https://www.tga.gov.au/publication/evaluation-biosimilars-1. Accessed 29 Jul 2016.
Biosimilars. Medicines and Medical Devices Safety Authority (Medsafe), Ministry of Health, New Zealand Government. 2014. http://www.medsafe.govt.nz/profs/RIss/Biosimilars.asp. Accessed 29 Jul 2016.
Guideline for the quality, safety, and efficacy assurance of follow-on biologics. Pharmaceuticals and Medical Devices Agency (PMDA), Japan. 2009. http://www.pmda.go.jp/files/000153851.pdf. Accessed 29 Jul 2016.
Guidelines on the evaluation of biosimilar products. Ministry of Food and Drug Safety (MFDS), South Korea. 2010. http://www.mfds.go.kr/jsp/common/download.jsp?fileinfo=S*1*%B5%BF%B5%EE%BB%FD%B9%B0%C0%C7%BE%E0%C7%B0%20%C6%F2%B0%A1%20%B0%A1%C0%CC%B5%E5%B6%F3%C0%CE%20(%BF%B5%B9%AE).pdf*e9a03e5980ec4520b888e54dbf6ee908*pdf*/files/upload/1/TB_F_INFODATA/13325/e9a03e5980ec4520b888e54dbf6ee908*265678*2012:08:29%2016:10:23. Accessed 29 Jul 2016.
Recent trend of pharmaceutical regulations in Taiwan. Taiwan Food & Drug Administration, Ministry of Health and Welfare, Taiwan. 2013. http://www.fda.gov.tw/upload/133/Content/2014033109035995639.pdf. Accessed 29 Jul 2016.
Guidance on registration of similar biological products in Singapore. Health Sciences Authority (HAS), Singapore Government. 2011. http://www.hsa.gov.sg/content/dam/HSA/HPRG/Western_Medicine/Overview_Framework_Policies/Guidelines_on_Drug_Registration/Appendix%2017_Guidance%20on%20Registration%20of%20Similar%20Biological%20Products%20in%20Singapore%202011.pdf. Accessed 29 Jul 2016.
Provisions for Drug Registration. China Food and Drug Administration, China. http://www.eng.sfda.gov.cn/WS03/CL0768/61645.html. Accessed 29 Jul 2016.
Guidelines on Similar biologics: regulatory requirements for marketing authorization in India. Ministry of Science and Technology and Ministry of Health and Family Welfare, Government of India. 2012. http://www.dbtbiosafety.nic.in/Files%5CCDSCO-DBTSimilarBiologicsfinal.pdf. Accessed 29 Jul 2016.
Abas A. Regulatory guidelines for biosimilars in Malaysia. Biol J Int Assoc Biol Stand. 2011;39(5):339–42.
Crommelin DJ, Shah VP, Klebovich I, McNeil SE, Weinstein V, Fluhmann B, et al. The similarity question for biologicals and non-biological complex drugs. Eur J Pharm Sci Off J Eur Fed Pharm Sci. 2015;76:10–7.
Pharmacovigilance. Geneva: World Health Organization. http://www.who.int/medicines/areas/quality_safety/safety_efficacy/pharmvigi/en/. Accessed 29 Jul 2016.
Bennett CL, Luminari S, Nissenson AR, Tallman MS, Klinge SA, McWilliams N, et al. Pure red-cell aplasia and epoetin therapy. New Engl J Med. 2004;351(14):1403–8.
Bennett CL, Evens AM, Andritsos LA, Balasubramanian L, Mai M, Fisher MJ, et al. Haematological malignancies developing in previously healthy individuals who received haematopoietic growth factors: report from the Research on Adverse Drug Events and Reports (RADAR) project. Br J Haematol. 2006;135(5):642–50.
Interchangeability of biosimilars—position of Finnish Medicines Agency Fimea. Finnish Medicines Agency Fimea (FIMEA), Finland. 2015. https://www.fimea.fi/documents/542809/838272/29197_Biosimilaarien_vaihtokelpoisuus_EN.pdf. Accessed 29 Jul 2016.
Schrijvers D, De Samblanx H, Roila F. Erythropoiesis-stimulating agents in the treatment of anaemia in cancer patients: ESMO Clinical Practice Guidelines for use. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2010;21(Suppl 5):v244–7.
Rizzo JD, Brouwers M, Hurley P, Seidenfeld J, Somerfield MR, Temin S. American society of clinical oncology/american society of hematology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. J Oncol Pract Am Soc Clin Oncol. 2010;6(6):317–20.
EMEA recommends a new warning for epoetins for their use in cancer patients. European Medicines Agency (EMEA) United Kingdom. 2008. http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2009/11/WC500015069.pdf. Accessed 29 Jul 2016.
Binocrit. European Medicines Agency United Kingdom. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000725/human_med_000675.jsp&mid=WC0b01ac058001d124. Accessed 29 Jul 2016.
Abseamed. European Medicines Agency, United Kingdom. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000727/human_med_000621.jsp&mid=WC0b01ac058001d124. Accessed 29 Jul 2016.
Epoetin Alfa Hexal. European Medicines Agency, United Kingdom. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000726/human_med_000768.jsp&mid=WC0b01ac058001d124. Accessed 29 Jul 2016.
Retacrit. European Medicines Agency, United Kingdom. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000872/human_med_001031.jsp&mid=WC0b01ac058001d124. Accessed 29 Jul 2016.
Silapo. European Medicines Agency, United Kingdom. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000760/human_med_001051.jsp&mid=WC0b01ac058001d124. Accessed 29 Jul 2016.
Mikhail A, Farouk M. Epoetin biosimilars in Europe: 5 years on. Adv Ther. 2013;30(1):28–40.
McKoy JM, Stonecash RE, Cournoyer D, Rossert J, Nissenson AR, Raisch DW, et al. Epoetin-associated pure red cell aplasia: past, present, and future considerations. Transfusion. 2008;48(8):1754–62.
Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol Off J Am Soc Clin Oncol. 2006;24(19):3187–205.
Ratiograstim. European Medicines Agency United Kingdom. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000825/human_med_001015.jsp&mid=WC0b01ac058001d124. Accessed 29 Jul 2016.
Tevagrastim. European Medicines Agency United Kingdom. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000827/human_med_001089.jsp&mid=WC0b01ac058001d124. Accessed 29 Jul 2016.
Biograstim. European Medicines Agency United Kingdom. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000826/human_med_000676.jsp&mid=WC0b01ac058001d124. Accessed 29 Jul 2016.
Filgrastim Hexal. European Medicines Agency United Kingdom. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000918/human_med_000791.jsp&mid=WC0b01ac058001d124. Accessed 29 Jul 2016.
Zarzio. European Medicines Agency United Kingdom. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000917/human_med_001170.jsp&mid=WC0b01ac058001d124. Accessed 29 Jul 2016.
Nivestim. European Medicines Agency United Kingdom. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/001142/human_med_001344.jsp&mid=WC0b01ac058001d124. Accessed 29 Jul 2016.
Grastofil. European Medicines Agency United Kingdom. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002150/human_med_001688.jsp&mid=WC0b01ac058001d124. Accessed 29 Jul 2016.
Accofil. European Medicines Agency United Kingdom. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003956/human_med_001798.jsp&mid=WC0b01ac058001d124. Accessed 29 Jul 2016.
del Giglio A, Eniu A, Ganea-Motan D, Topuzov E, Lubenau H. XM02 is superior to placebo and equivalent to Neupogen in reducing the duration of severe neutropenia and the incidence of febrile neutropenia in cycle 1 in breast cancer patients receiving docetaxel/doxorubicin chemotherapy. BMC Cancer. 2008;8:332.
Gatzemeier U, Ciuleanu T, Dediu M, Ganea-Motan E, Lubenau H, Del Giglio A. XM02, the first biosimilar G-CSF, is safe and effective in reducing the duration of severe neutropenia and incidence of febrile neutropenia in patients with small cell or non-small cell lung cancer receiving platinum-based chemotherapy. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2009;4(6):736–40.
Engert A, Griskevicius L, Zyuzgin Y, Lubenau H, del Giglio A. XM02, the first granulocyte colony-stimulating factor biosimilar, is safe and effective in reducing the duration of severe neutropenia and incidence of febrile neutropenia in patients with non-Hodgkin lymphoma receiving chemotherapy. Leuk Lymph. 2009;50(3):374–9.
Blackwell K, Semiglazov V, Krasnozhon D, Davidenko I, Nelyubina L, Nakov R, et al. Comparison of EP2006, a filgrastim biosimilar, to the reference: a phase III, randomized, double-blind clinical study in the prevention of severe neutropenia in patients with breast cancer receiving myelosuppressive chemotherapy. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2015;26(9):1948–53.
Waller CF, Semiglazov VF, Tjulandin S, Bentsion D, Chan S, Challand R. A phase III randomized equivalence study of biosimilar filgrastim versus Amgen filgrastim in patients receiving myelosuppressive chemotherapy for breast cancer. Onkologie. 2010;33(10):504–11.
FDA approves first biosimilar product Zarxio. US Food and Drug Administration, United States 2015. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm436648.htm. Accessed 29 Jul 2016.
Molineux G. Pegfilgrastim: using pegylation technology to improve neutropenia support in cancer patients. Anticancer Drugs. 2003;14(4):259–64.
Neulasta. European Medicines Agency United Kingdom. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000420/human_med_000924.jsp&mid=WC0b01ac058001d124. Accessed 29 Jul 2016.
Pegfilgrastim product approval information—licensing action. US Food and Drung Administration, United States. 2002. http://www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/therapeuticbiologicapplications/ucm080669.htm. Accessed 29 Jul 2016.
Biosimilars of pegfilgrastim. GaBI online—generics and biosimilars initiative. 2014. http://www.gabionline.net/Biosimilars/General/Biosimilars-of-pegfilgrastim. Accessed 29 Jul 2016.
FDA approval for trastuzumab. National Cancer Institute, United States http://www.cancer.gov/about-cancer/treatment/drugs/fda-trastuzumab. Accessed 29 Jul 2016.
Herceptin. European Medicines Agency, United Kingdom. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000278/human_med_000818.jsp&mid=WC0b01ac058001d124. 29 Jul 2016.
Biosimilars of trastuzumab. GaBI online—generics and biosimilars initiative. 2014. http://www.gabionline.net/Biosimilars/General/Biosimilars-of-trastuzumab. Accessed 29 Jul 2016.
Biosimilar trastuzumab approved in Korea. GaBI online—generics and biosimilars initiative. 2014. http://www.gabionline.net/Biosimilars/News/Biosimilar-trastuzumab-approved-in-Korea. 29 Jul 2016.
BIOCAD announces the first trastuzumab biosimilar approved by the Ministry of Health of the Russian Federation. Business Wire. 2016. http://www.businesswire.com/news/home/20160120005889/en/BIOCAD-Announces-Trastuzumab-Biosimilar-Approved-Ministry-Health. Accessed 29 Jul 2016.
Rituximab (marketed as Rituxan) Information. US Food and Drug Administration, United States http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm109106.htm. Accessed 29 Jul 2016.
MabThera. European Medicines Agency United Kingdom. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000165/human_med_000897.jsp&mid=WC0b01ac058001d124. Accessed 29 Jul 2016.
Biosimilars in emerging markets. GaBI online—generics and biosimilars initiative. 2015. http://www.gabi-journal.net/news/biosimilars-in-emerging-markets. Accessed 29 Jul 2016.
CJSC BIOCAD announces AcellBia is first mAb biosimilar approved by the Ministry of Health of the Russian Federation. PR Newswire. 2014. http://www.prnewswire.com/news-releases/cjsc-biocad-announces-acellbia-is-first-mab-biosimilar-approved-by-the-ministry-of-health-of-the-russian-federation-259233961.html. Accessed 29 Jul 2016.
Kaplanov K, Zaritskiy A, Alexeev S, Volodicheva E, Loginov A, Orlova R, et al. Key Results of International Randomized Open-Label Clinical Study of BCD-020 (rituximab biosimilar candidate) in Patients with B-Cell Non-Hodgkin’s Lymphoma. Blood. 2014;124(21):5467.
Authors’ contributions
LKSL, KM, and CL drafted the manuscript. LKSL and SLC revised this paper. All authors read and approved the final manuscript.
Acknowledgements
None.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Leung, L.K.S., Mok, K., Liu, C. et al. What do oncologists need to know about biosimilar products?. Chin J Cancer 35, 91 (2016). https://doi.org/10.1186/s40880-016-0151-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40880-016-0151-x