Abstract
Introduction
ATP-binding cassette subfamily B member 1 (ABCB1) and subfamily C member 10 (ABCC10) proteins are efflux transporters that couple the energy derived from ATP hydrolysis to the translocation of toxic substances and chemotherapeutic drugs out of cells. Cabazitaxel is a novel taxane that differs from paclitaxel by its lower affinity for ATP-binding cassette (ABC) transporters.
Methods
We determined the effects of cabazitaxel, a novel tubulin-binding taxane, and paclitaxel on paclitaxel-resistant, ABCB1-overexpressing KB-C2 and LLC-MDR1-WT cells and paclitaxel-resistant, ABCC10-overexpressing HEK293/ABCC10 cells by calculating the degree of drug resistance and measuring ATPase activity of the ABCB1 transporter.
Results
Decreased resistance to cabazitaxel compared with paclitaxel was observed in KB-C2, LLC-MDR1-WT, and HEK293/ABCC10 cells. Moreover, cabazitaxel had low efficacy, whereas paclitaxel had high efficacy in stimulating the ATPase activity of ABCB1, indicating a direct interaction of both drugs with the transporter.
Conclusion
ABCB1 and ABCC10 are not primary resistance factors for cabazitaxel compared with paclitaxel, suggesting that cabazitaxel may have a low affinity for these efflux transporters.
Similar content being viewed by others
Background
Paclitaxel is a clinically used chemotherapeutic drug, but its use can elicit resistance to various anticancer drugs in certain types of cancers [1]. The efficacy of paclitaxel can be attenuated by the overexpression of multidrug efflux transporters, altered metabolism, decreased sensitivity to apoptosis, alterations in microtubule dynamics, diminished interactions of paclitaxel with its cellular target, and genetic polymorphisms [1,2]. These aforementioned mechanisms of resistance typically produce chemotherapeutic failure. ATP-binding cassette subfamily B member 1 [ABCB1, also called multidrug resistance 1 (MDR1) or P-glycoprotein (P-gp)] and ATP-binding cassette subfamily C member 10 [ABCC10, also called multidrug resistance protein 7 (MRP7)] have been well characterized in terms of their capacities to confer resistance to paclitaxel [1,3-5]. The human ABCB1 transporter, a product encoded by the ABCB1 gene, which is localized to chromosome 7p21, is the first identified mammalian ATP-binding cassette (ABC) transporter [6,7]. The ABCB1 transporter has a molecular weight of 170 kDa and comprises two transmembrane-binding domains (TMD1 and TMD2) and two nucleotide-binding domains (NBD1 and NBD2) [4,8]. The human ABCC10 transporter is encoded by the ABCC10 gene, which is localized to chromosome 6p21.1 [9,10]. The ABCC10 transporter is a 171-kDa protein, containing three membrane-spanning domains (MSD1, MSD2, and MSD3) and two NBDs. It belongs to the class of long ABCCs that includes ABCC1, ABCC2, ABCC3, and ABCC6 [11].
Cabazitaxel is a new semisynthetic taxane approved for use by the United States Food and Drug Administration and is derived from 10-deacetyl-baccatin III, which is extracted from European yew needles [12]. Mechanistically, cabazitaxel exerts its cytotoxic effects by 1) binding to tubulin and promoting its assembly into microtubules while simultaneously inhibiting microtubule disassembly and 2) stabilizing microtubules, resulting in the inhibition of mitotic and interphase cellular functions [13]. It has been postulated that cancer cells expressing ABCB1 become resistant to taxanes [14]. Apart from ABCB1, the ABCC10 transcript has also been detected in several adenocarcinomas, including breast, ovarian, and lung tumors. This is of potential interest because the latter tumors are treated with taxanes. The ABCC10 transcript and ABCC10 protein were reported to be induced by vincristine exposure in two salivary gland adenocarcinoma cell lines that are cross-resistant to docetaxel, and the ABCC10 transcript was reported to be increased in MCF7 cells by exposure to doxorubicin. Moreover, ABCC10 is induced by paclitaxel in a non-small cell lung cancer cell line [3,15]. Our previous studies reported that ABCB1 and ABCC10 confer resistance to two classes of drugs that target microtubules: Vinca alkaloids and taxanes [1,16,17]. It was therefore of interest to determine if ABCB1 and ABCC10 might also confer resistance to new anti-microtubule drugs. Thus, we hypothesized that ABCB1 and ABCC10 may confer differential resistance to paclitaxel and cabazitaxel. In this study, we determined the sensitivity of ABCB1- and ABCC10-overexpressing cells to paclitaxel and cabazitaxel in vitro.
Methods
Materials
Cabazitaxel was purchased from MedChemexpress (Manmouth Junction, NJ, USA). Paclitaxel was purchased from Tocris Bioscience (Ellisville, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), phosphate-buffered saline (PBS), 10,000 IU/mL penicillin and 10,000 μg/mL streptomycin, and 0.25% trypsin were purchased from HyClone (Waltham, MA, USA). 3-(4,5-Dimethylthiazol-yl)-2,5-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), ammonium molybdate, 2-(N-morpholino) ethanesulfonic acid (MES) hydrate, antimony potassium tartrate, sodium azide, and N-methyl-D-glucamine were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Potassium phosphate, ethylene glycol tetraacetic acid (EGTA), and adenosine triphosphate (ATP) were products of AMRESCO (Solon, OH, USA). Sulfuric acid solution (37 N) was purchased from Fisher Scientific (Pittsburgh, PA, USA). KCl was purchased from Avantor Performance Materials (Center Valley, PA, USA). Ouabain was purchased from Enzo Life Sciences, Inc. (Farmingdale, NY, USA). Dithiothreitol was purchased from Promega Corporation (Madison, WI, USA). MgCl2 was purchased from EMD Millipore (Billerica, MA, USA). Ascorbic acid was purchased from VWR International (West Chester, PA, USA). Sodium orthovanadate was purchased from Alfa Aesar (Ward Hill, MA, USA). The OPSYS microplate reader was purchased from Dynex Technologies (Chantilly, VA, USA).
Cell lines
The ABCB1-overexpressing KB-C2 cell line was established by a step-wise exposure of KB-3-1 cells, a parental human epidermoid carcinoma cell line, to increasing concentrations (up to 2 μg/mL) of colchicine [18]. The LLC-PK1 porcine renal epithelial cells were transfected with wild-type human ABCB1 cDNA plasmid as previously described, and the stable transfectant cell line was named LLC-MDR1-WT [19]. HEK293/pcDNA3.1 and HEK293/ABCC10 cells were generated by transfecting HEK293 cells with an empty vector and an ABCC10 expression vector, respectively [20]. We thank Dr. Shinichi Akiyama (Kagoshima University, Japan) for the KB-3-1 and KB-C2 cell lines, Dr. Michael M. Gottesman (NCI, NIH, USA) for the LLC-PK1 and LLC-MDR1-WT cell lines, and Dr. Gary D. Kruh (University of Illinois at Chicago, IL, USA) for the ABCC10 plasmid.
Drug sensitivity
To determine the drug sensitivities of the previously described ABCB1-overexpressing KB-C2 cells, LLC-MDR1-WT cells, and ABCC10-overexpressing HEK293/ABCC10 cells, with KB-3-1, LLC-PK1, and HEK293/pcDNA3.1 cells as the respective controls [1,16], a modified MTT assay was performed [1,21]. Approximately 4,000 KB-3-1 cells, 7,000 KB-C2 cells, and 5,000 LLC-PK1, LLC-MDR1-WT, HEK293/pcDNA3.1, and HEK293/ABCC10 cells were seeded in 180 μL of medium in each well of 96-well plates. After incubating for 24 h at 37°C, 20 μL of paclitaxel or cabazitaxel (0.01 to 10 μmol/L) was added. Subsequently, cells treated with paclitaxel or cabazitaxel in DMEM supplemented with 10% FBS were incubated at 37°C for 72 h. After 72 h, 20 μL MTT (4 mg/mL) was added to each well. The cells were incubated at 37°C for another 4 h. The MTT with medium was removed, and 100 μL of DMSO was added to each well. The absorbance was measured at 570 nm by an Opsys microplate reader (Dynex Technologies, VA, USA). The degree of resistance was calculated by dividing the 50% inhibition concentration (IC50) as calculated using the Bliss method for drug-resistant cells (KB-C2, LLC-MDR1-WT, and HEK293/ABCC10) by that of the parental drug-sensitive cells (KB-3-1, LLC-PK1, and HEK293/pcDNA3.1), respectively. Each MTT assay was run in triplicate.
ABCB1 ATPase assay
The ABCB1 transporter uses energy derived from the hydrolysis of ATP to efflux their substrates across the membrane against a concentration gradient; thus, the ATP consumption reflects the ATPase activity of the transporter. The vanadate (Vi)-sensitive ATPase activity of ABCB1 in the membrane vesicles of High Five insect cells [(His)-6-tagged ABCB1 expressed in Trichoplusia ni cells using the recombinant baculovirus system and purified by metal affinity chromatography] was measured as previously described [22,23]. The membrane vesicles (10–20 μg protein/reaction) were incubated in ATPase assay buffer (50 mmol/L MES-Tris, pH 6.8, 50 mmol/L KCl, 5 mmol/L sodium azide, 1 mmol/L EGTA, 1 mmol/L ouabain, 2 mmol/L dithiothreitol, and 10 mmol/L MgCl2) at 37°C for 5 min with or without 0.3 mmol/L vanadate. The membrane vesicles in ATPase assay buffer were incubated with different concentrations (0–10 μmol/L) of paclitaxel or cabazitaxel at 37°C for 3 min, and then 5 mmol/L ATP was added at 37°C. After 20 min of incubation, the reaction was terminated by adding 0.1 mL of 5% SDS solution. The amount of Pi released was quantified at 800 nm using a Bio-Rad SmartSpec Plus Spectrophotometer (Hercules, CA, USA) as previously described [23,24].
Statistical analyses
All experiments were repeated at least three times. The differences between runs were assessed using the two-tailed Student’s t-test, and statistical significance was determined at P < 0.05. Microsoft Office Excel 2010, licensed from Microsoft (Redmond, WA, USA), was used for data processing and analysis.
Results
Cytotoxicity of paclitaxel and cabazitaxel in ABCB1- and ABCC10-overexpressing cells
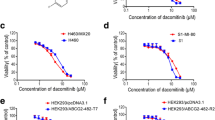
As shown in Table 1, significantly elevated resistance to paclitaxel was observed for the ABCB1-overexpressing, drug-selective KB-C2 cells and ABCB1-transfected LLC-MDR1-WT cells, which exhibited 21.2- and 25.6-fold resistance as compared with those of the parental KB-3-1 and LLC-PK1 cells, respectively; weakly elevated resistance to cabazitaxel was observed for KB-C2 and LLC-MDR1-WT cells, which exhibited 2.2- and 9.1-fold resistance as compared with those of KB-3-1 and LLC-PK1 cells, respectively. In addition, the resistance of ABCC10-overexpressing cells to paclitaxel and cabazitaxel was analyzed. The ABCC10-transfected HEK293/ABCC10 cells exhibited a 9.3-fold resistance to paclitaxel and 1.0-fold resistance to cabazitaxel as compared with those of HEK293/pcDNA3.1 cells. Representative concentration-response curves for paclitaxel and cabazitaxel are shown in Figure 1.
Cytotoxicity of paclitaxel and cabazitaxel in ABCB1- and ABCC10-overexpressing cells. ABCB1, ATP-binding cassette subfamily B member 1; ABCC10, ATP-binding cassette subfamily C member 10. The cytotoxicity of paclitaxel and cabazitaxel was determined by the MTT assay in ABCB1-overexpressing KB-C2 and LLC-MDR1-WT cells, ABCC10-overexpressing HEK293/ABCC10 cells, and their parental KB-3-1, LLC-PK1, and HEK293/pcDNA3.1 cells. Error bars indicate the standard deviation (SD). Significantly elevated resistance of KB-C2 (A) and LLC-MDR1-WT cells (C) to paclitaxel, none or low-level resistance of KB-C2 (B) and LLC-MDR1-WT cells (D) to cabazitaxel, significantly elevated resistance of HEK293/ABCC10 cells to paclitaxel (E), and no resistance of HEK293/ABCC10 cells to cabazitaxel (F) are observed as compared with those of their parental cells.
Effects of paclitaxel and cabazitaxel on ABCB1 ATP hydrolysis
Paclitaxel and cabazitaxel stimulated the ATPase activity of ABCB1 (Figure 2), suggesting that paclitaxel and cabazitaxel interact at the drug substrate-binding site and increase the ATPase activity of ABCB1. However, less stimulation of the ATPase activity of ABCB1 was observed with cabazitaxel as compared with paclitaxel, suggesting that cabazitaxel has a lower affinity for ABCB1, consistent with the cytotoxicity results.
Discussion
In the present study, ABCB1-overexpressing, drug-selective KB-C2 cells and ABCB1-transfected LLC-MDR1-WT cells showed low resistance to cabazitaxel. ABCC10-overexpressing HEK293/ABCC10 cells exhibited no resistance to cabazitaxel as compared with their resistance to paclitaxel. Furthermore, cabazitaxel stimulated the ATPase activity of ABCB1 to a lesser magnitude than paclitaxel.
The present analysis of ABCB1 and ABCC10 efflux transporters provides important and new information on the resistance profile related to these pumps. A notable feature of ABCB1 and ABCC10 that emerged from this line of investigation is that these pumps are able to confer little or no resistance to cabazitaxel. Of the microtubule-stabilizing drugs recently used in clinical development, cabazitaxel is the most advanced one [12]. As such, cabazitaxel exhibits a low affinity for ABCB1 or any previously tested drug efflux pump [25]. Our results suggest that the differential resistance properties of ABCB1- and ABCC10-overexpressing cells to paclitaxel and cabazitaxel may be attributed to structural differences of these drugs, although this remains to be determined.
Duran et al. [26] reported several potential biomarkers for predicting cabazitaxel resistance in cell lines that did not express the ABCB1 protein; these biomarkers included reduced breast cancer type 1 susceptibility protein (BRCA1) expression and increased class III β-tubulin isotype expression. Moreover, alterations in microtubule dynamics and induction of the epithelial-mesenchymal transition have been linked to the resistance mechanism of cabazitaxel [26]. Thus, it is possible that the drug-resistant cell lines used in our study may express the aforementioned markers and confer resistance to cabazitaxel. Therefore, future experiments will be directed toward determining the mechanisms that mediate cabazitaxel resistance by various methods, such as measuring the cellular retention of cabazitaxel in cell lines that are positive and negative for ABC transporters.
Conclusions
In conclusion, cabazitaxel, unlike paclitaxel, sensitizes ABCB1- and ABCC10-overexpressing cells. It is possible that cabazitaxel is a low-affinity substrate of ABCB1 and ABCC10 and that these transporters may not play a role in the development of drug resistance in the clinic. However, further preclinical studies are warranted to establish this fact.
References
Kathawala RJ, Sodani K, Chen K, Patel A, Abuznait AH, Anreddy N, et al. Masitinib antagonizes ATP-binding cassette subfamily C member 10-mediated paclitaxel resistance: a preclinical study. Mol Cancer Ther. 2014;13:714–23.
Yin S, Bhattacharya R, Cabral F. Human mutations that confer paclitaxel resistance. Mol Cancer Ther. 2010;9:327–35.
Kathawala RJ, Wang YJ, Ashby Jr CR, Chen ZS. Recent advances regarding the role of ABC subfamily C member 10 (ABCC10) in the efflux of antitumor drugs. Chin J Cancer. 2014;33:223–30.
Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58.
Dumontet C, Sikic BI. Mechanisms of action of and resistance to antitubulin agents: microtubule dynamics, drug transport, and cell death. J Clin Oncol. 1999;17:1061–70.
Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976;455:152–62.
Ueda K, Cornwell MM, Gottesman MM, Pastan I, Roninson IB, Ling V, et al. The mdr1 gene, responsible for multidrug-resistance, codes for P-glycoprotein. Biochem Biophys Res Commun. 1986;141:956–62.
Sauna ZE, Smith MM, Muller M, Kerr KM, Ambudkar SV. The mechanism of action of multidrug-resistance-linked P-glycoprotein. J Bioenerg Biomembr. 2001;33:481–91.
Kruh GD, Guo Y, Hopper-Borge E, Belinsky MG, Chen ZS. ABCC10, ABCC11, and ABCC12. Pflugers Arch. 2007;453:675–84.
Hopper E, Belinsky MG, Zeng H, Tosolini A, Testa JR, Kruh GD. Analysis of the structure and expression pattern of MRP7 (ABCC10), a new member of the MRP subfamily. Cancer Lett. 2001;162:181–91.
Sodani K, Patel A, Kathawala RJ, Chen ZS. Multidrug resistance associated proteins in multidrug resistance. Chin J Cancer. 2012;31:58–72.
Vrignaud P, Semiond D, Lejeune P, Bouchard H, Calvet L, Combeau C, et al. Preclinical antitumor activity of cabazitaxel, a semisynthetic taxane active in taxane-resistant tumors. Clin Cancer Res. 2013;19:2973–83.
Galsky MD, Dritselis A, Kirkpatrick P, Oh WK. Cabazitaxel. Nat Rev Drug Discov. 2010;9:677–8.
Kathawala RJ, Gupta P, Ashby Jr CR, Chen ZS. The modulation of ABC transporter-mediated multidrug resistance in cancer: a review of the past decade. Drug Resist Updat. 2014;Dec 10. doi:10.1016/j.drup.2014.11.002. [Epub ahead of print]
Hopper-Borge E, Xu X, Shen T, Shi Z, Chen ZS, Kruh GD. Human multidrug resistance protein 7 (ABCC10) is a resistance factor for nucleoside analogues and epothilone B. Cancer Res. 2009;69:178–84.
Wang YJ, Kathawala RJ, Zhang YK, Patel A, Kumar P, Shukla S, et al. Motesanib (AMG706), a potent multikinase inhibitor, antagonizes multidrug resistance by inhibiting the efflux activity of the ABCB1. Biochem Pharmacol. 2014;90:367–78.
Kathawala RJ, Wei L, Anreddy N, Chen K, Patel A, Alqahtani S, et al. The small molecule tyrosine kinase inhibitor NVP-BHG712 antagonizes ABCC10-mediated paclitaxel resistance: a preclinical and pharmacokinetic study. Oncotarget. 2014. [Epub ahead of print]
Akiyama S, Fojo A, Hanover JA, Pastan I, Gottesman MM. Isolation and genetic characterization of human KB cell lines resistant to multiple drugs. Somat Cell Mol Genet. 1985;11:117–26.
Fung KL, Pan J, Ohnuma S, Lund PE, Pixley JN, Kimchi-Sarfaty C, et al. MDR1 synonymous polymorphisms alter transporter specificity and protein stability in a stable epithelial monolayer. Cancer Res. 2014;74:598–608.
Chen ZS, Hopper-Borge E, Belinsky MG, Shchaveleva I, Kotova E, Kruh GD. Characterization of the transport properties of human multidrug resistance protein 7 (MRP7, ABCC10). Mol Pharmacol. 2003;63:351–8.
Kathawala RJ, Chen JJ, Zhang YK, Wang YJ, Patel A, Wang DS, et al. Masitinib antagonizes ATP-binding cassette subfamily G member 2-mediated multidrug resistance. Int J Oncol. 2014;44:1634–42.
Ramachandra M, Ambudkar SV, Chen D, Hrycyna CA, Dey S, Gottesman MM, et al. Human P-glycoprotein exhibits reduced affinity for substrates during a catalytic transition state. Biochemistry. 1998;37:5010–9.
Ambudkar SV. Drug-stimulatable ATPase activity in crude membranes of human MDR1-transfected mammalian cells. Methods Enzymol. 1998;292:504–14.
Yang D, Kathawala RJ, Chufan EE, Patel A, Ambudkar SV, Chen ZS, et al. Tivozanib reverses multidrug resistance mediated by ABCB1 (P-glycoprotein) and ABCG2 (BCRP). Future Oncol. 2014;10:1827–41.
Nightingale G, Ryu J. Cabazitaxel (jevtana): a novel agent for metastatic castration-resistant prostate cancer. P T. 2012;37:440–8.
Duran GE, Wang YC, Francisco EB, Rose JC, Martinez FJ, Coller J, et al. Mechanisms of resistance to cabazitaxel. Mol Cancer Ther. 2015;14:193–201.
Acknowledgements
This work was supported by funds from the National Institutes of Health (1R15CA143701) and St. John's University Research Seed Grant (579-1110-7002) to Dr. Zhe-Sheng Chen. Drs. Suneet Shukla and Suresh V. Ambudkar were supported by the Intramural Research Program, Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
Conception and design: RJK, CRA Jr, and Z-SC. Development of methodology: RJK, AK, SVA, and Z-SC. Acquisition of data: RJK, Y-JW, SA, CRA Jr, and Z-SC. Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): RJK, Y-JW, SS, SVA, CRA Jr, and Z-SC. Writing, review, and/or revision of the manuscript: RJK, SVA, CRA Jr, and Z-SC. Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): RJK, Y-JW, Y-KZ, SVA, CRA Jr, and Z-SC. Study supervision: RJK, SVA, CRA Jr, and Z-SC. All authors read and approved the final manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kathawala, R.J., Wang, YJ., Shukla, S. et al. ATP-binding cassette subfamily B member 1 (ABCB1) and subfamily C member 10 (ABCC10) are not primary resistance factors for cabazitaxel. Chin J Cancer 34, 5 (2015). https://doi.org/10.1186/s40880-015-0003-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40880-015-0003-0