Abstract
Background
The animal ecology literature proposes the view that predation risk induces fear in prey animals, but it is also possible that behavioral and physiological changes induced by predation risk are not associated with fear at all. If we view fear as a state indicated by measurable changes in behavior and physiology caused by threats, then it is valid to search for a link between markers of fearfulness and predation risk. I predicted that domestic fowls (Gallus gallus domesticus) foraging alone, and thus exposed to higher predation risk, would show higher vigilance (a behavioral marker of fearfulness) and lower external body temperature (a physiological marker of fearfulness) than domestic fowls foraging in pairs. These adjustments should become less prominent in the continued absence of threats during a trial.
Results
Domestic fowls that foraged alone rather than in pairs showed higher vigilance and lower external body temperature. While external body temperature returned to baseline values during a trial, vigilance unexpectedly increased. The results thus provide mixed support for an association between markers of fearfulness and predation risk.
Conclusions
I argue that vigilance is not always a sensitive marker of fearfulness because hunger can keep vigilance low even in risky settings. By contrast, external body temperature varied with group size and time during a trial, suggesting that this marker is more sensitive. Future studies are needed to validate the relationship between markers of fearfulness and predation risk.
Similar content being viewed by others
Background
Foraging exposes prey animals to threats from predators. Predation risk, the actual probability of attack from predators in a given environment [1], is associated with several behavioral and physiological changes in prey animals [2, 3]. At the behavioral level, prey animals can allocate more time to vigilance to gather information about potential threats and detect them more quickly [4]. At the physiological level, threats can initiate changes within seconds. The release of norepinephrine, for instance, induces a rapid increase in heart rate [5]. These short-term changes allow prey animals to focus their attention on threats and prepare their body for an eventual escape or fight. Other hormones released after a threat, such as glucocorticoids, produce their effects later and can be present for days [6].
The animal ecology literature proposes the view that predation risk induces fear in prey animals, and the aforementioned behavioral and physiological adjustments represent responses to fear [3, 7, 8]. The term fear is used evocatively because it is rarely measured empirically in this literature. In addition, it is not clear whether prey animals experience fear the same way that humans do [9]. Some researchers even argue that many changes in prey behavior and physiology in response to predation risk need not imply fear at all [10]. Nevertheless, if we view fear as a state indicated by measurable changes in behavior and physiology caused by current or anticipated threats [11, 12], then it is valid to search for a link between markers of fearfulness and predation risk.
To track the relationship between fearfulness and predation risk, it is important to identify ecological contexts associated with marked changes in predation risk. Group size is one of the main factors affecting predation risk for prey animals that forage in groups [13, 14]. Group foragers benefit from the multiplicity of senses available to detect predators. In addition, other group members dilute the risk of capture if the predator can only capture one individual during an attack [15], thus leading to reduced predation risk in groups. In addition to group size, predation risk is also expected to vary on a temporal basis as more information about predators becomes available [16, 17]. If prey animals regularly update their information about predation risk over time, the continued absence of threats might indicate a lower predation risk [18, 19]. If fearfulness is associated with predation risk, markers of fearfulness should match variation in predation risk induced by changes in group size and track temporal changes in predation risk.
In order to identify the links between fearfulness and predation risk, it is also pertinent to examine the efficacy of fearfulness markers. One putative marker of fearfulness is vigilance. Vigilance represents an allocation of time to monitor the surroundings for signs of current or anticipated danger [4]. Vigilance can be directed at predators or competitors within the group. As vigilance often detracts from the ability to acquire resources, costly vigilance should be indicative of the level of fear experienced by animals [20]. Consistent with expectations of reduced fearfulness in group settings, individuals in many species of birds and mammals decrease their investment in vigilance as group size increases [21, 22]. Nevertheless, vigilance does not always match the degree of threat from predation [23, 24] and, thus, is a likely less sensitive marker of fearfulness.
Another putative marker of fearfulness is external body temperature. During the rapid reaction to threats, blood flow is diverted to internal organs, which often leads to a decrease in temperature at the body surface [23]. A decrease in external body temperature has been shown in response to various stimuli in animals including air puffs [24] and handling [25]. Temperature in the eye area also responds to handling [24, 26], the administration of air puffs [27] or hormone injection [28]. External body temperature at various locations on the body might thus be a convenient physiological marker of fearfulness, but it has not been linked thus far with correlates of predation risk.
I aimed to determine whether vigilance and external body temperature tracked changes in predation risk over time and in groups of different sizes. Few studies have shown whether markers of fearfulness other than vigilance match changes in predation risk. To this end, I combined behavioral observations and thermal imaging to examine changes in vigilance and external body temperature in domestic fowls (Gallus gallus domesticus) that foraged alone or in pairs. Domestic fowls have bare patches on the comb and cheeks, which allow us to get precise measurements of external body temperature without interference from feathers. I predicted that domestic fowls foraging alone would show higher vigilance and lower external body temperature than those foraging in pairs. These adjustments should become less prominent during a trial in the continued absence of threats. These relationships should not prevail if fearfulness and predation risk are not associated, if these specific markers of fearfulness are not sensitive to variation in predation risk over time or in groups of different sizes or if fear was not successfully induced.
Methods
Subjects
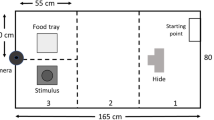
Twelve adult female domestic fowls of a layer breed served as experimental subjects. These birds were obtained from a commercial hatchery and raised together from day old in the same indoor pen. Colored rings on the legs allowed individual identification. Subjects were kept in a 3x3x3 m indoor pen under a 13 L:11D photoperiod regime. This pen connected through a small door to a similar sized covered outdoor pen exposed to natural light. The wire mesh on the outdoor pen allowed visual and auditory contact but no direct interaction with potential threats including foxes, dogs, cats, and hawks. Perches, patches for dust bathing, water, and a commercial layer feed were available at all times. Prior to the experiment, all birds had extensive experience with feeding in groups of various sizes in the outdoor pen.
Experimental procedure
Thermal imaging
This phase took place in two consecutive parts. The first part in the indoor pen established baseline external body temperature in a group setting while the second part documented changes in external body temperature when hens foraged alone in the outdoor pen. For the first part, I removed food for 3 h in the morning to increase feeding motivation. During the last half hour of food deprivation, I introduced the thermal imaging camera (Flir™ E40) to allow birds to get familiar with the equipment. I then took images of the head of each individual in a previously established random order. I took images as individuals walked around the indoor pen and positioned the camera at about 1 m from the head of each bird.
Immediately after taking all images, I tested individuals singly in the outdoor pen in a previously established random order. Each individual in turn was caught and transferred to the outdoor pen using the trap door. Birds were familiar with this procedure, and it took less than 10 s to catch, handle, and release a bird in the outdoor pen. The handling phase lasted about 5 s. One min after transferring one bird to the outdoor pen, I removed the cover over the food patch located there to signal the beginning of a food trial. Individuals immediately started to feed and the trial ended 3 min later. During the trial, I took images of the head of each bird from a distance of about 1 m. It proved difficult to obtain sharp images of the head during the short vigilance bouts. Therefore, I took images of the individuals when they fed head down. I took one image at the beginning of the trial and another during the last minute of the trial. After completion of a trial, I returned the bird to the indoor pen.
I repeated the full procedure on a second day, but this time after taking images of the birds in the indoor pen, I tested them in the outdoor pen in randomly formed pairs. The two trials thus produced paired observations (indoor-outdoor) for two different group sizes. Ambient temperature differed on the two days of thermal imaging by several degree Celsius. Indoor and outdoor temperatures on a given day, however, differed by less than one degree Celsius and relative humidity was similar.
Handling effect
Handling can cause transient changes in external body temperature [24, 29]. To determine how long these effects persisted, I carried out an experiment in which I took images of the head of each bird in the indoor pen before and shortly after capture and handling. After capture, each bird was held for 5 s (the typical handling time in the previous thermal imaging study) and then released on the ground after which a second image was taken (delay to obtain a sharp image ranged between 18 and 130 s with a median of 33 s).
Vigilance
I evaluated vigilance in the outdoor pen in single birds on one day and in pairs on another day. I kept the same testing order and formed the same pairs as in the thermal imaging trials. Prior to testing, I removed food in the morning in the indoor pen for 3 h to increase feeding motivation. Individuals were moved as before to the outdoor pen for food trials. Trials lasted 3 min and were videotaped at a distance of about 1 m.
Data collection
Temperature measurements
For each thermal image, I obtained external body temperature measurements at three different positions: the base of the comb near the middle of the head, the eye, and a bare patch on the cheek (Fig. 1).
Vigilance measurements
During food trials, all birds handled the crumbly feed in the head down position, from which I assumed that feeding interruptions represented vigilance. I played videos one frame at a time (1 frame = 33 ms) to get the timing and duration of each vigilance bout. For the duration of a vigilance bout, I counted the number of frames between the moment the hen maintained the bill at the horizontal level after raising its head from the food patch and the moment the hen started to peck at food. At the end of a trial, I obtained the frequency of vigilance bouts (number of vigilance bouts per min), the geometric mean duration of all vigilance bouts (durations were right skewed), and the total time spent vigilant in a trial expressed as a percentage of trial duration.
Statistical analyses
Temperature
Because external body temperature varies with ambient temperature in domestic fowls [30], I analyzed external body temperature measurements in the outdoor pen using the percentage change from the baseline measurements obtained in the indoor pen. For each bird, I thus calculated the difference between the temperature at one body position (comb, eye or cheek) in the outdoor pen and the temperature at the same position obtained minutes earlier in the indoor pen on the same day. This difference was then expressed at a percentage of the indoor measurement. Negative values indicate a decrease in relative external body temperature in the outdoor pen with respect to baseline.
For each body position, I used a linear mixed model with group size (1 and 2) and timing of the external body temperature measurement (early or late in the trial) as within-subject factors. I relied on t-tests based on model least-squares means to determine whether the mean percentage change was significantly different from 0 adjusting the alpha level downward with the Benjamini-Hochberg procedure. For the effect of handling, I used a paired t-test to compare external body temperature at each body position before and after handling.
Vigilance
For the effect of group size on the three measures of vigilance, I used mixed linear models with bird id as a random factor and group size as a fixed factor. I used the delay (in minutes) from the onset of testing on a given day to the start of a trial as a co-factor to control for potential unevenness in feeding motivation across trials carried out successively on a given day. To normalize distributions, I used the logit transformation for time spent vigilant and the logarithm base 10 transformation for the frequency and duration of vigilance bouts.
To examine changes in vigilance as a function of time during a trial, I obtained the timing and duration of each vigilance bout and the timing and duration of each feeding bout during each trial. To examine how the duration of these bouts changed during a trial, I used a linear mixed model with the logarithm base 10-transformed duration of a bout as the dependent variable, the timing of that bout in the trial (in seconds) as a co-factor, and group size as the independent variable. Bird id and bird id nested within group size served as random factors to control for autocorrelation. Least-squares means (SEM) in the transformed units of analysis are shown below.
Results
Temperature
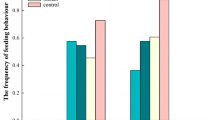
When birds foraged alone, external body temperature at the onset of a food trial was lower than at baseline for the comb (t test: t11 = − 3.7, P = 0.002), the eye (t test: t11 = − 3.8, P = 0.003), and the cheek (t test: t11 = − 4.4, P = 0.001) (Fig. 2). Later in the trial, external body temperature returned to baseline values for the comb (t test: t11 = − 1.9, P = 0.09), the eye (t test: t11 = − 0.66, P = 0.52), and the cheek (t test: t11 = − 0.76, P = 0.46).
The mean percentage change from baseline values in external body temperatures obtained at three different positions on the head when hens foraged alone or in pairs early or late in a food trial. Stars indicate deviations that were significantly different from the value of 0. Bars show one standard error
When birds foraged in pairs, external body temperature at the onset of a food trial did not differ from baseline for the comb (t test: t11 = − 1.1, P = 0.28), the eye (t test: t11 = − 1.2, P = 0.27) or the cheek (t test: t11 = − 2.7, P = 0.022; not significant after the adjustment) (Fig. 2). Later in the trial, the external body temperature remained at baseline values for the comb (t test: t11 = 0.23, P = 0.82), the eye (t test: t11 = 2.4, P = 0.037; not significant after the adjustment), and the cheek (t test: t11 = 1.3, P = 0.22).
Brief handling did not cause any significant changes in external body temperature for the comb (Paired t test: t11 = 0.46, P = 0.66), the eye (Paired t test: t11 = − 1.1, P = 0.31) or the cheek (Paired t test: t11 = 1.2, P = 0.25; Fig. 3).
Vigilance
No external disturbances occurred during the vigilance trials. Mean (SEM) time spent vigilant was higher when birds foraged alone [− 1.19 (0.17)] than in pairs [− 1.8 (0.18); ANOVA: F1,10 = 6.4, P = 0.03], controlling for the non-significant effect of delay (ANOVA: F1,10 = 0.57, P = 0.47). Mean (SEM) frequency of vigilance was higher when birds foraged alone [0.95 (0.042)] than in pairs [0.63 (0.044); ANOVA: F1,10 = 27.7, P = 0.0004], controlling for the non-significant effect of delay (ANOVA: F1,10 = 2.7, P = 0.13). Mean (SEM) duration of vigilance bouts did not differ in birds that foraged alone [0.12 (0.039)] or in pairs [0.21 (0.040); ANOVA: F1,10 = 2.7, P = 0.14], controlling for the non-significant effect of delay (ANOVA: F1,10 = 0.24, P = 0.64).
The duration of a vigilance bout increased during a food trial (ANOVA: F1,374 = 18.7, P < 0.0001; Fig. 4) with no overall effect of group size (ANOVA: F1,9 = 0.42, P = 0.53). The pattern of increase was not statistically different in the two different group sizes (ANOVA: F1,374 = 1.13, P = 0.29). The duration of a feeding bout did not vary during a food trial (ANOVA: F1,398 = 1.5, P = 0.23; Fig. 4), but was lower when birds foraged alone rather than in pairs (ANOVA: F1,9 = 25.7, P = 0.0007; Fig. 4). The effect of group size did not change during the duration of a trial (ANOVA: F1,398 = 1.8, P = 0.18). Together, these results show that birds maintained more vigilance alone than in pairs, and became more vigilant later in a food trial.
Discussion
Domestic fowls that foraged alone rather than in pairs showed higher vigilance and lower external body temperature during the early phases of a food trial. Later in a trial, external body temperature for solitary birds returned to baseline values despite an increase in vigilance. The results thus provide mixed support for an association between markers of fearfulness and predation risk.
A decrease in vigilance as group size increases has been noted in many species of birds and mammals [21, 22], including domestic fowls in a recent study [31]. Extra vigilance in smaller groups is costly for domestic fowls because feeding interferes with vigilance although some vigilance might be carried out while feeding [32]. This adjustment is vigilance is thus compatible with a food-safety trade-off. However, predation risk is not the only factor that can cause changes in vigilance in response to variation in group size [33]. As feeding competition increases in larger groups, models show that a decrease in vigilance is adaptive to increase the relative share of limited resources, and competition alone might explain why vigilance decreases with group size [33, 34]. Nevertheless, if competition acted alone, it is not clear why external body temperature would change at all (fear is not involved) and why it would increase as a trial progressed in solitary birds that are not competing at all. I conclude that variation in predation risk must be involved in the responses to changes in group size.
Changes in vigilance occurred as a food trial progressed suggesting dynamic adjustments in the trade-off between food and safety. Birds in this study allocated more time to vigilance in the later phases of a trial mainly by increasing the duration of vigilance bouts. Models typically predict a decrease in vigilance in the continued absence of threats over time [18, 19], and there is some support for this prediction [35,36,37,38]. However, domestic fowls showed the opposite pattern. A simple explanation is that birds became more vigilant because they were less hungry as the food trial progressed. State-dependent models of vigilance predict that more satiated animals should allocate more time to vigilance [39], which is in line with the present findings.
External body temperature proved sensitive to variation in group size at three different body positions. Earlier studies with domestic fowls subjected to handling or air puffs showed that external body temperature decreased substantially in these areas [24, 40]. Studies with other species also showed that external body temperature measured at different positions can decrease considerably in handled animals [26, 28]. Here, I showed that foraging alone was sufficient to decrease external body temperature by about 3 degree Celsius in the comb area and by about 1 degree Celsius in the eye area and the cheek. These changes in external body temperature reflect the effect of group size rather than handling because brief handling did not cause any short-term changes in external body temperature. In this study, the presence of only one companion was sufficient to bring back external body temperature to baseline values. External body temperature also increased during a trial in solitary birds, which is in line with the prediction that predation risk should become lower in the continued absence of threats during a trial.
Several physiological factors can alter external body temperature irrespective of predation risk. I corrected the effect of ambient temperature by using the percentage change from baseline. There were little changes in temperature between indoor and outdoor pens for each test, and all tests took place at the same time of day, ruling out circadian effects on body temperature [41]. Digestion can increase core body temperature in domestic fowls [41], but it should lead to a decrease and not an increase in external body temperature if any digestion took place at all over the brief trials. Head position of the birds varied between temperature measurements at baseline (head up) and during food trials (head down). In a previous study with domestic fowls, head position did not influence external body temperature in the comb area [24]. Eye temperature, however, was slightly higher when birds fed head down, which could potentially explain the smaller effect size of group size at that position. Nevertheless, changes in head position cannot explain temporal trends in external body temperature during a trial because all measurements in food trials were taken head down. Recent work suggests that anticipation of a reward, such as food in the present study, can cause a significant drop in external body temperature [42]. Because all birds, whether alone or in pairs, expected a food reward, this theory cannot explain why individuals in pairs did not show the same drop in external temperature as solitary birds. From this, I conclude that changes in external body temperature reflected primarily the effect of group size, and that the fearful state changed in dynamic fashion over a food trial.
The two markers of fearfulness were not consistent in their indications. Solitary individuals increased their vigilance during a trial, but their external body temperature returned to baseline values. Similar inconsistencies between vigilance and physiological markers of fear, such as heart rate and glucocorticoid production, are common in the literature (see [43] for a review). Here, I argue that vigilance measurements did not always match predation risk because domestic fowls were hungry, and vigilance in the early part of a trial interfered with the acquisition of resources.
While vigilance can be simpler to document in the field than physiological measurements, this study shows that vigilance might not always be sensitive to variation in predation risk. External body temperature, by contrast, closely matched predation risk, as manipulated by variation in group size, and tracked temporal variation in predation risk. External body temperature as a marker of fearfulness should be further evaluated in other species and in other contexts known to affect predation risk to determine its sensitivity and specificity.
Thermal imaging proved useful to track changes in external body temperature in unrestrained live birds, and should be considered in future studies. Although other species may not have areas on the body as convenient to record temperature as the patches without feathers in domestic fowl, results from this and other studies show that eye temperature can be a good marker. While it might be difficult to approach timid animals to get close-up images, it is possible to get individuals to approach the camera on their own to obtain temperature measurements [44].
Conclusions
Variation in predation risk induced changes in two putative markers of fearfulness, with external body temperature being more sensitive than vigilance. At least with respect to responses associated with temporal changes and variation in group size, I infer that an increase in predation risk was reflected by greater fearfulness.
References
Lank DB, Ydenberg RC. Death and danger at migratory stopovers: problems with "predation risk". J Avian Biol. 2003;34:225–8.
Lima SL. Nonlethal effects in the ecology of predator-prey interactions: what are the ecological effects of anti-predator decision-making? BioScience. 1998;48:25–34.
Clinchy M, Sheriff MJ, Zanette LY. Predator-induced stress and the ecology of fear. Funct Ecol. 2013;27:56–65.
Beauchamp G. Animal vigilance: monitoring predators and competitors. London: Academic Press; 2015.
Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol. 2004;67:259–84.
Harris BN, Carr JA. The role of the hypothalamus-pituitary-adrenal/interrenal axis in mediating predator-avoidance trade-offs. Gen Comp Endocrino. 2016;230–231:110–42.
Brown JS, Laundré JW, Gurung M. The ecology of fear: optimal foraging, game theory, and trophic interactions. J Mammal. 1999;80:385–99.
Stankowich T, Blumstein DT. Fear in animals: a meta-analysis and review of fear assessment. Proc R Soc Lond B Biol Sci. 2005;272:2627–34.
LeDoux JE. Coming to terms with fear. Proc Natl Acad Sci. 2014;111:2871–8.
Creel S, Winnie JA, Christianson D. Glucocorticoid stress hormones and the effect of predation risk on elk reproduction. Proc Natl Acad Sci. 2009;106:12388–93.
Gross CT, Canteras NS. The many paths to fear. Nat Rev Neurosci. 2012;13:651–8.
Broom DM. Sentience and animal welfare. Wallingford: CABI; 2014.
Beauchamp G. Social predation: how group living benefits predators and prey. New York: Academic Press; 2014.
Caro TM. Antipredator defenses in birds and mammals. Chicago: University of Chicago Press; 2005.
Bertram BCR. Living in groups: predator and prey. In: Krebs JR, Davies NB, editors. Behavioural Ecology. Oxford: Blackwell; 1978. p. 64–96.
Périquet S, Todd-Jones L, Valeix M, Stapelkamp B, Elliot N, Wijers M, Pays O, Fortin D, Madzikanda H, Fritz H, et al. Influence of immediate predation risk by lions on the vigilance of prey of different body size. Behav Ecol. 2012;23:970–6.
Creel S, Schuette P, Christianson D. Effects of predation risk on group size, vigilance, and foraging behavior in an African ungulate community. Behav Ecol. 2014;25:773–84.
Beauchamp G, Ruxton GD. Changes in anti-predator vigilance over time caused by a war of attrition between predator and prey. Behav Ecol. 2012;23:368–74.
Sirot E, Pays O. On the dynamics of predation risk perception for a vigilant forager. J Theor Biol. 2011;276:1–7.
Welp T, Rushen J, Kramer DL, Festa-Bianchet M, de Passillé A-M. Vigilance as a measure of fear in dairy cattle. Appl Anim Behav Sci. 2004;87:1–13.
Beauchamp G. What is the magnitude of the group-size effect on vigilance? Behav Ecol. 2008;19:1361–8.
Elgar MA. Predator vigilance and group size in mammals and birds: a critical review of the empirical evidence. Biol Rev. 1989;64:13–33.
Oka T, Oka K, Hori T. Mechanisms and mediators of psychological stress-induced rise in core temperature. Psychosom Med. 2001;63:476–86.
Edgar JL, Nicol CJ, Pugh CA, Paul ES. Surface temperature changes in response to handling in domestic chickens. Physiol Behav. 2013;119:195–200.
Herborn KA, Graves JL, Jerem P, Evans NP, Nager R, McCafferty DJ, McKeegan DEF. Skin temperature reveals the intensity of acute stress. Physiol Behav. 2015;152:225–30.
Ikkatai Y, Watanabe S. Eye surface temperature detects stress response in budgerigars (Melopsittacus undulatus). NeuroReport. 2015;26:642–6.
Edgar JL, Held S, Paul ES, Pettersson I, I'Anson Price R, Nicol CJ. Social buffering in a bird. Anim Behav. 2015;105:11–9.
Stewart M, Webster JR, Verkerk GA, Schaefer AL, Colyn JJ, Stafford KJ. Non-invasive measurement of stress in dairy cows using infrared thermography. Physiol Behav. 2007;92:520–5.
Lewden A, Nord A, Petit M, Vézina F. Body temperature responses to handling stress in wintering black-capped chickadees (Poecile atricapillus L.). Physiol Behav. 2017;179:49–54.
Richards SA. The significance of changes in the temperature of the skin and body core of the chicken in the regulation of heat loss. J Physiol. 1971;216:1–10.
Beauchamp G. Difficulties in monitoring conspecifics mediate the effects of visual obstruction on the level and synchronization of vigilance. Front Ecol Evol. 2017;5:12.
Rogers LJ, Zucca P, Vallortigara G. Advantages of having a lateralized brain. Proc R Soc Lond B Biol Sci. 2004;271:S420–2.
Beauchamp G, Ruxton GD. Changes in vigilance with group size under scramble competition. Am Nat. 2003;161:672–5.
Bednekoff PA, Lima SL. Risk allocation and competition in foraging groups: reversed effects of competition if group size varies under risk of predation. Proc R Soc Lond B Biol Sci. 2004;271:1491–6.
Beauchamp G. Timing of attacks by a predator at a prey hotspot. Behav Ecol Sociobiol. 2016;70:269–76.
Beauchamp G, Ruxton GD. Vigilance decreases with time at loafing sites in gulls (Larus spp.). Ethology. 2012;118:733–9.
Wheeler HC, Hik DS. Giving-up densities and foraging behaviour indicate possible effects of shrub encroachment on arctic ground squirrels. Anim Behav. 2014;95:1–8.
Trouilloud W, Delisle A, Kramer DL. Head raising during foraging and pausing during intermittent locomotion as components of antipredator vigilance in chipmunks. Anim Behav. 2004;67:789–97.
McNamara JM, Houston AI. Evolutionarily stable levels of vigilance as a function of group size. Anim Behav. 1992;43:641–58.
Edgar JL, Lowe JC, Paul ES, Nicol CJ. Avian maternal response to chick distress. Proc R Soc Lond B Biol Sci. 2011;2010(2701).
Savory CJ, Kostal L, Nevison IM. Circadian variation in heart rate, blood pressure, body temperature and EEG of immature broiler breeder chickens in restricted-fed and ad libitum-fed states. Brit Poultry Sci. 2006;47:599–606.
Moe RO, Stubsjøen SM, Bohlin J, Flø A, Bakken M. Peripheral temperature drop in response to anticipation and consumption of a signaled palatable reward in laying hens (Gallus domesticus). Physiol Behav. 2012;106:527–33.
Beauchamp G. What can vigilance tell us about fear? Animal Sentience. 2017;15.
Jerem P, Herborn K, McCafferty DJ, McKeegan DEF, Nager R. Thermal imaging to study stress non-invasively in unrestrained birds. J Vis Exp : JoVE. 2015;53184.
Acknowledgements
I thank Martine Boulianne for lending the thermal imaging equipment and Éric Parent for operating tips, and two anonymous reviewers for useful comments.
Availability of data
All data are provided in Additional file 1.
Funding
There was no funding for this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Animal Care Committee of the Faculty of Veterinary Medicine, University of Montréal, Canada.
Consent for publication
Not applicable for this paper.
Competing interests
The author declares that he has no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Table S1. Data used for this project in excel format. (XLSX 42 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Beauchamp, G. External body temperature and vigilance to a lesser extent track variation in predation risk in domestic fowls. BMC Zool 4, 1 (2019). https://doi.org/10.1186/s40850-019-0039-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40850-019-0039-8