Abstract
Background
Cystic fibrosis (CF) is a life-limiting genetic condition in which daily therapies to maintain lung health are critical, yet treatment adherence is low. Previous interventions to increase adherence have been largely unsuccessful and this is likely due to a lack of focus on behavioural evidence and theory alongside input from people with CF. This intervention is based on a digital platform that collects and displays objective nebuliser adherence data. The purpose of this paper is to identify the specific components of an intervention to increase and maintain adherence to nebuliser treatments in adults with CF with a focus on reducing effort and treatment burden.
Methods
Intervention development was informed by the Behaviour Change Wheel (BCW) and person-based approach (PBA). A multidisciplinary team conducted qualitative research to inform a needs analysis, selected, and refined intervention components and methods of delivery, mapped adherence-related barriers and facilitators, associated intervention functions and behaviour change techniques, and utilised iterative feedback to develop and refine content and processes.
Results
Results indicated that people with CF need to understand their treatment, be able to monitor adherence, have treatment goals and feedback and confidence in their ability to adhere, have a treatment plan to develop habits for treatment, and be able to solve problems around treatment adherence. Behaviour change techniques were selected to address each of these needs and were incorporated into the digital intervention developed iteratively, alongside a manual and training for health professionals. Feedback from people with CF and clinicians helped to refine the intervention which could be tailored to individual patient needs.
Conclusions
The intervention development process is underpinned by a strong theoretical framework and evidence base and was developed by a multidisciplinary team with a range of skills and expertise integrated with substantial input from patients and clinicians. This multifaceted development strategy has ensured that the intervention is usable and acceptable to people with CF and clinicians, providing the best chance of success in supporting people with CF with different needs to increase and maintain their adherence. The intervention is being tested in a randomised controlled trial across 19 UK sites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Key messages regarding feasibility
-
In order to develop an intervention to increase adherence to treatment in people with cystic fibrosis (CF), we needed to develop an intervention using behavioural science theory and evidence and informed by people from our target population.
-
We developed and refined a complex intervention underpinned by a strong theoretical framework and evidence base. The CFHealthHub intervention is usable and acceptable to people with CF, providing support for people with CF with different needs to increase and maintain their adherence.
-
We have a digital platform, intervention manual, and training package for use in the main trial of the CFHealthHub intervention
Background
Cystic fibrosis (CF) is an inherited genetic condition that affects approximately 10,500 people in the UK and 100,000 worldwide [1]. The condition causes the build-up of thick sticky mucus in the digestive system and lungs which can result in recurrent lung infections, progressive lung damage, and respiratory failure [2]. People with CF require a time-consuming regimen of treatment in order to maintain their health [3, 4].
There are effective inhaled treatments for CF, usually delivered via a nebuliser, that include antibiotics to reduce infections and mucolytics to thin mucus and to keep airways clear. However, consistent with other long-term conditions, adherence to nebuliser treatments is low [5,6,7]. Low adherence is associated with increased lung damage and additional need for treatment with intravenous (IV) antibiotics, with higher associated treatment costs [6, 8,9,10,11], and significant impacts on quality of life [12]. There is a need for effective interventions to increase adherence to treatment in this population.
Interventions have so far shown limited success in increasing adherence in people with CF [13,14,15]. There are a number of potential reasons for this. First, the interventions may not be targeting the most appropriate factors [16]. Second, there is a lack of studies using a theory and evidence-based approach [17]. Third, interventions may assume that one-size fits all despite evidence that the factors affecting adherence may be person-specific [18]. Even where there have been reported successes, adherence outcomes have not been measured objectively and therefore the findings may not be reliable. Adherence is often measured by either self-report or medicine possession ratio (MPR). In the UK, objective estimates of median adherence are in the region of 36% [5], whereas MPR for inhaled therapy are in the region of 65% [11] and self-report around 80% [5, 11]. To be sure of success, we need to be able to assess the impact of an intervention on sensitive, objectively measured adherence.
With the advent of nebuliser devices (eTrack™, Pari and I-neb™, Phillips Respironics) that record time and date stamped treatments and support data transfer, we now have a means to capture objective treatment adherence data. This is important not only as an outcome measure for any intervention, but also to inform patients and clinicians of current adherence, given evidence of the effectiveness of feedback in order to change adherence behaviour [19, 20]. A key aim of the research programme was to develop a digital platform that could capture and display objectively measured nebuliser adherence and ‘make adherence visible’ and then to develop an associated behaviour change intervention to promote and support increases in adherence and the maintenance of adherence in the longer term. This paper describes the process of the development of the CFHealthHub digital platform and the associated behaviour change intervention to support adherence to nebulised treatment in adults with CF.
Intervention development approach
The approach to intervention development that we employed was the combined approach identified in a taxonomy of intervention development; a ‘theory and evidence-based approach’ with a ‘target population-based’ approach [21]. The Behaviour Change Wheel (BCW) [22, 23] is a theory and evidence-based approach [20] selected because of the need to change the behaviour of people with CF. The person-based approach (PBA) to intervention development [24] is a target population-based approach [21] in which feedback from the target population is collected. It is complementary to BCW [24] and has been previously used alongside the BCW [25].
The BCW was devised following a systematic evaluation and synthesis of 19 frameworks of behaviour change and considers Capability, Opportunity, and Motivation in relation to Behaviour (COM-B model) [22]. The approach follows three stages: (i) Understanding the behaviour, identifying clear and specific target behaviours, and analysing the factors that impact on that behaviour and the need for change. This stage often uses the complementary Theoretical Domains Framework (TDF) [26, 27] that specifies 14 key domains from 33 behaviour change theories, that each influence capability, opportunity, or motivation [22, 28]. (ii) Identifying intervention functions and policy categories, i.e. ways to enact interventions, to achieve behaviour change, and (iii) identifying specific behaviour change techniques (BCTs), i.e. the specific active ingredients to change behaviour as described in the behaviour change technique taxonomy [29], and modes of delivery. The approach incorporates a systematic assessment of the available options and choices and has been widely used in the development of behaviour change interventions in settings including adherence (e.g. [30, 31]).
The PBA was devised from experience of developing digital interventions and utilises mixed methods with people from the target population to inform all stages of the intervention development in an iterative process. Given that we started with a plan to include a digital platform to display adherence data, PBA was an appropriate approach to the development of this digital intervention.
Conceptual framework and aims of the intervention
We started this intervention development process with some initial ideas about what the intervention might include and the kinds of resources we might have to deliver it. Early work by the team using quality improvement and process mapping [32] had highlighted the need for objective data on medication adherence in CF, and we explored how this could positively impact on clinical practice [33,34,35]. Therefore, we aimed to develop a digital platform that could capture and display objective nebuliser adherence data to patients and clinicians. We understood the important role that the clinical teams play in CF and that adherence support and therefore intervention delivery would be supported by a trained healthcare professional [34].
Early work by members of the team [36, 37] had also considered barriers to adherence in CF and this fed into a conceptual framework of the broad factors influencing adherence and how an intervention might act on these factors to produce and then maintain change. A particular focus of this early work was a consideration of how adherence could be maintained without increasing perceived effort or burden. This conceptual framework drew on the COM-B model and also on other models of adherence and behaviour change and is presented in Fig. 1 (and see [22] p. 81).
The conceptual framework proposes that adherence behaviour is influenced by reflective motivation, i.e. a rational weighing up of the perceived necessity against the perceived concerns about treatment [38]. For some people, an intervention would need to address motivation before any other strategies could be successful since without this people with CF would not start to initiate attempts to adhere to treatment. Those who want to increase adherence to treatment will make attempts to do so but in many cases these attempts will be hindered by a range of capability and opportunity barriers. An intervention needs to support people to overcome these barriers so that they can adhere to their treatment. Self-regulation is one way in which people can sustain the life-long adherence to preventative inhaled treatment required to maintain lung health. However, there is evidence that self-regulation is difficult to maintain [39] and requires effortful self-control [40] and self-regulatory capacity [41]. Habit theory [42] proposes that habits formed through regular repetition of a specific behaviour in response to a cue over time (initially maintained through self-regulation) comes to trigger the behaviour (automatic motivation) such that habit strength then predicts the likelihood of the behaviour and motivation-driven self-regulation becomes less important. Habits have been proposed to be one of the key mechanisms by which behaviour change can be maintained in the longer term [43] with less perceived effort and burden, and thus a key aim of the intervention is to promote habit formation.
Having a conceptual framework from the start of the project provided a structure that guided intervention development. We recognised the potentially important role of capability, opportunity, and motivation and the overall aim of the intervention. However, we needed to understand the specific barriers and facilitators to adherence for people with CF and how we could best develop an intervention within this conceptual framework that would enable people to adhere and make habits.
Methods
The team
The core intervention development team included people with different perspectives, skills, and expertise: MA is a health psychologist with expertise in the development of theory-based behaviour change interventions. PW is a computer scientist and health informatician with expertise in the design and development of digital health platforms. SD and AOC are health services researchers with experience in undertaking qualitative research with behaviour change interventions. MH and JB are research physiotherapists with expertise in respiratory health and supporting patients with CF with their adherence. MW is a consultant in respiratory medicine working with adults with CF and with expertise in quality improvement. DB has cystic fibrosis and is a health services researcher and co-ordinated the patient and public involvement (PPI) group.
Dynamic and iterative approach
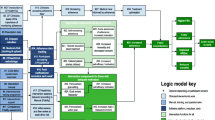
Intervention development is not a simple linear process. Different methods and actions are taken at different stages but they are used in a dynamic way in that they overlap and are revisited throughout the process [44]. The team followed an intervention development process with stages that fed into each other as illustrated in Fig. 2. Software development used the Agile process [45]. This involved the continuous delivery of working software to meet the shifting requirements identified by the intervention development team. The process required close collaboration between the technical and intervention development teams.
Ethical approval was gained for all studies [REC references: 15/YH/0332; 15/WS/0089] and all participants gave written informed consent.
Stages of development
There are seven identified domains of actions taken across different approaches to intervention development: conception, planning, designing, creating, refining, documenting, and planning for future evaluation [21].
Stage 1: Planning
Understanding practicalities of delivery
Input from members of the team working in the NHS context enabled us to understand that the intervention would need to be delivered flexibly by different members of the multidisciplinary team (MDT) or by health professionals recruited from outside of the MDT, due to NHS shortages in staff [46]. Thus, the intervention that was developed needed to be able to be delivered by a range of health professionals in order to ensure that future implementation was feasible.
Understanding the behaviour
We undertook a needs analysis for the intervention informed by the following.
Literature review
We reviewed the literature to identify key barriers to nebuliser adherence in adults with CF. This included a systematic review of qualitative studies [47], and we updated our knowledge with key papers published during the course of the development phase (e.g. [48]).
Qualitative research with patients
We conducted semi-structured interviews with 18 adults with cystic fibrosis from a single CF centre in the UK sampled by objective adherence, gender, age, and deprivation index. The data-prompted interviews [49] included the presentation of a graph showing each person their nebuliser adherence data over the last 6 months. The topic guide was informed by the literature review and based around understanding adherence in the context of cystic fibrosis and life in general, and the COM-B model [22] and TDF [26]. The data from these interviews were analysed using two different approaches; a framework analysis using the TDF, including a comparison of factors identified by higher and lower adherence [50], and a discursive analysis [51].
Survey with health professionals
We consulted health professionals to understand their perceptions of the barriers to adherence and possible solutions to address those barriers. Fourteen clinicians working at five adult CF centres across the UK were sent an email survey which was completed and returned by six clinicians.
PPI
Findings and interpretation of the interviews were provided to the patient co-applicant who led the PPI group to ensure that they were plausible and realistic. DB was involved from a very early stage in discussions around the proposed intervention, including the rationale for its use, as well as the design and functionality of the proposed website.
Stage 2: Designing and creating the prototype intervention
Stage two of the intervention development process involved the development of a prototype intervention. There were two parts to the CFHealthHub intervention: (i) the digital platform displaying adherence data and online content and tools and (ii) the interventionist-delivered aspects of the intervention delivered during contact with a health professional. Frequent meetings of the intervention development team, informed by parallel discussions of the patient and public involvement group, were held during stages 2 and 3. At these meetings, we discussed input from each of the following activities.
Design of the prototype intervention using the Behaviour Change Wheel approach
Following the Behaviour Change Wheel approachFootnote 1, we mapped intervention functions and behaviour change techniques to the identified needs of the intervention. Options were considered and discussed during meetings of the intervention development team, and decisions were informed by the APEASE criteria [23]: affordability refers to the cost of the intervention, which must be within budget; practicability refers to the extent to which the intervention can be delivered as designed to the target population; effectiveness/cost-effectiveness refers to effectiveness of the intervention in a real-world context in relation to that which is most cost-effective; acceptability refers to the extent to which the intervention is judged to be appropriate by different stakeholders; side-effects/safety includes unintended consequences of the intervention; and equity refers to the extent to which the intervention impacts on disparities in living standards, health, and wellbeing. We also considered the mode of delivery of each of the behaviour change techniques, whether they were delivered via the digital platform or whether they were delivered by a health professional acting as an interventionist. MH, a physiotherapist experienced in the delivery of adherence support in CF care, informed the development of the interventionist-delivered components.
Design of the CFHealthHub digital platform
The first phase of technical development was to develop the process and mechanisms by which inhalation data (time-stamped nebulisations) could be automatically captured from third party devices and software, transferred securely, and displayed in a usable way on a digital platform in relation to prescription data (i.e. treatment taken/treatment prescribed). The data transfer flow is shown in Fig. 3. From the eTrack nebuliser, inhalation data was automatically sent to a Qualcomm Life 2net Hub located in the participant’s home. Data was transferred from the Qualcomm Hub to a secure server maintained by Pari and then forwarded on to the CFHealthHub server for display and use in the CFHealthHub digital platform. Data transfer was in real-time and required no additional actions by the participant over and above normal nebuliser usage, assuming all devices maintained connectivity with the required networks. This phase also involved a 6-week development and testing phase, where the data transfer mechanism was tested, and the data quality of the transmitted data was validated (see Fig. 2, prototype intervention 1). This was refined over a number of iterations. The need for specific content and tools that arose from the intervention development work fed into further technical developments and prototype releases.
Stage 3: Iterative refinement of the prototype using the person-based approach
Five participants were people with CF who were aged 16 or older, on the CF registry, provided with an eTrack nebuliser and Qualcomm hub, and given access to the CFHealthHub platform in order to assess the ability of the system to successfully record and display nebulisations. They were followed up after 1 week to troubleshoot any data upload issues and interviewed after a period of 1 month to give them time to use the prototype intervention.
Twenty-two participants were recruited into the iterative development study. Participants were people with CF who were aged 16 or older, on the CF registry, and willing to take inhaled mucolytics via a chipped nebuliser (eTrack). They were provided with an eTrack nebuliser and Qualcomm hub. They received four sessions of intervention delivery from a physiotherapist and were given independent access to the CFHealthHub web platform. We conducted 18 semi-structured telephone interviews with participants in different cycles of the software development (see Fig. 2, protoypes 1–5) to ask about acceptability of the appearance and functionality of the digital platform and potential improvements. Additionally, we conducted six in-depth think aloud interviews [52, 53] with participants whilst they were using a version of the CFHealthHub website. The screen and audio of the interview was recorded using Camtasia™ software. This allowed the software team to identify technical and navigational issues with the website that were corrected in subsequent releases. We also interviewed the physiotherapist delivering the intervention at two time points for wider views about how to deliver the intervention to patients and how that linked to the clinician view of the website.
PPI
Input was provided by DB and wider PPI reference group throughout the early development phase of the intervention. Initially, this involved providing input into the proposed content for the textual parts of the website, in terms of the type and level of information that was felt appropriate, not only for people with CF, but others involved in their care.
As the digital platform was developed, PPI input was again provided at regular intervals. Members of the PPI reference group were given the opportunity, on a number of separate occasions, to explore iterations of the website through a demonstration version of the website. Feedback was then provided in meetings of the group, which was then passed back to the wider study team.
Aside from input on the design, group members also provided comment on practical issues around data sharing within the website, and the user guide that had been produced to accompany it.
Stage 4: Documenting the intervention
At the end of this process, in readiness for the pilot trial, we created an intervention manual that outlined the key components of the intervention, how to use the CFHealthHub digital platform, and the manner and structure of delivery, and an associated training programme for interventionists as well as a user guide for participants.
Stage 5: Further refinement of the intervention following piloting
Whilst descriptions of the intervention development process often stop before piloting and feasibility testing [44, 54], we utilised the pilot and feasibility study [55] to identify further refinements that were made to the intervention before it was used in the final randomised controlled trial.
The pilot and feasibility study consisted of a mixed methods process evaluation undertaken concurrently with a pilot RCT in two UK CF centres. Participants were people with CF who were aged 16 or older, on the CF registry, and willing to take inhaled mucolytics and/or antibiotics via an eTrack nebuliser. Three interventionists were trained to deliver the intervention in six face-to-face meetings over 5 months to 32 participants who had access to the CFHealthHub (CFHH) website throughout. We conducted 25 semi-structured face-to-face interviews with patients in the intervention arm of the RCT (n = 14), interventionists delivering the intervention (n = 3 at 2 time points), and members of the wider multidisciplinary team (MDT) (n = 5).
The findings from the quantitative [55] and qualitative [56] aspects of the study were triangulated [57] and the implications for the further refinement of the intervention discussed by the development team with input from PPI representatives. As the software was more mature at this stage, changes became more costly in terms of implementation effort and therefore regular prioritisation meetings were conducted where the team agreed on which requirements would be implemented. Decisions about which work to prioritise were made using the MoSCoW criteria of prioritisation: Must have, Should have, Could have, and Won’t have [58].
A full description of the intervention was written following the TiDieR (Template for Intervention Description and Replication) checklist [59].
Results
Stage 1: Planning the intervention
The research confirmed that different factors influenced different people’s ability to adhere and it was therefore important, for reasons of equity, to develop an intervention that addressed multiple capability, opportunity, and motivation barriers to adherence. Our behavioural needs analysis enabled us to identify the factors that the intervention needed to address (see Table 1) and the team considered and discussed whether the intervention should be designed to address each of these needs. Three domains were excluded at this stage (see Table 1 for rationale).
Stage 2: Designing and creating the intervention
The stage 1 analysis indicated that a number of needs were replicated across different TDF domains (e.g. need to address treatment concerns), and the intervention development team therefore generated ‘themes of need’ for the intervention (see Table 2). The selection of intervention functions matched to each theme of needs is described in Table 2 along with the reasons for inclusion/exclusion according to the APEASE criteria. Table 2 also displays the selection of behaviour change techniques (BCTs) to match the needs and intervention functions selected. BCTs that were considered but rejected by the team according to the APEASE criteria are also shown.
Discussions about the intervention considered the needs of different types of patients with different barriers to adherence, as indicated in the analysis of the stage 1 qualitative work. We therefore considered how the intervention could be tailored to meet the needs of individuals and to reduce the possibility of overwhelming an individual with lots of BCTs that might not be useful or relevant. There were two key aspects of tailoring that we incorporated early on: modules of content and paths through the intervention. In relation to the need to understand treatment, people had a range of necessity and concern beliefs about the treatments that they had been prescribed, and we only needed to address their specific beliefs related to lower adherence. To address this, we identified the need for a personalised area where we could locate specific targeted and tailored content which we named the Toolkit. Participants could access their toolkit directly from the home page. Educational and persuasive content was grouped into six themed modules of content informed by the literature and our qualitative work, e.g. Why is it important that I do my nebuliser treatments every day? and I have concerns about my nebuliser treatments. We devised an algorithm to automatically prioritise up to three modules of content for each participant based on their responses to matched items in the BMQ-SpecificFootnote 2 [38]. For example, a ‘strongly disagree’ response to the beliefs statement, This nebuliser treatment protects me from becoming worse, made it more likely that they would be matched to the I’m not convinced that my nebuliser treatment works module. Interventionists could override and change modules if discussions with participants indicated other or changed priorities over the course of intervention delivery identified during review sessions. Following consultations, interventionists could also select specific modules of problem-solving content and videos matched to the specific needs of the participant and place these in the Toolkit area.
The educational/persuasive content of the modules was created to ensure that the information was accessible and meaningful to people with different needs and different perceptions of ‘credible sources’. It included simple animated videos of treatment action, patient stories and links to external websites (e.g. CF Trust, NHS) and Cochrane reviews about drug treatments. All of the content was reviewed by the PPI groups and feedback was sought throughout the iterative development process.
We understood that setting specific goals and plans for treatment would only be effective if people were sufficiently motivated to increase their adherence, and if not, then the intervention should follow a different path that replaced goal setting/planning with ensuring a need to understand treatment and confidence building using the modules and videos available on CFHealthHub and open, non-judgmental discussions with patients.
Some aspects of the intervention were less about the tools and content of the CFHH website and more about how the interventionists interacted with participants. For example, the self-efficacy intervention components required that interventionists focused on times when treatment had been taken rather than times of non-adherence during discussions about treatment graphs.
Stage 3: Refining the intervention
Feedback from discussions about the behaviour change techniques and the iterative development study fed into both the technical development of the CFHealthHub digital platform and how the intervention would be delivered by health professionals (the interventionist-delivered components). The versions of CFHealthHub that the participants received changed over time as new content and tools were added into the digital platform (see Table 3). Table 4 provides the main feedback from participants and interventionists from this process along with how these were responded to in the development process.
Stage 4: Documenting the intervention
The final intervention was documented in a manual for interventionists. This included the following sections that focused on motivating health professionals about the value of the intervention as well as the knowledge and skills that they needed to deliver it:
-
Description of the overall intervention and the rationale for its development
-
Orientation to the CFHealthHub digital platform including the data displays, behaviour change content and tools, and how to use them
-
Plans for different types of intervention delivery sessions including how to prepare for them
-
Information about how to tailor the intervention to suit different patients’ needs
-
Intervention delivery using a person-centred approach
An associated training programme for interventionists was developed.
Stage 5: Further refinement following piloting
The outcomes of the pilot and feasibility study are described in detail elsewhere [55, 56]. Key outcomes that fed into further development of the intervention and how they were responded to in subsequent developments are described in Table 5, and the final intervention that was used in the randomised controlled trial are described in Table 6.
The intervention manual was revised to address the identified need for change and to include information about the CFHealthHub mobile apps (iOS and Android) and associated functions. We developed worksheets for interventionists to follow during delivery of the intervention sessions. These worksheets included step by step instructions about how to interact with the CFHH content and the participant and included hints about how to phrase questions. An example worksheet for the first intervention visit is included in supplementary files. The associated training programme for interventionists was revised to be delivered in 2 face-to-face training days with 4 days of independent online training delivered via a virtual learning environment and ongoing tutorial support.
Modifications and adjustments to how the intervention was tailored and personalised were also considered and these are described in Tables 7 and 8.
Discussion
The CFHealthHub intervention which comprised a digital platform and delivery by a health professional (with an associated manual and training) was developed through a rigorous and systematic development process. It was shown to be usable and acceptable to people with CF and the clinical community. Our development work demonstrated that inhalation data could be automatically transferred from a third party nebuliser device and displayed, in combination with prescription data, to provide visibility of a participant’s adherence in real-life settings. Participants and clinicians were able to understand and interpret the data display quickly and easily. The intervention includes behaviour change techniques to address adherence to nebuliser treatment in people with CF, which we have been able to tailor and personalise, so that it is appropriate for people with a range of different barriers to adherence.
A key value of our approach is that it has incorporated theory- and evidence-based approaches (Behaviour Change Wheel [22, 23]) and target population-based approaches (person-based approach [24, 31]) [21]. Throughout the development process, we have been responsive to feedback and have changed and refined the intervention and the way in which it is delivered. The iterative process allowed issues to be identified quickly and updated rapidly, often during the early design phases, which minimised the cost and resources required to make the changes. Whilst pilot and feasibility studies are often considered to be outside of the intervention development process [44, 54], we utilised this opportunity to make further refinements to the intervention and its implementation in readiness for the full randomised controlled trial. We anticipate that the refinement process will continue following the randomised controlled trial and process evaluation in which it is currently being tested (trial registration, ISRCTN55504164) to inform implementation in clinical practice (trial registration, ISRCTN14464661).
Models for intervention development offer a pathway for intervention development but not a solution. Multiple decisions have to be made along the way, and the way in which they are made and the rationale behind those decisions depends in part on the intervention development team and their skills, experiences and expertise. The APEASE criteria [22] informed decisions and MoSCoW [58] enabled us to prioritise later changes, but the information that fed into the assessment of those criteria required the expertise of the development team. Ideas that seemed practical from the perspective of a health psychologist were not always practical from the perspective of a computer scientist. In addition, in terms of the likely effectiveness of the selected components, this required reference to our conceptual framework (see Fig. 1) and a good understanding of the theories and principles that underpinned different aspects of it; thus, the intervention drew on a range of different theories and evidence. The My treatment module (see Table 7) reflected the necessity-concerns framework [38], given that knowledge and beliefs about the consequences of treatment largely related to perceptions about the necessities and concerns for nebuliser treatment and social cognitive theory [60, 61] in terms of the role of outcome expectancies in behaviour. Confidence building also drew on social cognitive theory, employing a number of established strategies to increase self-efficacy (mastery, vicarious experiences, etc.). The self-monitoring, goal setting and planning modules drew on control theory [62], to explain how increased awareness of adherence behaviour and the identification of a discrepancy between current behaviour and goal might result in self-regulated behaviour change through action planning. Habit theory [42, 63] influenced the structure of action planning used in the intervention, i.e. the identification of a cue or prompt for nebuliser treatment and the use of an implementation intention [64] if cue then nebuliser treatment-based action plan. Coping plans were used as part of problem solving [65] in order to address the issue of capability and opportunity barriers to treatment adherence and to help maintain adherence [43].
The development process was highly dependent on multidisciplinary working involving a team comprised of academics, clinicians, patients with CF, research software engineers, and UX designers. There were varying levels of understanding and experience of each other’s roles, working practices, and workload. The initial model was based on obtaining and analysing participant feedback, agreeing design and software development requirements followed by development sprints, giving a new release of CFHH for each cycle of participants. However, difficulties in providing qualitative feedback in a timely manner identified previously [66] and the time required for design changes, programming, and testing meant this was not a viable working model. Regular meetings to facilitate clear communication, less technical and clinical language, and patience were required in order to develop the intervention, and many of the desired changes required significant development time such that some of the changes identified in stage 2 of the process were not actually included in the CFHealthHub digital platform until the beginning of the RCT. We recommend that future projects have early and ongoing team discussions so that expectations from all involved parties are realistic.
Limitations
There are some limitations of the approach. The data that informed stages 1 and 3 of the process stemmed from patients and clinicians from a single CF centre, and in stage 5 (the pilot study), from a further two centres and their associated clinical teams. This means that the intervention was based on a relatively small sample, and it is feasible that these centres may have differed from others in key ways, not least of which might have been high levels of motivation, and the clear commitment of the principal investigators at each site. The intervention development process was driven by a particular development team, made up of a particular mix of skills and expertise, and it is conceivable that a different team with different members may have made different decisions and arrived at a very different intervention. Whilst the input from patients, clinicians, and the multidisciplinary team was valuable, the process was resource and time intensive.
The intervention that we have developed is a complex one with multiple components tailored to meet the needs of different patients. Whilst this is a potential strength, it is also a potential limitation in that intervention delivery is quite long, and selection of the appropriate components for a particular patient relies on the skills and training of the interventionist. An alternative approach would have been to focus on one aspect of need (e.g. planning and habit formation) and develop an intervention just focused on these components. However, this would have meant that the intervention was not suitable for patients with lower motivation. Given that this group is of particular concern to clinicians (because they tend to be the least well), we felt that it would not be equitable or acceptable to design an intervention which did not target adherence increases across different patient groups. Following the RCT, we will be able to undertake analysis to explore which aspects of the intervention produced the intended changes in process outcomes and which did not, and if there are particular groups of patients for whom the intervention worked more or less well. This will enable us to pare down and refine the intervention further so that in the future, the intervention can be more tailored and can incorporate just those components found to be successful in improving adherence and this will likely reduce the length and complexity.
The initial qualitative work undertaken in stage 1 indicated that for patients, nebuliser use was seen as an integral part of their CF treatment alongside chest physiotherapy, diet, and enzymatic treatment for digestive issues, and for many patients, other treatments for co-morbid conditions including diabetes and liver conditions. It was beyond the scope of this programme of work to develop a system that could support adherence to all of these aspects of care.
Conclusion
We have devised an intervention to increase adherence to nebuliser treatment in adults with CF with substantial input from patients and clinicians and which has a strong theoretical and evidence base. The intervention comprised a digital platform (www.cfhealthhub.com) and components delivered in patient consultations with an interventionist. It is usable and acceptable to people with CF and clinicians. The intervention is currently being tested in a randomised controlled trial across 19 UK sites.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Notes
We omitted the selection of policy categories stage of the BCW process as the decision to focus on service provision had been made previously during the development of the programme aims.
We also included an additional belief item identified as important in our qualitative work: Nebulised antibiotic treatments are more important for my health than IV antibiotics
Abbreviations
- APEASE:
-
Affordability, Practicability, Effectiveness/Cost-effectiveness, Acceptability, Side-effects/Safety, Equity
- BCTs:
-
Behaviour change techniques
- BCW:
-
Behaviour Change Wheel
- CF:
-
Cystic fibrosis
- CFHH:
-
CFHealthHub
- COM-B:
-
Capability, opportunity, and motivation—behaviour
- MDT:
-
Multidisciplinary team
- MoSCoW:
-
Must have, should have, could have, won’t have
- PBA:
-
Person-based approach
- PPI:
-
Patient and public involvement
- RCT:
-
Randomised controlled trial
- TiDieR:
-
Template for Intervention Description and Replication
- TDF:
-
Theoretical domains framework
References
Cystic Fibrosis Trust [Internet] London, UK: What is cystic fibrosis? [cited 2019 Nov 18] Available from https://www.cysticfibrosis.org.uk/what-is-cystic-fibrosis.
O’Sullivan B, Freedman S. Cystic fibrosis. The Lancet. 2009;373(9678):1891–904.
Sawicki G, Sellers D, Robinson W. High treatment burden in adults with cystic fibrosis: challenges to disease self-management. Journal of Cystic Fibrosis. 2009;8(2):91–6.
Sawicki G, Tiddens H. Managing treatment complexity in cystic fibrosis: challenges and opportunities. Pediatric Pulmonology. 2012;47(6):523–33.
Daniels T, Goodacre L, Sutton C, Pollard K, Conway S, Peckham D. Accurate assessment of adherence. Chest. 2011;140(2):425–32.
Eakin M, Bilderback A, Boyle M, Mogayzel P, Riekert K. Longitudinal association between medication adherence and lung health in people with cystic fibrosis. Journal of Cystic Fibrosis. 2011;10(4):258–64.
Hoo ZH, Curley R, Walters SJ, Campbell MJ, Wildman MJ. Exploring the implications of different approaches to estimate centre-level adherence using objective adherence data in an adult cystic fibrosis centre–a retrospective observational study. J Cystic Fibrosis. 2019.
Briesacher BA, Quittner AL, Saiman L, Sacco P, Fouayzi H, Quittell LM. Adherence with tobramycin inhaled solution and health care utilization. BMC pulmonary medicine. 2011 Dec;11(1):5.
Hoo Z, Campbell MJ, Curley R, Wildman MJ. Rescue and prevention in cystic fibrosis: an exploration of the impact of adherence to preventative nebulised therapy upon subsequent rescue therapy with IV antibiotics in adults with CF. InJournal of Cystic Fibrosis. 2016;15(Suppl 1):S120 Elsevier.
Quittner AL, Zhang J, Marynchenko M, Chopra PA, Signorovitch J, Yushkina Y, Riekert KA. Pulmonary medication adherence and health-care use in cystic fibrosis. Chest. 2014 Jul 1;146(1):142–51.
White H, Shaw N, Denman S, Pollard K, Wynne S, Peckham DG. Variation in lung function as a marker of adherence to oral and inhaled medication in cystic fibrosis. European Respiratory Journal. 2017 Mar 1;49(3):1600987.
Quittner AL, Saez-Flores E, Barton JD. The psychological burden of cystic fibrosis. Current opinion in pulmonary medicine. 2016 Mar 1;22(2):187–91.
Goldbeck L, Fidika A, Herle M, Quittner A. Psychological interventions for individuals with cystic fibrosis and their families. Cochrane Database of Syst Rev. 2014.
Quittner AL, Eakin MN, Alpern AN, Ridge AK, McLean KA, Bilderback A, Criado KK, Chung SE, Riekert KA. Clustered randomized controlled trial of a clinic-based problem-solving intervention to improve adherence in adolescents with cystic fibrosis. Journal of Cystic Fibrosis. 2019.
Jones S, Curley R, Wildman M, Morton RW, Elphick HE. Interventions for improving adherence to treatment in cystic fibrosis. Cochrane database of systematic reviews. 2015;4.
Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. Bmj. 2008 Sep 29;337:a1655.
Michie S, Prestwich A. Are interventions theory-based? Development of a theory coding scheme. Health psychology. 2010 Jan;29(1):1.
Easthall C, Barnett N. Using theory to explore the determinants of medication adherence; moving away from a one-size-fits-all approach. Pharmacy. 2017 Sep;5(3):50.
Ivers N, Jamtvedt G, Flottorp S, Young JM, Odgaard-Jensen J, French SD, O’Brien MA, Johansen M, Grimshaw J, Oxman AD. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane database of systematic reviews. 2012;6.
Ivers NM, Grimshaw JM, Jamtvedt G, Flottorp S, O’Brien MA, French SD, Young J, Odgaard-Jensen J. Growing literature, stagnant science? Systematic review, meta-regression and cumulative analysis of audit and feedback interventions in health care. Journal of general internal medicine. 2014 Nov 1;29(11):1534–41.
O’Cathain A, Croot L, Sworn K, Duncan E, Rousseau N, Turner K, Yardley L, Hoddinott P. Taxonomy of approaches to developing interventions to improve health: a systematic methods overview. Pilot and feasibility studies. 2019 Dec;5(1):41.
Michie S, Van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implementation science. 2011 Dec;6(1):42.
Michie S, Atkins L, West R. The behaviour change wheel. A guide to designing interventions. 1st ed. Great Britain: Silverback Publishing; 2014. p. 1003–10.
Yardley L, Morrison L, Bradbury K, Muller I. The person-based approach to intervention development: application to digital health-related behavior change interventions. J Med Internet Res. 2015;17(1):e30.
Bowers HM, Kendrick T, Glowacka M, Williams S, Leydon G, May C, Dowrick C, Moncrieff J, Laine R, Nestoriuc Y, Andersson G. Supporting antidepressant discontinuation: the development and optimisation of a digital intervention for patients in UK primary care using a theory, evidence and person-based approach. BMJ open. 2020;10(3):e032312.
Cane J, O’Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implementation science. 2012 Dec;7(1):37.
French SD, Green SE, O’Connor DA, McKenzie JE, Francis JJ, Michie S, et al. Developing theory-informed behaviour change interventions to implement evidence into practice: a systematic approach using the Theoretical Domains Framework. Implement Sci. 2012;7:38.
Atkins L, Michie S. Changing eating behaviour: what can we learn from behavioural science? Nutrition Bulletin. 2013 Mar;38(1):30–5.
Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, Eccles MP, Cane J, Wood CE. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Annals of behavioral medicine. 2013;46(1):81–95.
Barker F, Atkins L, de Lusignan S. Applying the COM-B behaviour model and behaviour change wheel to develop an intervention to improve hearing-aid use in adult auditory rehabilitation. International journal of audiology. 2016;55(sup3):S90–8.
Chiang N, Guo M, Amico KR, Atkins L, Lester RT. Interactive two-way mHealth interventions for improving medication adherence: an evaluation using the behaviour change wheel framework. JMIR mHealth and uHealth. 2018;6(4):e87.
Barach P, Johnson JK. Understanding the complexity of redesigning care around the clinical microsystem. Qual Saf Heal Care. 2006;15(suppl_1):i10–6.
Hoo Z, Gardner B, Curley R, Wildman M. Part I : Understanding the variation in adherence with nebulised treatment in cystic fibrosis. J Improv Sci. 2013;9(January):1–10.
Wildman MJ, Hoo ZH. Moving cystic fibrosis care from rescue to prevention by embedding adherence measurement in routine care. Paediatr Respir Rev. 2014;15(S1):16–8.
Hoo ZH, Curley R, Campbell MJ, Walters SJ, Hind D, Wildman MJ. Accurate reporting of adherence to inhaled therapies in adults with cystic fibrosis: methods to calculate normative adherence. Patient Prefer Adherence. 2016;887.
Milne A, Rose C, Thornton S, Curley R, Hoo ZH, Wildman M. 275 understanding barriers to weight gain, nebuliser use and exercise in CF. J Cyst Fibros. 2014;13:S118.
Jones S, Babiker N, Gardner E, Royle J, Curley R, Hoo ZH, et al. Promoting adherence to nebulized therapy in cystic fibrosis: poster development and a qualitative exploration of adherence. Patient Prefer Adherence. 2015;9:1109–20.
Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychology and health. 1999 Jan 1;14(1):1–24.
Rothman, A. J., Baldwin, A. S., Hertel, A. W., & Fuglestad, P. T. Self-regulation and behavior change: disentangling behavioral initiation and behavioral maintenance. In K. D. Vohs & R. F. Baumeister (Eds.), Handbook of self-regulation: Research, theory, and applications 2011(pp. 106-122). New York, NY, US: Guilford Press.
Baumeister RF, Heatherton TF. Self-regulation failure: an overview. Psychological inquiry. 1996 Jan 1;7(1):1–5.
Hall PA, Fong GT. Temporal self-regulation theory: a model for individual health behavior. Health Psychology Review. 2007 Mar 1;1(1):6–52.
Gardner B, Rebar AL. Habit formation and behavior change. In Oxford Research Encyclopedia of Psychology; 2019.
Kwasnicka D, Dombrowski SU, White M, Sniehotta F. Theoretical explanations for maintenance of behaviour change: a systematic review of behaviour theories. Health Psychology Rev. 2016s;10(3):277–96.
O’Cathain A, Croot L, Duncan E, Rousseau N, Sworn K, Turner KM, Yardley L, Hoddinott P. Guidance on how to develop complex interventions to improve health and healthcare. BMJ open. 2019 Aug 1;9(8):e029954.
Highsmith J. Agile project management: creating innovative products. Pearson education; 2009.
King’s Fund. The health care workforce in England: make or break?.2018. Nov. https://www.kingsfund.org.uk/sites/default/files/2018-11/The%20health%20care%20workforce%20in%20England.pdf.
Jones S, Curley R, Wildman M. 345 Systematic review of qualitative studies investigating barriers to adherence in patients with cystic fibrosis using framework analysis structured by a conceptual framework of behaviour change. Journal of Cystic Fibrosis. 2013 Jun 1;12:S136.
Sawicki GS, Heller KS, Demars N, Robinson WM. Motivating adherence among adolescents with cystic fibrosis: youth and parent perspectives. Pediatric Pulmonology. 2015 Feb;50(2):127–36.
Kwasnicka D, Dombrowski SU, White M, Sniehotta F. Theoretical explanations for maintenance of behaviour change: a systematic review of behaviour theories. Health psychology review. 2016 Jul 2;10(3):277–96.
Arden MA, Drabble S, O’Cathain A, Hutchings M, Wildman M. Adherence to medication in adults with cystic fibrosis: an investigation using objective adherence data and the theoretical domains framework. Brit J of Health Psychol. 2019;24(2):357–80.
Drabble SJ, O’Cathain A, Arden MA, Hutchings M, Beever D, Wildman M. When is forgetting not forgetting? A discursive analysis of differences in forgetting talk between adults with cystic fibrosis with different levels of adherence to nebulizer treatments. Qualitative health research. 2019 Jul;13:1049732319856580.
Ericsson KA, Simon HA. How to study thinking in everyday life: contrasting think-aloud protocols with descriptions and explanations of thinking. Mind, Culture, and Activity. 1998 Jul 1;5(3):178–86.
Van den Haak MJ, De Jong MD, Schellens PJ. Evaluation of an informational web site: three variants of the think-aloud method compared. Technical Communication. 2007 Feb 1;54(1):58–71.
Hoddinott P. A new era for intervention development studies. Pilot and Feasibility Studies. 2015;1:36–6.
Hind D, Drabble SJ, Arden MA, Mandefield L, Waterhouse S, Maguire C, Cantrill H, Robinson L, Beever D, Scott AJ, Keating S. Supporting medication adherence for adults with cystic fibrosis: a randomised feasibility study. BMC pulmonary medicine. 2019 Dec;19(1):77.
Drabble SJ, O’Cathain A, Scott AJ, Arden MA, Keating SM, Hutchings M, Maguire C & Wildman R. How the mechanisms of relationship, visibility, and fit operated in a web-based intervention with health professional support to increase nebulizer adherence in adults with CF: a qualitative process evaluation study of a pilot randomized controlled trial. Journal of Medical Internet Research. Under review.
O’Cathain A, Murphy E, Nicholl J. Three techniques for integrating data in mixed methods studies. Bmj. 2010 Sep 17;341:c4587.
Clegg D, Barker R. Case method fast-track: a RAD approach: Addison-Wesley Longman Publishing Co, Inc..; 1994.
Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, Altman DG, Barbour V, Macdonald H, Johnston M, Lamb SE. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. Bmj. 2014 Mar 7;348:g1687.
Bandura A. Social foundations of thought and action. Englewood Cliffs, NJ; 1986.
Bandura A. Social cognitive theory of self-regulation. Organizational behavior and human decision processes. 1991 Dec 1;50(2):248–87.
Carver CS, Scheier MF. Control theory: a useful conceptual framework for personality–social, clinical, and health psychology. Psychological bulletin. 1982 Jul;92(1):111.
Gardner B. A review and analysis of the use of ‘habit’ in understanding, predicting and influencing health-related behaviour. Health psychology review. 2015 Aug 7;9(3):277–95.
Gollwitzer PM. Implementation intentions: strong effects of simple plans. American psychologist. 1999 Jul;54(7):493.
Sniehotta FF, Schwarzer R, Scholz U, Schüz B. Action planning and coping planning for long-term lifestyle change: theory and assessment. European Journal of Social Psychology. 2005 Jul;35(4):565–76.
Drabble SJ, & O’Cathain A. Moving from randomised controlled trials to mixed methods intervention evaluations in Hesse-Biber S, & Johnson B (eds.) Oxford Handbook of Mixed and Multimethod Research Inquiry. Oxford University Press. 2015.
Acknowledgements
We would like to thank Professor Susan Michie who provided helpful feedback on the Behaviour Change Wheel mapping stage of the process; and the clinicians and patients who took the time to provide feedback during intervention conception and development without whom this work could not have taken place. We would like to acknowledge the UX research and design input provided by Ricardo Ortega of KeepItUsable, who developed the early front-end designs of the CFHealthHub website and apps.
Funding
This study/project is funded by the National Institute for Health Research (NIHR) [ACtiF Programme (reference RP-PG-1212-20015)] Programme Grants for Applied Research. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Author information
Authors and Affiliations
Contributions
MA (Professor of Health Psychology), SD (Research Associate), PW (Digital Health Software Lead), MH (Physiotherapist) and DB (Patient and Public Involvement Representative) drafted the report. The following designed the research: MW (Consultant Respiratory Physician), AO (Professor of Health Services Research), MA, SD, PW, MH, JB (Professor of Physiotherapy), DH (Assistant Director of Clinical Trials Research Unit), and JA (Professor of Health Informatics). The following were involved in acquisition of data for the work: SD, MH, CM (Study Manager), HC (Study Manager), MA, and PW. The following were involved in the analysis and interpretation of the data: MA, SD, MH, MW, AO, PW, DB, JB, CM, HC, and DH. The following designed the intervention software, tools and manuals/training: MA, MH, PW, JA, and JB. All authors were involved in the final approval of the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was gained for all studies: from the Yorkshire and Humber ethics committee [REC reference 15/YH/0332] and West of Scotland ethics committee [REC reference 15/WS/0089]. All participants gave written informed consent.
Consent for publication
Not applicable
Competing interests
Martin Wildman received funding from Zambon and support from Philips Respironics for the early development work. This has not had any direct influence on the study reported here. Martin Wildman has worked with Pari to carry out studies using the eTrack. This has not had any direct influence on the study reported here. The University of Manchester software team received funding from Pari to create a medication reporting component within the CFHealthHub software. This has not had any direct influence on the study reported here.
The other authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
First Intervention Visit: Worksheet
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Arden, M.A., Hutchings, M., Whelan, P. et al. Development of an intervention to increase adherence to nebuliser treatment in adults with cystic fibrosis: CFHealthHub. Pilot Feasibility Stud 7, 1 (2021). https://doi.org/10.1186/s40814-020-00739-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40814-020-00739-2