Abstract
Background
Antimicrobial resistance is a serious concern. Although the widespread use of antimicrobials in livestock has exacerbated the emergence and dissemination of antimicrobial resistance genes (ARG) in farm environments, little is known about whether antimicrobial use affects distribution of ARG in livestock systems. This study compared the distribution of microbiomes and resistomes (collections of ARG) across different farm sectors in dairy herds that differed in their use of antimicrobials. Feces from heifers, non-lactating, and lactating cows, manure storage, and soil from three conventional (antimicrobials used to treat cows) and three organic (no antimicrobials used for at least four years) farms in Pennsylvania were sampled. Samples were extracted for genomic DNA, processed, sequenced on the Illumina NextSeq platform, and analyzed for microbial community and resistome profiles using established procedures.
Results
Microbial communities and resistome profiles clustered by sample type across all farms. Overall, abundance and diversity of ARG in feces was significantly higher in conventional herds compared to organic herds. The ARG conferring resistance to betalactams, macrolide-lincosamide-streptogramin (MLS), and tetracyclines were significantly higher in fecal samples of dairy cows from conventional herds compared to organic herds. Regardless of farm type, all manure storage samples had greater diversity (albeit low abundance) of ARG conferring resistance to aminoglycosides, tetracyclines, MLS, multidrug resistance, and phenicol. All soil samples had lower abundance of ARG compared to feces, manure, and lagoon samples and were comprised of ARG conferring resistance to aminoglycosides, glycopeptides, and multi-drug resistance. The distribution of ARG is likely driven by the composition of microbiota in the respective sample types.
Conclusions
Antimicrobial use on farms significantly influenced specific groups of ARG in feces but not in manure storage or soil samples.
Similar content being viewed by others
Background
Antimicrobial resistance (AMR) is one of the serious global public health threats of this century. It threatens the treatment and control of infections caused by microbial pathogens that are no longer susceptible to the antimicrobials commonly administered to treat them [1]. The widespread use of antimicrobials in human and animal settings has resulted in the emergence and rapid acquisition of antimicrobial resistance by pathogens, complicating disease treatments in humans and animals [2]. In 2018, the total amount of antimicrobials used in the U.S. livestock industry was approximately 11.6 million kg [3]. Of the antimicrobials approved by the Food and Drug Administration to treat livestock, 8 classes are considered to be medically relevant, meaning that they are used to treat human disease: aminoglycosides, cephalosporins, fluoroquinolones, lincosamides, macrolides, penicillins, sulfas, and tetracyclines [3]. Additionally, the World Health Organization (WHO) has developed criteria for the classification of antibiotics, based on their importance in the treatment of human disease, as “critically important,” “highly important,” and “important” [4]. The “critically important” category includes medications such as ceftiofur, a third-generation cephalosporin used in cattle to treat conditions such as bovine respiratory disease; this drug is considered critically important in human medicine because it is one of the few reliable treatments for infection with Escherichia coli and Salmonella spp. It is apparent, therefore, that the use of antimicrobials in livestock has the potential to impact human health.
As a result of the high frequency and large amounts of antimicrobials prescribed, livestock and their environment have become one of the large reservoirs of antimicrobial-resistant bacteria (ARB) and antimicrobial resistance genes (ARG) [5,6,7]. Notably, commensals and environmental microbes that are otherwise susceptible to antimicrobials may become resistant by acquiring ARG from resistant bacteria and thus may contribute to AMR dissemination across the food chain [8]. While the widespread use of antimicrobials has exacerbated dissemination of AMR in animals, humans, and the environment, there are only a few reports [9,10,11] that describe the prevalence, distribution, and dissemination pathways of ARG within agricultural sites in response to antimicrobial use on farms. How antimicrobial administration affects gut microbial populations in livestock, what resistance mechanisms are exhibited by microbiota, and whether these mechanisms disseminate to agricultural sites when voided in feces are all interesting topics for which sufficient information is lacking. Animals receiving antimicrobials induce the selection pressure such that specific bacteria that have gained resistance via mutation or horizontal gene transfer can survive [12]. Consequently, their excreta (feces and urine) can be enriched with ARG and ARB, along with antibiotic residues [13]. When manure (a mixture of feces, urine, bedding, and other materials) is subsequently applied to agricultural land, the soil then becomes a sink for the resistant pollutants [14]. The soil can also function as a source for dispersing the pollutants beyond the animal agroecosystem into and through the complex web of crops, water, wildlife, etc. [15]. Although knowledge is lacking about how and with what frequency the transfer of ARG to humans occurs, there is growing evidence that ARB and ARG from agricultural systems can affect humans through numerous routes [16,17,18].
Previous studies have investigated the fate of a limited number of ARG in farm sectors [19, 20]. In a previous study [21], we have demonstrated the distribution of ARG in feces, manure, and soil across dairy agroecosystems and identified a diverse pool of ARG in manure. Metagenomic approaches offer the possibility of exploring the complete spectrum of ARG within a microbial ecosystem [13] and have been increasingly applied to survey livestock [22] and the broader livestock environment [13, 23] to expand our understanding of the distribution of ARB and ARG in animal production systems. The purpose of this study was to determine whether antimicrobial use on dairy farms influences microbial community structure and composition and distribution of antimicrobial resistant genes in feces, manure storage, and soil in dairy agroecosystems. Our hypothesis was that antimicrobial use on farms would differentially affect the prevalence and distribution of ARG in different farm sectors. To test this, we compared certified-organic (antimicrobials were not used to treat animals for at least 4 years) and conventional herds (antimicrobials have been used to treat animals) matched by farm size for the distribution of ARG using metagenomic approaches.
Methods
Selection of dairy herds and sample collection
A list of certified organic dairy operations was obtained from the USDA Agricultural Marketing Service (https://www.ams.usda.gov/services/organic-certification/certifying-agents) from which 3 organic dairy herds were selected and matched with 3 conventional dairy herds based on size and geographic region. Five herds were located in southeastern Pennsylvania and 1 herd was located in northeastern Maryland. Herds were classified and matched by size based on the total number of mature cows on farm. Herd size classification was: i) small herds of 40–100 cows, ii) medium herds of 101–300 cows, and iii) large herds of 301–500 cows. The actual herd sizes were as follows: small organic – 40 cows, small conventional – 72 cows, medium organic – 190 cows, medium conventional – 210 cows, large organic – 500 cows, and large conventional – 490 cows.
Sampling of cow feces
The design of the experiment and animal sampling protocol were approved by the University of Pennsylvania Institutional Animal Care and Use Committee (IUCAC protocol number 806194). All animal sampling was conducted on private farms with the permission of the farm owners. Fecal samples were collected via rectum from 3 lactating cows, 3 non-lactating cows, and 3 heifers between the ages of 6–12 months for each of the 6 farms. Fecal samples were individually collected and placed in 50 mL conical tubes. About 250 g of sample was collected for each sample type. Within 2 h of sampling, the samples were transported on ice to the lab where they were archived at − 80 °C. All samples were extracted for DNA in one batch.
Sampling of manure storage
Manure storage samples were obtained from farm-specific manure collection systems. All farms utilized a permanent manure storage structure (lagoon or concrete pit) to store all or a portion of manure. A container was lowered into the manure storage structure to collect manure samples that were composited for analysis. On farms that stacked manure deposits as a pile, a composite of the pile was sampled to represent a manure sample. The manure sample was transferred into 50 mL conical tubes, placed on ice and transported to the laboratory along with fecal samples.
Sampling of soil
Soils from the 3 organic and 3 conventional dairy farms were sampled with a stainless-steel, 1.6 cm diameter probe. Twenty to 30 soil cores were taken in a grid pattern in a representative area of the pastures (2 farms) and crop fields (4 farms) and these samples were composited for analysis. Samples were collected between 8 March and 26 June 2017. The most recent manure applications to these fields occurred in fall 2016, prior to planting the winter cover crop. In addition, soil samples, one from a pristine forest and the other from an agricultural experimental field that has had no known manure application, served as comparisons. Bulk soil samples were crumbled and air-dried, then sieved to pass through a 2 mm mesh screen and stored at − 20 °C until use in the laboratory.
Sample processing and sequencing
The genomic DNA was extracted from all samples using PowerSoil DNA Isolation Kit (MOBIO Laboratories, Inc., Carlsbad, CA), as described by Pitta et al. [21]. Library preparation for shotgun sequencing was performed using Nextera XT DNA Library Preparation Kit (FC-131-1024, Illumina, San Diego, CA, USA) following the manufacturer’s protocol. Briefly, 1 ng of extracted DNA from each sample was subjected to tagmentation followed by addition of indexes and adapters using a limited-cycle PCR program. The generated libraries were cleaned using AMPure XP beads (Beckman Coulter; Brea, CA), normalized, and pooled. Shotgun sequencing was performed on the Illumina NextSeq500 platform (tight insert size of 250 bp for high-throughput sequencing from both ends by 2 × 150 bp).
Bioinformatics analysis
Raw sequences were subjected to quality trimming using Trimmomatic (0.36) [24] according to the following parameters: starting from either end of the sequence, bases were trimmed off if their Phred quality score was < 3 or if they appeared as N; bases were trimmed off if their average Phred quality score was < 15 when the sequence was analyzed on a 4-base sliding window; and sequences were removed if they were shorter than 36 bases in length. Reads aligning to the host genome (ARS-UCD1.2/bosTau9) were identified and removed using Bowtie2 (v2.2.7) [25] with parameters set by the flag “--very sensitive local --un-conc”.
To identify ARG sequences, quality-controlled reads were aligned to MEGARes 2.0 (a hand-curated antimicrobial resistance genes database containing 7868 ARG) [26], using BWA v0.7.13 [27]. The aligned (BAM) files were then sorted and converted to a SAM file using Samtools v1.7 [28]. The MEGARes database contains specific annotation of genes (sequence headers contain ‘RequiresSNPConfirmation’) that require the presence of single nucleotide polymorphisms (SNP) at specific loci in order to confer resistance [29]. Therefore, alignments labeled with “RequiresSNPconfirmation” in the SAM-formatted file were extracted into a single fasta file which was further subjected to a secondary validation analysis by using Resistance Gene Identifier (RGI) (v 5.1.1) with “Perfect” and “Strict” algorithms [30]. Sequencing reads that did not survive the RGI-based confirmation were excluded from the SAM file, and the remaining alignments were then analyzed through ResistomeAnalyzer (−t 80; at least 80% of nucleotides in the reference sequence that were aligned to by at least one sequence read) to parse the number of resistance genes within each sample (https://github.com/cdeanj/resistomeanalyzer). We also performed at 100% gene comparison (Additional Table 5) but presented all our comparisons at 80%.
Taxonomic labels were assigned to quality-controlled reads by mapping sequences to a low-complexity masked database of bacterial, archaeal, viral, fungal, and protozoal sequences from NCBI complete genomes (downloaded 22 October 2020). The relative abundance of the bacterial genus was estimated using Bracken [31]. In order to identify the bacterial hosts (ARB) of identified ARG, for each sample, the kraken2 output file, which contains the sequence read ID and the assigned taxonomy label, was merged with each ARG output file using the sequence read ID as the key in R. The combined output was used to assign ARG to their corresponding bacterial hosts.
The taxonomy and ARG sequence abundances were normalized to the counts per million approach accounting for various sequence depths across samples using the ‘cpm’ function available in edgeR library in R. The Bray-Curtis dissimilarity index was calculated on the normalized data and was visualized using non-metric multidimensional scaling (NMDS) using the metaMDS function available in vegan R package. A non-parametric permutational multivariate analysis of variance test (PERMANOVA), implemented in the vegan package for R [32], was used to test the effects of farm type (conventional or organic), sample type (feces, lagoon, manure, or soil), and the interactions of these variables on overall community composition. PERMANOVA tests were done on Bray-Curtis dissimilarity index. Comparisons of ARG (cpm) between farm type, sample type and their interactions were tested using generalized linear model.
Results
Sequencing information
In this study, a total of 33 metagenomic libraries inclusive of cow feces, manure, lagoon, and soil samples (hereafter referred to as sample type) collected from 3 conventional and 3 organic farms (hereafter referred as farm type) in southeastern Pennsylvania were analyzed for their composition of microbial communities, distribution of ARG, and bacterial hosts carrying ARG. Antibiotic use on conventional farms is reported in Additional Table 1.
A total of 1 billion paired end reads were sequenced. These raw sequences were trimmed and then quality filtered (about 5% of reads were eliminated; 4% Trimmomatic filtering and 1% host filtering) resulting in approximately 971 million reads (Additional Table 2). The average number of sequence reads per sample was 29,346,035 (min: 8,135,779-max: 112,217,060) A total of 9123 ARG were identified by comparing quality-filtered sequence reads to the MEGARes2 database.
Community clustering patterns based on microbiome and resistome profiles
Based on nonmetric multidimensional scaling (NMDS) analysis (Fig. 1), microbial communities clustered by sample type irrespective of farm type. Within each sample type, manure and lagoon microbiomes between different farms showed greater variation whereas fecal and soil microbiomes remained homogenous except for one soil sample. Interestingly, the soil microbiome in reference soil samples (i.e. soils from the pristine forest as well as the experimental field receiving no manure) was similar to those soil samples collected on dairy farms.
Resistomes (collections of ARG) also clustered by sample type and were consistent with the clustering patterns of microbiomes (Fig. 2). In fecal samples of both farm types, resistomes were predominated by ARG that conferred resistance to tetracyclines, MLS, and betalactams. Across all manure and lagoon samples, resistomes were diverse and contained ARG that conferred resistance to several antimicrobials. Soil resistomes, including reference soil samples, clustered together with ARG that conferred resistance to aminocoumarins, glycopeptides, and multidrug resistance.
Based on PERMANOVA (Additional Table 3), no differences in microbiomes and resistomes were observed between organic and conventional herds but significant differences were observed between sample types (P < 0.05).
Microbial community composition
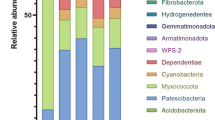
A total 37 different bacterial phyla were identified across all samples (Table 1). The most dominant phyla were Proteobacteria (35%), Firmicutes (22%), Bacteroidetes (21%), and Actinobacteria (16%) that collectively contributed to the greatest bacterial abundance. Fecal samples were dominated by Firmicutes (37%) and Bacteroidetes (31%) followed by Proteobacteria (17%) and Actinobacteria (10%) across both conventional and organic dairy cows. Proteobacteria constituted the majority (57%) of bacterial abundance in manure and lagoon samples across both farm types whereas Proteobacteria (55%) and Actinobacteria (35%) together comprised the majority of bacterial populations in soil samples of both farm types.
Identification and characterization of ARG
A total of 401 unique ARG were identified from 9123 sequences across all samples (Additional Table 4). The means of ARG (CPM) conferring resistance to different classes of antimicrobials across different sample types in both farm operations are presented in Table 2. For each class of antimicrobials, the type of resistance mechanism (Fig. 3) and the corresponding gene(s) are also presented (Additional Table 4). Overall, 38 different resistance mechanisms conferring resistance to 15 classes of antimicrobials were described. Of the 401 unique ARG, 305 representing 34 different mechanisms were identified on conventional farms, whereas 307 unique ARG representing 34 different mechanisms were identified on organic farms. The total number of ARG identified in conventional herds was 7020 while on organic farms, 2048 ARG were identified. Only 3 unique ARG were identified in pristine soil environments used as controls (soil from an agricultural field that had never received manure application and forest soil, respectively).
Heat map of resistance mechanisms. Heat map showing resistance mechanisms for each class of antimicrobials identified in fecal, lagoon, manure, and soil sample types of conventional and organic farms. Relative abundance of each mechanism in each sample is given by the color code in upper right-hand corner of figure. Ref: reference; LactCow: lactating cow; NonLactCow: non-lactating cow
ARG profiles in different sample types of organic and conventional dairy systems
Overall, feces sampled from animal groups in conventional herds had a greater abundance as well as more diverse ARG compared to feces from animal groups in organic herds (Fig. 3; Additional Table 4). There was an abundance of ARG conferring resistance to aminoglycosides, betalactams, tetracyclines (ribosomal protection proteins), and macrolide-lincosamide-streptogramin (MLS; lincosamide nucleotidyltransferases) across feces from all animal groups from both organic and conventional herds. However, these ARG were significantly more abundant in conventional herds compared to organic herds. In organic herds, heifers and non-lactating cows did not carry any additional ARG in their fecal samples. The fecal samples of lactating cows in large and medium organic herds had an abundance of ARG conferring resistance to rifampin (rpoB) whereas feces from lactating cows in the small organic herd had a very low abundance of ARG conferring resistance to class A betalactamases and aminoglycoside-O-phosphotransferases (AG-O-PT). Notably, in the conventional herds, ARG conferring resistance to class A betalactamases (cfx) were detected in feces from all animal groups. The feces from the heifer group in the medium conventional herd had multiple ARG including those conferring resistance to class A betalactamases (cblA, ctx, rob), class C betalactamases (bLaEC), cationic peptides, fluoroquinolones, MLS, multidrug resistance, and tetracyclines (tetX, tet32, tet44, tet40, tetQ, and tetR). The feces from the non-lactating and lactating cows in the medium herd did not have any additional ARG except those that were common to all fecal samples in conventional herds. Feces from the heifers from the small conventional herd also had diverse ARG but in low abundance.
Regardless of the farm type, the manure and lagoon samples were predominated by ARG conferring resistance to tetracyclines, MLS, and aminoglycosides. Although the same type of ARG was detected in the manure and lagoon samples of both farm types, their abundance was higher in conventional farms compared to organic farms; however, there was a large variation between farms. The ARG conferring resistance to class D betalactamases (blaOXA) was present in 3 of 4 samples in the conventional herd whereas it was present only in the medium organic herd but in very low abundance.
The soil ARG were distinct from feces and manure, with soil being predominated by ARG conferring resistance to aminocoumarins, aminoglycosides, glycopeptides, and multi-drug resistance. The overall abundance of soil ARG was much lower than feces and manure but very similar between organic and conventional herds. The ARG profiles of soils collected on farms were similar to forest soil.
Taxonomy associated with antimicrobial classes
The resistance-encoding ARG were mainly harbored in lineages belonging to the bacterial phyla Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria (Fig. 4); those ARG for which the bacterial host was not identified were grouped as unclassified phylum (about 23% of ARG reads were unclassified bacteria).
Among the aminoglycoside ARG, the bacterial hosts for efflux pump resistance mechanisms were exclusive to Proteobacteria. Among the aminoglycoside-N-acetyltransferases, the aac3 ARG was abundant in the dairy cow feces from the medium conventional farm. The host carrying this gene was the Enterobacteriaceae family. The aac(6′) ARG was present in dairy cow feces across both farm types and was carried in almost all bacterial lineages. While most of the bacterial hosts carrying aminoglycoside-O-nucleotidyltransferase (AG-O-NT) genes were not identified, a few of the genes for AG-O-NT, particularly ant(3′), were distributed in manure and lagoon samples and were mostly carried by Proteobacteria lineages. However, ant6 was distributed in fecal samples and was carried by Clostridia and Bacilli from Firmicutes, Actinomycetaceae from Actinobacteria, and a few Bacteroidetes members. The ant6 identified in soil samples from the small organic herd was carried by Bacteroidetes members only. Interestingly, ant9 genes were distributed in fecal and manure samples of both herds and were predominantly carried by Bacteroides members. The acetyl-O-phosphotransferases (AG-O-PT) were distributed in feces and manure samples for which the host was not determined for a majority of ARG. Among the identified AG-O-PT, the hosts were mostly Proteobacteria and a few Firmicutes members.
In the betalactam class, among the class A betalactamases, which were detected only in fecal samples, the blaCFX ARG were detected only in Bacteroidetes members. The other class C betalactamases that were in lower abundance were blaACI, detected in Firmicutes, and blaCTX, detected in Proteobacteria members. Class D (blaOXA) betalactamases that were detected in manure and lagoon samples were mostly identified in Bacteroidetes and Proteobacteria members. The bacterial hosts carrying ARG conferring resistance to glycopeptides in soil samples were identified as Actinobacteria members.
Among the ARG conferring resistance to MLS, the lnuC gene was the most abundant ARG and was carried predominantly by Clostridia and Bacilli (Firmicutes) followed by Proteobacteria and Spirochaete members. A small portion of ARG conferring resistance to MLS was also contributed by lnuA which was carried by Bacteroidetes members in the fecal samples of dairy cows from both farm types. The efflux pumps for macrolides and streptogramins were mostly found in Bacilli and Clostridiales in manure samples. The ARG responsible for efflux pump-based resistance in the multi-drug resistance category were mostly identified in Proteobacteria. The bacterial hosts for ARG conferring resistance to rifampin (rpoB) were mostly Actinobacteria followed by Firmicutes in feces from all animal groups, whereas the rph ARG identified in soil samples were carried by Actinobacteria members.
Among the genes conferring resistance to tetracyclines, the bacterial hosts for tet32 and tet40 detected in feces from all animal groups were Firmicutes members, tet36 and tetQ were specifically found in Bacteroidetes members, tet44 and tetT were found in Firmicutes and Proteobacteria members, and tetW was detected in several unidentified phyla and across lineages of Firmicutes, Proteobacteria, and Bacteroidetes. The tetR and tetY genes identified in manure samples was restricted to Proteobacteria members only. The sulI and sulII ARG conferring resistance to sulfonamides were also identified in Proteobacteria lineages only.
Discussion
There is strong evidence that livestock and their environments have become large reservoirs for ARB and ARG [18, 21]. As the frequent use of antimicrobials in livestock has been listed as one of the major causal factors, adhering to organic standards by restricting antimicrobial use [33] may prevent or reduce AMR dissemination on farms [34]. The purpose of this study was to determine whether there were differences in the distribution of microbiota and ARG on dairy farms that differed in their use of antimicrobials. Overall, we found that the abundance and diversity of ARG was much higher in the conventional herds compared to organic herds. Feces in particular had a higher concentration of commonly detected ARG and those that conferred resistance to some of the critically and highly important antimicrobials used to treat human infections. Similar patterns were reported by Rovira et al. [35], who found that a higher number of ARG were detected in feces and waste water samples in conventional herds compared to organic herds, although the number of unique ARG identified in this study was lower (95 vs. 144) than those reported in Rovira et al. [35].
At the community level, composition of microbiomes and resistomes did not differ between organic and conventionally managed dairy herds. We found that the microbiome and resistome differed between sample types (P < 0.05) which agrees with our previous findings [21] and those of Noyes et al. [13], where both studies investigated different agricultural sites and reported that the associated respective resistomes were different. It was reported by Wang et al. [36] that changes in the core resistome identified in manure samples were highly correlated with the microbial phylogeny of the same sample indicating that presence and activity of ARG may be influenced by microbial populations in the samples. Similar clustering patterns between microbiomes and resistomes of the same sample types as observed in this study may indicate that phylogenetic composition of the microbiome has an influence on ARG profiles.
Within each sample type, fecal resistomes differed by farm type in their relative abundance and distribution, but no such differences were observed for manure and soil samples, suggesting that overall differences in resistomes between organic and conventional farms were driven mostly by fecal resistomes and not those from manure and soil samples. The type of antimicrobials that were administered on the 3 conventional herds in this study were recorded as cephalosporins, penicillins, and streptomycins for dry cow therapy; cephalosporins for mastitis; macrolides, fluoroquinolones, and chloramphenicols for pneumonia; and ampicillin and cephalosporins for metritis (Additional Table 1). Accordingly, feces from conventional animal groups had diverse (198 out of 401 unique ARG) ARG that contributed to a higher abundance (total ARG counts: 6037 vs.1429) compared to their counterparts from organic herds. Among the animal groups, feces from non-lactating cows and heifers had a higher abundance of ARG compared to lactating cows in all 3 conventional dairy herds. Feces from all animals had ARG conferring resistance to cephalosporin (cfx, ctx), penicillins (ctx, rob, aci, cblA), MLS, and phenicols. These results are in agreement with those of Rovira et al. [35] indicating that ARG abundance in fecal samples correspond to the types of antimicrobials administered to animals. Lactating cows from conventional dairy herds that were sampled for this study had not been given antimicrobials at the time of sampling, and this may explain why the ARG abundance and diversity was lower in feces from lactating cows than those from non-lactating cows and heifers. We also found that feces from the heifers in the medium conventional herd had an exceptionally higher abundance and greater diversity of ARG than other animal groups across all conventional herds. The exact cause for this high abundance of ARG in heifers on this farm is beyond the scope of this study. The fecal samples from individual cows were pooled by type and farm for this study and therefore it is not clear if one or more cows were enriched in ARG. Furthermore, heifers from this particular farm were raised on a different site for efficient use of feed and other resources.
Among the organic herds, the large and medium herds stopped using antimicrobials more than 20 years prior to sample collection for this study and the small herd had not used antimicrobials since 2013. Fecal samples from all animal groups from both conventional and organic herds had ARG conferring resistance to betalactamases and MLS as well as tetracycline ribosomal protection proteins (tetW, tet32, and tet44), indicating that these may form the core resistome in dairy cow feces. Fecal samples from animal groups from organic herds were relatively clean compared to those from conventional herds, particularly non-lactating cows and heifers. Lactating cows from the large and medium organic herds had ARG conferring resistance to rifampins in fecal samples which were not detected in other animal groups but were detected at much lower concentration in conventional herds. Rifampin is used to treat certain types of infections in horses [37] and is occasionally used in dogs and cats [38] but is not prescribed for use in livestock. It has been reported that ARG conferring resistance to rifampin, particularly rpoB, contribute to 35% of the abundance of annotated ARG across diverse environmental samples such as aquaculture sediment, sludge, biofilm, and river water environments [39]. It is interesting to note that the microbiome of lactating cows on organic herds for unknown reasons may select for bacteria with rifampin resistance. Rifampin is a broad-spectrum antimicrobial that is the drug of choice for the treatment of tuberculosis in humans. Therefore, the findings of this study highlight the need for further investigations into the prevalence, persistence, distribution, and functional role of ARG conferring resistance to rifampin on organic farms. Furthermore, while the bacterial hosts that carried these genes were identified as Clostridia in feces and Gammaproteobacteria members in manure and lagoon samples, several of the bacterial hosts carrying rifampin resistance are yet to be identified reinforcing the need for further research on rifampin resistance. In contrast, lactating cows from the small organic herd did not have ARG conferring resistance to rifampin in their fecal samples but carried low numbers of ARG conferring resistance to aminoglycosides and class A betalactamases. It is not clear if the history of withholding antimicrobials only for 4 years or other management factors on this farm contributed to low levels of ARG conferring resistance to commonly used antimicrobials in feces. Collectively, these data indicate the need for research on the functional role of ARG on organic operations and how these functions change with different bacterial hosts.
The ARG profiles of manure and lagoon samples between conventional and organic herds were similar, although the organic herds had lower abundance than the conventional herds. We have reported that similar to feces, manure is a hotspot for ARG and is enriched in a diverse ARG gene pool [21] which is in agreement with this study. A history of organic management practices on farms did not seem to affect the ARG profiles of manure or lagoon samples in organic herds. There were ARG conferring resistance to multiple antimicrobials including aminoglycosides, betalactamases (particularly class D), MLS, multidrug resistance, cationic antimicrobial peptides, phenicols, sulfonamides, and tetracyclines. The presence of extended-spectrum ARG conferring resistance to betalactamases, such as blaOXA, a class D betalactamase, on dairy sectors at a very low abundance in this study was also reported by Rovira et al. [35] and Vikram et al. [40] using metagenomic approaches. Interestingly, we found that most of these betalactamases were identified only in Bacteroides spp in this study. The Bacteroides group of bacteria carries the most unique and diverse resistance mechanisms [41] and the occurrence of ARG conferring resistance to betalactamases in these bacteria in diverse environments has been increasing. The blaCFXA gene has been reported in Bacteroides isolates from human infections and also in the Bacteroides genus identified in cows treated with ceftiofur [42]. The blaCFXA gene was found to be highly resistant to first and second generation cephalosporins and moderately resistant to third and fourth generation cephalosporins [43].
Among the MLS resistance genes, it is interesting to note that in feces, commensal bacteria such as members of Bacteroidetes and Firmicutes carried ARG conferring resistance to lincosamide antimicrobials whereas in manure samples, ARG conferring resistance to macrolides, lincosamides, and streptogramins were detected. Interestingly, only Bacilli in manure samples carried ARG for streptogramins. The ubiquitous presence of ARG conferring resistance to MLS in beef cattle and their environment has been documented [44], although these MLS resistance genes can also occur frequently by mutation [45]. Nevertheless, differences in resistance mechanisms between feces and manure for MLS resistance needs further research and may shed light on the prevalence and dissemination pathways of MLS resistance in livestock farm environments.
Across both farm types, soil samples were enriched in genes conferring resistance to aminocoumarins, glycopeptides, multidrug resistance, rifampin, tetracyclines, and trimethoprim. Among soil samples, the sample from the small organic herd had a diverse pool of ARG including efflux pumps for multidrug resistance, MLS, rifampins, and tetracyclines. As per the history obtained from producers, the soils sampled during spring/summer received manure application about 6–9 months prior to sampling time thus indicating the influence of environmental factors on the distribution of diverse ARG in the soil sample; this is in agreement with the findings of Hurst et al. [46] who reported that the abundance of ARG can be influenced by environmental factors such as location, antimicrobial use, farm type, antimicrobial concentrations, and storage of manure samples.
The ubiquitous distribution of tetracycline resistance in cows and their excrements has been well documented [21, 42]. There is evidence to show that the presence of these ARG even in the absence of tetracycline administration is linked to improved health and growth promotion of dairy cattle [47]. These authors stated that tetO, tetW, and tetQ constitute a core tetracycline-specific resistome in heifers and their excrements. Similar findings were also observed in the current study where tetW and tetQ were among the most abundant ARG across most fecal, manure, and lagoon samples, suggesting that these genes co-occur with other genes or are needed for microbial metabolism. Notably, the similarity between specific gene sequences in Butyrivibrio, a commensal rumen bacterial genus, and the tetO gene in Streptococcus pyogenes indicate a possible transfer of these ARG between microbes of different origin. Further, tetW in Butyrivibrio has a higher G + C content than its genome, once again suggesting its possible acquisition from other higher G + C genomes [48]. Interestingly, we found that bacterial hosts carrying tetQ were Bacteroidetes members and those carrying tetW were mostly Clostridia members, whereas other ARG conferring resistance to tetracycline were detected in Enterobacteriaceae members of Proteobacteria. The tet36 and tet39 ARG were unique to manure and lagoon samples and were identified only in Bacteroides spp and Acinetobacter baumanii, respectively. The detection of the tet39 ARG on plasmids of several strains of Acinetobacter may indicate its possible dissemination to other Gram-negative bacteria [49] whereas the tet36 gene, a ribosomal protection gene, failed to disseminate between different species of Bacteroides under laboratory conditions [50]. Collectively, these data reveal that while a majority of ARG conferring resistance to tetracycline have become a part of the genomes of commensal bacteria there may be other ARG, such as tet36 and tet39, that are restricted to only a few species. Further investigations are needed to understand the prevalence, persistence, function, and dissemination mechanisms of these ARG.
Conclusions
Microbial community composition was influenced by sample type but no significant differences were observed between conventional and organic farms for each sample type. The use of antimicrobials had an independent effect on distribution of ARG. Antimicrobial use on farms influenced the distribution of specific ARG such as those conferring resistance to betalactams, tetracyclines, and MLS in conventionally managed cows. These ARG were detected in organically managed operations but at a very low abundance and were carried in commensal bacteria. Irrespective of farm type, all manure and lagoon samples had diverse ARG revealing that antimicrobial use has no effect on the distribution of resistomes in manure samples. All soil samples appeared to have a core resistome that may be highly conserved; however, the total abundance and distribution of ARG may be influenced by environmental factors. Further studies are needed to investigate the prevalence, distribution, and functional significance of ARG on organic farms both on temporal and spatial scales.
Availability of data and materials
The datasets generated and/or analysed during the current study are available in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) under accession number PRJNA588263.
Abbreviations
- AG-O-NT:
-
Aminoglycoside-O-nucleotidyltransferases
- AG-O-PT:
-
Aminoglycoside-O-phosphotransferases
- AMR:
-
Antimicrobial resistance
- ARB:
-
Antimicrobial resistant bacteria
- ARG:
-
Antimicrobial resistance genes
- MDR:
-
Multi-drug resistance
- MLS:
-
Macrolide-lincosamide-streptogramin
- SNP:
-
Single nucleotide polymorphism
References
Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health. 2015;109(7):309–18. https://doi.org/10.1179/2047773215Y.0000000030.
Allen HK. Alternatives to antibiotics: Why and how. NAM Perspectives. 2017. Discussion Paper, National Academy of Medicine, Washington, DC. doi:https://doi.org/10.31478/201707g.
United States Food and Drug Administration (FDA) Center for Veterinary Medicine. Summary report on antimicrobials sold or distributed for use in food-producing animals. 2018. https://www.fda.gov/media/133411/download. Accessed 14 Feb 2020.
WHO Advisory Group on Integrated Surveillance of Antimicrobial Resistance, World Health Organization (WHO). Critically important antimicrobials for human medicine. 3rd edition. 2012. http://apps.who.int/iris/bitstream/10665/77376/1/9789241504485_ eng.pdf?ua=1. Accessed 20 Jan 2020.
Kimera ZI, Mshana SE, Rweyemamu MM, Mboera LEG, Matee MIN. Antimicrobial use and resistance in food-producing animals and the environment: An African perspective. Antimicrob Resist Infect Control. 2020;9(37). https://doi.org/10.1186/s13756-020-0697-x.
Van Boeckel TP, Brower C, Gilbert M, Grenfell BT, Levin SA, Robinson TP, et al. Global trends in antimicrobial use in food animals. Proc Natl Acad Sci U S A. 2015;112(18):5649–54. https://doi.org/10.1073/pnas.1503141112.
Magouras I, Carmo LP, Stärk KD, Schüpback-Regula G. Antimicrobial usage and resistance in livestock: where should we focus? Front Vet Sci. 2017;15(4):148. https://doi.org/10.3389/fvets.2017.00148.
Thanner S, Drissner D, Walsh F. Antimicrobial resistance in agriculture. MBio. 2016;7(2):e02227–15. https://doi.org/10.1128/mBio.02227-15.
Chantziaras I, Boyen F, Callens B, Dewulf J. Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: a report on seven countries. J Antimicrob Chemother. 2014;69(3):827–34. https://doi.org/10.1093/jac/dkt443.
AbuOun M, O’Connor HM, Stubberfield EJ, Nunez-Garcia J, Sayers E, Crook DW, et al. Characterizing antimicrobial resistant Escherichia coli and associated risk factors in a cross-sectional study of pig farms in Great Britain. Front Microbiol. 2020;11:861. https://doi.org/10.3389/fmicb.2020.00861.
Scott L, Menzies P, Reid-Smith RJ, Avery BP, McEwen SA, Moon CS, et al. Antimicrobial resistance in fecal generic Escherichia coli and Salmonella spp. obtained from Ontario sheep flocks and associations between antimicrobial use and resistance. Can J Vet Res. 2012;76(2):109–19.
Modi SR, Lee HH, Spina CS, Collins J. Antibiotic treatment expands the resistance reservoir and ecological network of the phage metagenome. Nature. 2013;499:219–22. https://doi.org/10.1038/nature12212.
Noyes NR, Yang X, Linke LM, Magnuson RJ, Cook SR, Zaheer R, et al. Characterization of the resistome in manure, soil and wastewater from dairy and beef production systems. Sci Rep. 2016;6:24645. https://doi.org/10.1038/srep24645.
Zhu YG, Johnson TA, Su JQ, Qiao M, Guo GX, Stedtfeld RD, et al. Diverse and abundant antibiotic resistance genes in Chinese swine farms. Proc Natl Acad Sci. 2013;110(9):3435–40. https://doi.org/10.1073/pnas.1222743110.
Martinez JL. Effect of antibiotics on bacterial populations: a multi-hierarchical selection process. F1000Res. 2017;6:51. https://doi.org/10.12688/f1000research.9685.1.
Durso LM, Cook KL. Impacts of antibiotic use in agriculture: what are the benefits and risks? Curr Opinion Microbiol. 2014;19:37–44. https://doi.org/10.1016/j.mib.2014.05.019.
Durso LM, Cook KL. One health and antibiotic resistance in agroecosystems. EcoHealth. 2018;1:1–6. https://doi.org/10.1007/s10393-018-1324-7.
Franklin AM, Aga DS, Cytryn E, Durso LM, McLain JE, Pruden A, et al. Antibiotics in agroecosystems: introduction to the special section. J Environ Qual. 2016;45:377–93. https://doi.org/10.2134/jeq2016.01.0023.
Ben WW, Qiang Z, Adams C, Zhang H, Chen L. Simultaneous determination of sulfonamides, tetracyclines and tiamulin in swine wastewater by solid-phase extraction and liquid chromatography-mass spectrometry. J Chromatogr A. 2008;1202:173–80. https://doi.org/10.1016/j.chroma.2008.07.014.
Heuer H, Smalla K. Manure and sulfadiazine synergistically increased bacterial antibiotic resistance in soil over at least two months. Environ Microbiol. 2007;9(3):657–66. https://doi.org/10.1111/j.1462-2920.2006.01185.x.
Pitta DW, Dou Z, Kumar S, Indugu N, Toth JD, Vecchiarelli B, et al. Metagenomic evidence of the prevalence and distribution patterns of antimicrobial resistance genes in dairy agroecosystems. Foodborne Pathog Dis. 2016;13(6):296–302. https://doi.org/10.1089/fpd.2015.2092.
Durso LM, Harhay GP, Bono JL, Smith TPL. Virulence-associated and antibiotic resistance genes of microbial populations in cattle feces analyzed using a metagenomic approach. J Microbiol Methods. 2011;84(2):278–82. https://doi.org/10.1016/j.mimet.2010.12.008.
Wichmann F, Udikovic-Kolic N, Andrew S, Handelsman J. Diverse antibiotic resistance genes in dairy cow manure. MBio. 2014;5(2):e01017–3. https://doi.org/10.1128/mBio.01017-13.
Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–20. https://doi.org/10.1093/bioinformatics/btu170.
Langmead B, Salzberg SL. Fast gapped-read alignment with bowtie 2. Nat Methods. 2012;9(4):357. https://doi.org/10.1038/nmeth.1923.
Hyatt D, Chen GL, LoCascio PF, Land ML, Larimer FW, Hauser LJ. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics. 2010;11(1):119. https://doi.org/10.1186/1471-2105-11-119.
Li H, Durbin R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics. 2009;25(14):1754–60. https://doi.org/10.1093/bioinformatics/btp324.
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The sequence alignment/map (SAM) format and SAMtools. Bioinformatics. 2009;25(16):2078–9. https://doi.org/10.1093/bioinformatics/btp352.
Jia B, Raphenya AR, Alcock B, Waglechner N, Guo P, Tsank KK, et al. CARD 2017: expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res. 2017;45(D1):D566–73. https://doi.org/10.1093/nar/gkw1004.
Alcock BP, Raphenya AR, Lau TTY, Tsang KK, Bouchard M, Edalatmand A, et al. CARD 2020: antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2020;48(D1):D517–25. https://doi.org/10.1093/nar/gkz935.
Lu J, Breitwieser FP, Thielen P, Salzberg SL. Bracken: estimating species abundance in metagenomics data. Peerj Comput Sci. 2017;3:e104. https://doi.org/10.7717/peerj-cs.104.
Oksanen J, Blanchet FG, Kindt R, Legendre P, O’Hara RB, Simpson GL, et al. Vegan: community ecology package. R package version 1.17–4. http://cran.r-project.org>. Acesso Em. 2010;17(23):2010.
Gunnarsson S, Mie A. Organic animal production–a tool for reducing antibiotic resistance? In: Springer S, Grimm H, editors. Professionals in food chains. Wageningen: Academic Publishers; 2018. p. 13.
Misiewicz T, Shade J. Organic food and farming as a tool to combat antibiotic resistance and protect public health. The Organic Center. 2016;4. https://www.organic-center.org/sites/default/files/publication_files/2016/07/TOC_ Report_AntibioticResistance_FINAL.pdf. Accessed 9 Jan 2020.
Rovira P, McAllister T, Lakin SM, Cook SR, Doster E, Noyes NR, et al. Characterization of the microbial resistome in conventional and “raised without antibiotics” beef and dairy production systems. Front Microbiol. 2019;10:1980. https://doi.org/10.3389/fmicb.2019.01980.
Wang C, Dong D, Strong PJ, Zhu W, Ma Z, Qin Y, et al. Microbial phylogeny determines transcriptional response of resistome to dynamic composting processes. Microbiome. 2017;5(1):103. https://doi.org/10.1186/s40168-017-0324-0.
Burrows GE, MacAllister CG, Beckstrom DA, Nick JT. Rifampin in the horse: comparison of intravenous, intramuscular, and oral administrations. Am Journal Vet Res. 1985;46(2):442–6.
De Lucia M, Bardagi M, Fabbri E, Ferreira D, Ferrer L, Scarampella F, et al. Rifampicin treatment of canine pyoderma due to multidrug-resistant meticillin-resistant staphylococci: a retrospective study of 32 cases. Vet Dermatol. 2017;28(2):171–e36. https://doi.org/10.1111/vde.12404.
Ma L, Li B, Zhang T. Abundant rifampin resistance genes and significant correlations of antibiotic resistance genes and plasmids in various environments revealed by metagenomic analysis. Appl Microbiol Biotechnol. 2014;98(11):5195–204. https://doi.org/10.1007/s00253-014-5511-3.
Vikram A, Rovira P, Agga GE, Arthur TM, Bosilevac JM, Wheeler TL, et al. Impact of “raised without antibiotics” beef cattle production practices on occurrences of antimicrobial resistance. Appl Environ Microbiol. 2017;83(22):e01682–17. https://doi.org/10.1128/AEM.01682-17.
Edwards R. Resistance to β-lactam antibiotics in Bacteroides spp. J Med Microbiol. 1997;46(12):979–86. https://doi.org/10.1099/00222615-46-12-979.
Chambers L, Yang Y, Littier H, Ray P, Zhang T, Pruden A, et al. Metagenomic analysis of antibiotic resistance genes in dairy cow feces following therapeutic administration of third generation cephalosporin. P Natl Acad Sci USA. 2015;10(8):e0133764. https://doi.org/10.1371/journal.pone.0133764.
García N, Gutiérrez G, Lorenzo M, García JE, Píriz S, Quesada A. Genetic determinants for cfxA expression in Bacteroides strains isolated from human infections. J Antimicrob Chemother. 2008;62(5):942–7. https://doi.org/10.1093/jac/dkn347.
Agga GE, Cook KL, Netthisinghe AM, Gilfillen RA, Woosley PB, Sistani KR. Persistence of antibiotic resistance genes in beef cattle backgrounding environment over two years after cessation of operation. PLoS One. 2019;14(2):e0212510. https://doi.org/10.1371/journal.pone.0212510.
Roberts MC. Environmental macrolide–lincosamide–streptogramin and tetracycline resistant bacteria. Front Microbiol. 2011;2(2):40. https://doi.org/10.3389/fmicb.2011.00040.
Hurst JJ, Oliver JP, Schueler J, Gooch C, Lansing S, Crossette E, et al. Trends in antimicrobial resistance genes in manure blend pits and long-term storage across dairy farms with comparisons to antimicrobial usage and residual concentrations. Environ Sci Technol. 2019;53(5):2405–15. https://doi.org/10.1021/acs.est.8b05702.
Kyselková M, Jirout J, Vrchotová N, Schmitt H, Elhottová D. Spread of tetracycline resistance genes at a conventional dairy farm. Front Microbiol. 2015;29(6):536. https://doi.org/10.3389/fmicb.2015.00536.
Barbosa TM, Scott KP, Flint HJ. Evidence for recent intergeneric transfer of a new tetracycline resistance gene, tet (W), isolated from Butyrivibrio fibrisolvens, and the occurrence of tet (O) in ruminal bacteria. Environ Microbiol. 1999;1:53–64. https://doi.org/10.1046/j.1462-2920.1999.00004.x.
Agersø Y, Guardabassi L. Identification of Tet 39, a novel class of tetracycline resistance determinant in Acinetobacter spp. of environmental and clinical origin. J Antimicrob Chemother. 2005;55(4):566–9. https://doi.org/10.1093/jac/dki051.
Whittle G, Whitehead TR, Hamburger N, Shoemaker NB, Cotta MA, Salyers AA. Identification of a new ribosomal protection type of tetracycline resistance gene, tet (36), from swine manure pits. Appl Environ Microbiol. 2003;69(7):4151–8. https://doi.org/10.1128/AEM.69.7.4151-4158.2003.
Acknowledgements
We are grateful to the Center for Host-Microbe Interactions, School of Veterinary Medicine, University of Pennsylvania, for sequencing services.
Funding
This study was funded by a grant from the Pennsylvania Department of Agriculture (fund #44166102). The funders played no role in the design of the study, collection, analysis, or interpretation of data, or in writing the manuscript.
Author information
Authors and Affiliations
Contributions
DP and ZD obtained funding for and designed this study. HA assisted with study design and advised on the use of antimicrobials on farms. JT, JB, and LB obtained the samples and communicated with farmers. JT and BV performed all sample analysis. NI and DP performed all data analysis. DP and MH prepared the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The design of the experiment and animal sampling protocol were approved by the University of Pennsylvania Institutional Animal Care and Use Committee (IUCAC protocol 806194). All animal sampling was conducted on private farms with the permission of the farm owners.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1 Table S1
. Selected antimicrobials used on conventional dairy herds.
Additional file 2: Table S2
. Samples and sequencing information for organic and conventional farms. Ref: reference; Lact: lactating cow; NonLact: non-lactating cow; Org: organic; Conv: conventional.
Additional file 3: Table S3
. Results of PERMANOVA analysis for organic and conventional farms. R2 and P values given for farm type, sample type, and their interaction for microbiome and resistome.
Additional file 4: Table S4
. Distribution of antimicrobial resistance genes (ARG) on organic and conventional farms at 80% gene content.
Additional file 5: Table S5
. Distribution of antimicrobial resistance genes (ARG) on organic and conventional farms at 100% gene content.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pitta, D.W., Indugu, N., Toth, J.D. et al. The distribution of microbiomes and resistomes across farm environments in conventional and organic dairy herds in Pennsylvania. Environmental Microbiome 15, 21 (2020). https://doi.org/10.1186/s40793-020-00368-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40793-020-00368-5