Abstract
Background
Cutaneous metastases of colorectal cancer (CRC) are rare, occurring in 0.7% to 5% of cancer patients. Furthermore, the molecular subtypes of cutaneous metastasis of CRC are unclear. Here, we present a rare case of cutaneous metastasis of high-frequency microsatellite instability (MSI-high)/BRAFV600E-mutant cecum cancer.
Case presentation
A 77-year-old woman presented at the outpatient clinic with a subcutaneous mass on her left back. An excisional biopsy was performed and metastatic cutaneous adenocarcinoma was diagnosed. A computed tomography scan of the thorax and abdomen showed thickening of the cecum wall, the presence of pericolic lymph nodes, multiple masses in the liver, and a single nodule in the right lung. Right colectomy with D2 lymphadenectomy and functional end-to-end anastomosis was performed because of the almost-complete intestinal obstruction. The expression of KRAS wild type, BRAFV600E mutation, and MSI-high was detected in the cecum cancer using molecular pathological examination. She received chemotherapy with XELOX + BEV regimen (capecitabine + oxaliplatin + bevacizumab). After four administrations, a computed tomography scan showed reduction of distant metastases, which suggested partial response.
Conclusions
We encountered a rare case of cutaneous metastasis of MSI-high and BRAFV600E-mutant cecum cancer. In the future, it will be necessary to accumulate more cases to identify clinical features and more effective treatments for CRCs with cutaneous metastasis.
Similar content being viewed by others
Background
Colorectal cancer (CRC) is a common malignancy worldwide and is one of the leading causes of cancer-related deaths. More than 10% of patients with CRC have metastases at the time of diagnosis. CRC commonly metastasizes to the regional lymph nodes, lungs, liver, and peritoneum, but cutaneous metastases are rare. At the molecular level, CRC is a heterogeneous disease with several molecular subtypes that harbor distinct molecular, genetic, pathologic, and clinical characteristics. Here, we present a rare case of a 77-year-old female with cutaneous metastasis in high-frequency microsatellite instability (MSI-high)/B-type Raf kinase (BRAF) V600E mutant cecum cancer.
Case presentation
A 77-year-old woman presented at the outpatient clinic with a subcutaneous mass on her left back (Fig. 1a, b). She experienced severe weight loss and loss of appetite a few months before visiting the outpatient clinic. Excisional biopsy was performed, and histopathology showed a malignant neoplasm composed of well-formed ductal structures that were growing irregularly and invasively (Fig. 1c–e). Metastatic cutaneous cancer was suspected, because columnar epithelial cells formed a fused ductal structure, mucus was found in the lumen, and extensive necrosis of the lesion was noted. She was referred to our department for further examination and treatment.
a, b There was a polypoid lesion with redness on the left back. c Lesions were present from the dermis to the subcutaneous fatty tissue. d Necrosis was found in a wide area of the lesion. e Columnar epithelial cells formed a fused ductal structure. There were also ducts that produce mucus in the lumen (hematoxylin–eosin staining, c × loupe. d ×20 magnification. e ×200 magnification)
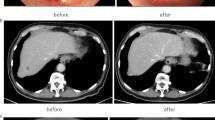
The patient had no significant medical history, allergies, and was not on medication. Laboratory tests showed: Hb 13.4 g/dL, CEA 2.4 ng/mL (normal value < 4.5 ng/mL), CA19-9 < 0.6 U/mL, CA125 128.4 U/mL (normal value < 35 U/mL). Computed tomography (CT) scan of the thorax to the abdomen showed thickening of the cecum wall, the presence of pericolic lymph nodes, multiple masses in the liver, and a single nodule in the right lung (Fig. 2a–c). 18F-fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT) revealed increased uptake in the cecum, the masses in the liver, and the right lung with standardized uptake values of 23.7, 8.6/5.9, and 4.9, respectively (Fig. 2d–f). Colonoscopy revealed a circumferential type 2 tumor of the cecum, and endoscopic biopsy showed moderately differentiated adenocarcinoma.
CT scan showed thickening of the cecum wall (a) and multiple masses in the liver (b), a single nodule in right lung (c). FDG-PET/CT revealed elevated uptake in the cecum and masses in the liver and a right lung with a standardized uptake value of 23.7 (d) and 8.6/5.9 (e), 4.9 (f), respectively. CT = computed tomography. FDG-PET/CT = 18F-fluorodeoxyglucose-positron emission tomography/computed tomography
Although she presented with unresectable distant metastases, because of the almost-complete intestinal obstruction, right colectomy with D2 lymphadenectomy and functional end-to-end anastomosis was performed (Fig. 3). The pathological diagnosis was moderately differentiated tubular adenocarcinoma with metastases in 5 of 13 resected lymph nodes. Both the primary site and the cutaneous metastatic lesion were not found in these histopathological findings, such as tumor-infiltrating lymphocytes, a Crohn’s-like lymphocytic reaction, mucinous or signet ring differentiation. The final pathological stage was pT4bN2aM1b, Stage IVb according to the Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma 9th edition. Furthermore, the expression of KRAS wild type, BRAFV600E mutation, and MSI-high was detected in the cecum cancer using molecular pathological examination with the PCR-reverse sequence-specific oligonucleotide probe method. After surgery, the patient received chemotherapy with XELOX + BEV regimen (capecitabine + oxaliplatin + bevacizumab). After four administrations of the XELOX + BEV regimen, a CT scan showed reduction of distant metastases, which suggested partial response (Fig. 4). The patient is alive 8 months after surgery.
Conclusions
Cutaneous metastasis of cancer is rare, occurring in 0.7%–5% of cancer patients [1]. Higher rates of cutaneous metastasis occur in melanomas, breast and lung cancers, and mucosal carcinomas of the head and neck [2]. The incidence of cutaneous metastasis of CRC is only 2.3% [3]. Furthermore, there are few reports on the genetic background of cutaneous metastasis of CRC. The most common site of cutaneous metastasis in CRC is the abdominal skin, often on surgical incision scars. Other cutaneous sites include the pelvis, back, chest, upper extremities, head, and neck [4]. The exact mechanisms of cutaneous metastasis are still unknown; however, four categories of mechanisms have been reported including the direct extension of primary cancer, lymphatic or hematogenous spread, and surgical implantation [5]. Identification of cutaneous metastasis indicates a poor prognosis. The average survival of patients after the diagnosis of cutaneous metastasis of colon carcinoma is 18 months. In cases of multiple metastases or unresectable lesions, chemotherapy could be considered. When the lesion is resectable and painful, local excision is the preferred treatment option.
MSI is a genetic change caused by a deficiency in mismatch repair (MMR) systems. MSI-high is present in approximately 15% of patients with CRC [4]. MSI-high is more common among those with stage II (20.2%) and stage III CRC (10.9%) [6] but is less frequent among those with stage IV CRC (3.5%) [7]. MSI-high CRC is associated with better survival, right-sided primary tumors, and poorly differentiated tumors with mucinous histological feature [8]. Another report showed that MSI-high CRC is diagnosed at a younger age and has fewer metastases to the liver and lungs than microsatellite stable (MSS) CRC [9]. The most frequent site of metastasis in MSI-high CRC is the perineum (43.8%), followed by the liver (22.9%) and distant lymph nodes (18.8%) [7]. To the best of our knowledge, this is the first report of cutaneous metastasis of MSI-high CRC. MSI-high tumors are heavily infiltrated by activated cytotoxic T-cell lymphocytes and Th1 lymphocytic cells [9]. Pages et al. showed that compared with tumors with vascular emboli and lymphatic and perineural invasion, tumors without these early steps of the metastatic processes had increased infiltration of immune cells [10]. These facts suggest that lymphocytic infiltration may make MSI-high CRC less likely to have distant metastases. There is greater immunoreactivity in deficient mismatch repair tumors 11,12,13] in stage II/III CRC; therefore, patients with MSI-high tumors have a better 5-year overall survival rate than those with MSS or MSI-low tumors [14]. A previous study showed that lymphocyte infiltration into primary tumors is a strong independent predictor of relapse and overall survival, with high lymphocyte infiltration being a positive prognostic factor in CRC [15]. A Buckowitz et al. reported that MSI-high CRCs with Crohn’s-like lymphocytic reaction are associated with fewer distant metastases [16]. In this case, since there is neither high lymphocyte infiltration nor Crohn’s-like lymphocytic reaction, it may have led to distant metastases. In contrast, MSI-high is a poor prognostic factor in stage IV CRC [17, 18]. Compared with MSS or MSI-low CRCs, MSI-high CRC has the lowest rate of liver metastases and the highest rate of peritoneal metastases, which are related to prognosis [19]. Tran et al. reported that BRAF mutant CRCs were observed to have significantly poorer survival compared with BRAF wild CRCs in MSI-high CRCs [18]. BRAF mutations may contribute to the poor prognosis of stage IV MSI-high CRC.
BRAF is an RAS-regulated serine/threonine kinase in the RAS/RAF/MEK/ERK mitogen-activated protein kinase (M) signaling pathway, which governs proliferation, differentiation, migration, and apoptosis. BRAF mutations are found in approximately 5% of patients with metastatic CRC in our country [20] and 34.6% with MSI-high CRC [17]. BRAF mutation is considered a poor prognostic factor. The most frequent site of metastasis in BRAF-mutant CRC is the liver (63%), followed by distant lymph nodes (56%) and the perineum (46%) [18]. Lianggong et al. reported the first case of cutaneous metastases of BRAF-mutant CRC [21]. In this case, FOLFIRI (irinotecan, calcium leucovorin, and 5-FU) in combination with cetuximab and the BRAF inhibitor vemurafenib caused cutaneous and liver metastases to shrink.
Pembrolizumab [22] and nivolmab + ipilimumab [23] have been confirmed to be effective treatments for MSI-high mCRC. In the KEYNOTE-177 study, pembrolizumab improved progression-free survival in patients with MSI-high metastatic CRC compared with the current chemotherapy regimen for first-line treatment of MSI-high metastatic CRC [24]. For BRAF-mutant metastatic CRC, FOLFOXIRI + bevacizumab was reported as the first-line chemotherapy in the subgroup analysis of the TRIBE study [25]. Furthermore, the effectiveness of the triple therapy of encorafenib, cetuximab, and binimetinib is reported [26]. Chemotherapy including BRAF inhibitors is expected to treat CRC with distant metastases. However, it is unclear whether chemotherapy for BRAF mutations or chemotherapy for high MSI is prioritized for distant metastases with BRAF-mutant and MSI-high CRC. That is the research task hereafter. In our case, XELOX + bevacizumab combination was selected as the treatment because of her poor performance status and based on the domestic drug approval status. It could be one of the treatment options for BRAF-mutant and MSI-high CRC, because the therapeutic effect was confirmed.
We encountered a rare case of cutaneous metastasis of CRC that had MSI-high and a BRAFV600E mutation. In future, it will be necessary to accumulate more cases to identify clinical features and more effective treatments for cutaneous metastatic CRC.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author, TY, upon reasonable request.
Abbreviations
- CRC:
-
Colorectal cancer
- MSI-high:
-
High-frequency microsatellite instability
- CT:
-
Computed tomography
- FDG-PET/CT:
-
18F-fluorodeoxyglucose-positron emission tomography/computed tomography, XELOX + BEV: capecitabine + oxaliplatin + bevacizumab, MMR: mismatch repair
- MSS:
-
Microsatellite stable
- FOLFIRI:
-
Irinotecan + calcium leucovorin + 5-FU
- FOLFOXIRI + BEV:
-
5-Fluorouracil + oxaliplatin + irinotecan + bevacizumab
References
Nesseris I, Tsamakis C, Gregoriou S, Ditsos I, Christofidou E, Rigopoulos D. Cutaneous metastasis of colon adenocarcinoma: case report and review of the literature. An Bras Dermatol. 2013;88:56–8.
Bachanova V, Burns LJ, McKenna DH, et al. Allogeneic natural killer cells for refractory lymphoma. Cancer Immunol Immunother. 2010;59:1739–44.
Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma. A retrospective study of 7316 cancer patients. J Am Acad Dermatol. 1990;22:19–26.
Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29:228–36.
Mehregan AH. Metastatic carcinoma to the skin. Dermatologica. 1961;123:311–25.
Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28:3219–26.
Koopman M, Kortman GA, Mekenkamp L, et al. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br J Cancer. 2009;100:266–73.
Kim H, Jen J, Vogelstein B, Hamilton SR. Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol. 1994;145:148–56.
He WZ, Hu WM, Wang F, et al. Comparison of mismatch repair status between primary and matched metastatic sites in patients with colorectal cancer. J Natl Compr Canc Netw. 2019;17:1174–83.
Frank P, Anne B, Matthieu C, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–66.
Marisa L, Svrcek M, Collura A, et al. The balance between cytotoxic T-cell lymphocytes and immune checkpoint expression in the prognosis of colon tumors. J Natl Cancer Inst. 2018. https://doi.org/10.1093/jnci/djx136.
Ishikawa T, Fujita T, Suzuki Y, et al. Tumor-specific immunological recognition of frameshift-mutated peptides in colon cancer with microsatellite instability. Cancer Res. 2003;63:5564–72.
Kloor M, Becker C, Benner A, et al. Immunoselective pressure and human leukocyte antigen class I antigen machinery defects in microsatellite unstable colorectal cancers. Cancer Res. 2005;65:6418–24.
Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247–57.
Llosa NJ, Cruise M, Tam A, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5:43–51.
Buckowitz A, Knabel H-P, Benner A, et al. Microsatellite instability in colorectal cancer is associated with local lymphocyte infiltration and low frequency of distant metastases. Br J Cancer. 2005;92:1746–53.
Venderbosch S, Nagtegaal ID, Maughan TS, et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res. 2014;20:5322–30.
Tran B, Kopetz S, Tie J, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 2011;117:4623–32.
Sherman SK, Schuitevoerder D, Chan CHF, Turaga KK. Metastatic colorectal cancers with mismatch repair deficiency result in worse survival regardless of peritoneal metastases. Ann Surg Oncol. 2020;27:5074–83.
Hashiguchi Y, Muro K, Saito Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25:1–42.
Liao L, Cheng Q, Zhu G, Pei F, Ye S. Cutaneous metastasis of ascending colon cancer harboring a BRAF V600E mutation: a rare case report. Medicine (Baltimore). 2020;99:e20026.
Le DT, Kim TW, Van Cutsem E, et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol. 2020;38:11–9.
Overman MJ, Lonardi S, Wong KYM, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36:773–9.
Andre T, et al. Pembrolizumab versus chemotherapy for microsatellite instability-high/mismatch repair deficient metastatic colorectal cancer: the phase 3 KEYNOTE-177 Study. J Clin Oncol. 2020;38:LBA4–LBA4. https://doi.org/10.1200/JCO.2020.38.18_suppl.LBA4.
Loupakis F, Cremolini C, Salvatore L, et al. FOLFOXIRI plus bevacizumab as first-line treatment in BRAF mutant metastatic colorectal cancer. Eur J Cancer. 2014;50:57–63.
Kopetz S, Grothey A, Yaeger R, et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E-Mutated colorectal cancer. N Engl J Med. 2019;381:1632–43.
Acknowledgements
We thank Dr. Hideki Noda for providing cutaneous metastasis information. We would like to thank Editage (www.editage.jp) for the English language editing.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
KY drafted the manuscript and treated the patient. TY treated the patient and helped draft the manuscript. MY, KO, TK, MI, DS, YC, KN, YS, and HM treated the patient. HI, SS, and MO determined the treatment plan and revised the manuscript. All authors read and approved the final manuscript.
Authors' information
KY, TY, MY, KO, TK, MI, DS, YC, KN, YS, and HM are staff surgeons of the Department of Surgery, Hiroshima City Hiroshima Citizens Hospital. HI is the chief general manager of the Department of Surgery, Hiroshima City Hiroshima Citizens Hospital. SS and MO are Vice President of Hiroshima City Hiroshima Citizens Hospital.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yunoki, K., Yano, T., Yoshimitsu, M. et al. Cutaneous metastasis of cecum cancer with MSI-high and BRAFV600E mutation: a case report. surg case rep 7, 185 (2021). https://doi.org/10.1186/s40792-021-01265-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40792-021-01265-w