Abstract
Background
Esophageal perforation after aortic replacement/stenting for aortic dissection or aneurysm is a rare but severe complication. However, its cause, standard treatment, and prognosis are unclear. We analyzed the treatment and outcome retrospectively from seven cases experienced at our hospital.
Case presentation
The median age of the patients was 70 years (range, 41–86), and six of the seven cases were male. As the first treatment, aortic replacement techniques were performed in five, and thoracic endovascular aortic repair (TEVAR) procedure was performed in two. We evaluated the treatment of the perforation, the cause of death, and the median survival time after reparative surgery (esophagectomy).
Initial treatment of the perforation was esophagectomy without reconstruction in six and esophagogastric bypass (later, esophagectomy was performed) in one. Three of seven cases could be discharged from hospital or moved to another hospital, but two of these three cases died of major bleeding on postoperative days 320 and 645. The other four esophagectomy cases died in hospital because of sepsis on postoperative days 14, 30, and 41 and major bleeding on postoperative day 54. The one surviving case was a 65-year-old man who underwent reconstruction, and was still alive without signs of infection at 424 days postoperatively.
Conclusion
The prognosis of esophageal perforation cases after aortic replacement/stenting for thoracic aortic dissection or aneurysm is poor, though there were some cases with relatively long survival. Therefore, the indication for invasive esophagectomy should be decided carefully. Control of infection including regional infection is essential for successful treatment.
Similar content being viewed by others
Background
Esophageal perforation after aortic replacement or stent grafting for aortic dissection or aneurysm is a rare but potentially fatal complication. However, its cause, standard treatment, and prognosis are unclear [1,2,3].
There are some reports with the key word of aorto-esophageal fistula (AEF) between the thoracic aorta and the esophagus. However, it is unclear whether the definition of AEF includes esophageal perforation after aortic replacement, as artificial grafts do not fistulize. In fact, most AEF-related papers are about cases after thoracic endovascular aortic repair (TEVAR) procedure [3,4,5].
The development of esophageal perforation indicates the occurrence and persistence of infection leading to mediastinitis or sepsis. However, it is interesting to consider the cause of the infection and to speculate on what happened at the site of the local lesion, that is, whether esophageal perforation is caused by localized infection including artificial graft infection or the infection is caused by esophageal perforation. In any case, perforation becomes the cause of continuous infection, and esophagectomy may be necessary for definitive treatment. However, esophagectomy is highly invasive surgery, and the indication should be carefully considered, particularly after major cardiovascular surgery. It is not also sure whether localized infection including graft/stent infection is improved after esophagectomy.
We retrospectively reviewed our seven cases of esophageal perforation after aortic replacement/stenting and analyzed their treatment and outcome.
Case presentation
We experienced seven esophageal perforation cases after aortic replacement/stenting for thoracic aortic dissection or aneurysm from 2013 to 2015 (Table 1). The median age of patients was 70 years (range, 41–86), and six of the seven cases were male. The cardiovascular surgical procedures included two cases of total arch replacement for aortic dissection, two cases of replacement techniques, and two cases of endovascular aneurysm repair for aortic aneurysm. Valve-sparing aortic root replacement was initially performed for acute aortic dissection with Marfan syndrome case. Several operations were performed in patient nos. 3, 5, and 7 because of disease progression. Continuous localized infection after cardiovascular surgery was observed in patient nos. 2, 3, and 6. The symptoms leading to the diagnosis of eshophageal perforation and the period from cardiovascular surgical procedure to esophageal perforation are shown in Table 1. We evaluated the treatment of perforation, cause of death, and median survival time after reparative surgery (esophagectomy).
Initial treatment of the perforation was esophagectomy without reconstruction in six and esophagogastric bypass (with later esophagectomy) in one. Gastrostomy for enteral alimentation was performed in all six esophagectomy cases. Aortic replacement of the descending aorta was performed at the same time in patient no. 3, and evacuation of hematoma in the left thoracic cavity caused by dissection was needed in patient no. 7. Median operation time was 375 min (range, 327–638 min), and blood loss was 500 ml (range, 85–2374 ml). Postoperative complications were observed in six of seven cases (Table 2).
Patient nos. 1, 6, and 7 could be discharged from hospital or moved to another hospital, but patient nos. 6 and 7 died of major bleeding on postoperative days 320 and 645. These two cases experienced chronic regional infection of the artificial graft/stent. The other four esophagectomy cases died in hospital because of sepsis on postoperative days 14, 30, and 41 and major bleeding on postoperative day 54.
The one surviving case (no. 1) was a 65-year-old man who underwent reconstruction without severe complications, and was still alive without signs of infection at 424 days postoperatively.
Conclusions
Delayed esophageal perforation secondary to thoracic aortic replacement or thoracic endovascular aortic repair (TEVAR) is a rare but potentially fatal condition [2, 4,5,6,7].
Seto et al. reported that although the exact mechanism of secondary esophageal perforation after stent grafting remains unknown, hypotheses include (1) direct erosion of the stent graft into the esophagus, (2) pressure necrosis caused by the self-expanding endoprosthesis, (3) ischemic esophageal necrosis due to disruption of the arteries that feed the esophagus, (4) infection of the stent-graft prosthesis (artificial graft for aortic replacement was included in our case), (5) pseudoaneurysm development, and (6) endoleakage into the residual aneurysmal sac [7].
In our cases that was performed aortic replacement procedure (nos. 1–6), (3) and (4) were thought to be a possible cause. In addition, no. 3 case had potential for (2), and no. 5 had potential for (2) and (5). No. 7 with Marfan syndrome had highly potential for (5) and (6). There was no equivalent case for (1).
We consider the hypothesis that ischemic esophageal necrosis due to disruption of the arteries that feed the esophagus should be focused. The thoracic esophagus is fed by bronchial and esophageal branches of the thoracic aorta. Aortic replacement or stent grafting can potentially damage these feeding arteries of the thoracic esophagus. We consider that the relatively long period from cardiovascular surgery to esophageal perforation supports this hypothesis. Uncontrolled continuous localized infection including artificial graft infection seems certain to aggravate esophageal wall ischemia and disruption in a similar way.
Eggebrecht et al. reported that they observed mild erosive lesions in the esophagus that led to perforation on endoscopy [4]. This suggested that the lesion took some time to progress to perforation, and ischemic change of the esophageal wall occurred gradually. They also mentioned that recognition of this pre-perforation state could have prompted early triage and/or surgical repair before esophageal perforation.
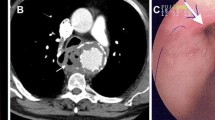
In no. 5 case, we had recognized redness and erosive change of the esophagus on endoscopy before the aortic replacement. If we had decided esophagectomy at that point, we might avoid poor prognosis of the case.
The prognosis of esophageal perforation cases after aortic replacement/stenting for thoracic aortic dissection or aneurysm is extremely poor especially in the elderly cases. In the elderly cases (over 80 years old), nos. 3, 4, and 5 died 41, 14, and 30 days with sepsis and other severe complications after the esophagectomy, respectively (Table 2.). Therefore, the indication for highly invasive esophagectomy should be decided carefully. We surgeons should restrict the esophagectomy to sustainable patients for invasive surgery in consideration of age and complications. We want to suggest elderly cases over 80 years old should be refrained from the esophagectomy.
It is important to control infection including regional infection and progression of cardiovascular disease for successful treatment as the result of a survival case (no. 1).
Artificial graft/stent with chronic infections was considered to be removed for long survival. We also consider that it is important to perform cardiovascular surgery with attention to maintaining esophageal blood flow.
Vascular-rich tissue filling (muscle flap or omental) to the infection site after esophagectomy may be useful for infection control. Our surviving case underwent intercostal muscle flap filling which could control prosthetic graft infection.
References
Kieffer E, Chiche L, Gomes D. Aortoesophageal fistula: value of in situ aortic allograft replacement. Ann Surg. 2003;238(2):283–90. Epub 2003/08/02.
Kaneda T, Onoe M, Asai T, Mohri Y, Saga T. Delayed esophageal necrosis and perforation secondary to thoracic aortic rupture: a case report and review of the literature. Thorac Cardiovasc Surg. 2005;53(6):380–2. Epub 2005/11/29.
Wada T, Takeuchi H, Fujimura N, et al. Intractable esophago-mesiastinal fistula as a rare complication following thoracoabdominal aortic replacement. Esophagus. 2011;8:277–81.
Eggebrecht H, Mehta RH, Dechene A, Tsagakis K, Kuhl H, Huptas S, et al. Aortoesophageal fistula after thoracic aortic stent-graft placement: a rare but catastrophic complication of a novel emerging technique. J Am Coll Cardiol Intv. 2009;2(6):570–6. Epub 2009/06/23.
Chiesa R, Tshomba Y, Kahlberg A, Marone EM, Civilini E, Coppi G, et al. Management of thoracic endograft infection. J Cardiovasc Surg. 2010;51(1):15–31. Epub 2010/01/19.
Akashi H, Kawamoto S, Saiki Y, Sakamoto T, Sawa Y, Tsukube T, et al. Therapeutic strategy for treating aortoesophageal fistulas. Gen Thorac Cardiovasc Surg. 2014;62(10):573–80. Epub 2014/08/27.
Seto T, Fukui D, Tanaka H, Komatsu K, Ohtsu Y, Terasaki T, et al. Tracheo-bronchial obstruction and esophageal perforation after TEVAR for thoracic aortic rupture. Ann Vasc Dis. 2014;7(4):421–5. Epub 2015/01/17.
Authors’ contributions
All the authors contributed intellectual content of this paper as mentioned. YY, YK, MH, and TK participated in the design and acquisition of the data. TI conceived of this study and participated in the design and coordination, and RF acted as the moderator of this work. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This report was in accordance with the Helsinki Declaration of 1975, as revised in 2000 and 2008. We gave consideration to the privacy of the patient, and the manuscript does not include any identifying information.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Yaguchi, Y., Kumata, Y., Horikawa, M. et al. Seven esophageal perforation cases after aortic replacement/stenting for thoracic aortic dissection or aneurysm. surg case rep 3, 77 (2017). https://doi.org/10.1186/s40792-017-0354-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40792-017-0354-7