Abstract
Purpose
This review aimed to reveal the influence of implant guides on surgical accuracy with regard to supporting types, manufacturing methods and design (including fixation screws and sleeves).
Methods
A literature search related to accuracy of surgical guides for dental implantation was performed in Web of Science and PubMed. Studies with in vivo or in vitro deviation data published in recent 5 years (2018–2022) were included and assessed by Newcastle–Ottawa Scale with regard to risk of bias and reliability degree of clinical studies. Accuracy-related deviation data were summarized as forest plots and normal distributions.
Results
Forty-one articles were included with high degree of credibility. Data showed that implant surgery accuracy can be achieved with mean distance deviation < 2 mm (most < 1 mm) and angular deviation < 8° (most < 5°).
Conclusions
Bilateral tooth-supported guides exhibited highest in vitro accuracy and similar in vivo accuracy to unilateral tooth-supported guides; mucosa-supported guides exhibit lowest in vivo accuracy, while its in vitro data showed low credibility due to mechanical complexity of living mucosa tissue. Milling exhibited higher in vivo accuracy of guides than 3d-printing, though further data support was needed. Design of fixation screws and sleeves of implant guides affected the surgical accuracy and might remain a research focus in near future. However, lack of universal evaluation standards for implantation accuracy remained a major problem in this field. The influence of implant guides on surgical accuracy revealed in this review might shed light on future development of dental implantology.
Similar content being viewed by others
Introduction
Global population aging results in increasing demand for dental implant surgery, emphasizing the necessity of improving the surgical accuracy, which directly increases the success rate and reduces surgical trauma [1]. In recent years, digital technology that realizes the visualization of planting schemes significantly raises the surgical accuracy of dental implantation [2]. Digital surgical guides, as the information carrier of implant direction, position, angle, can effectively enhance the surgical accuracy, reduce surgical time and complications [3]. The surgical accuracy of dental implantation is influenced by data acquisition method, manufacturing procedure, supporting types, fixation screws and sleeve design of the surgical guide.
Accuracy of implanting guide comprises trueness and precision (ISO 5725–1:1994). Trueness refers to the deviation between postoperative placement and preoperative plan of the implant; precision refers to the deviation of repetitive test results. Generally, accuracy discussed in clinical studies refers to trueness, while in vitro studies (e.g., implant on plaster models) may involve both trueness and precision. The accuracy compared and discussed in this review mainly refers to trueness. Despite the lack of a universal evaluation standard for implantation surgical accuracy, common indicators including coronal deviation (mm), apical deviation (mm), depth deviation/vertical deviation (mm), angular deviation (°) are applied in existing literatures and discussed in this review (Fig. 1).
Preoperative data acquisition, including intraoral (collected via intraoral scanning or extraoral scanning of impressions) and CBCT data, is the prerequisite for implant guide design [4]. Existing studies indicated a higher accuracy of intraoral scanners (IOSs) than extraoral scanning of impressions [5], and IOSs are the developing trend in the future and are highly accepted among the patients due to its flexibility and simplicity [6]. Accuracy of commercial IOSs ranges from 20 to100 μm for dentition, and 50–250 μm for edentulous jaws [7, 8]. By contrast, CBCT exhibits lower accuracy: within an applicable range of radiation dose (20–100 μSv, generally considered as a balance of safety and accuracy), the voxel size of CBCT-constructed 3D models ranges from 0.1 to 0.5 mm3, and the accuracy ranges from 200 to 1000 μm [9]. Considering the accuracy range of CBCT, it is generally recommended to maintain a safety margin of 2 mm from adjacent anatomical structures in clinical practice [10]. As the accuracy of dentition model obtained by CBCT is relatively low to meet the requirements of surgical guide design, integration of CBCT-constructed model and scanned dentition model is commonly applied [4]. As data acquisition accuracy will be projected onto design of surgical guides, and current evaluation of implant surgery accuracy is mainly based on CBCT data acquisition, comparability of the accuracy indicator values in different literatures should be reviewed dialectically.
Two common narrative aspects of existing reviews on the accuracy of digital surgical guides are comparing static surgical guides to dynamic or augmented reality (AR) navigation systems [11] and analyzing the accuracy of surgical guides for specific conditions. Examples of the later include comparison of the applicability, accuracy and clinical effects of digital and traditional surgical guides in flapless implant surgery reviewed by Emitis Natali Naeini in 2020 [12] demonstrating higher accuracy of digital surgical guides than traditional ones; comparison of the accuracy of digital surgical guides between flap and flapless implant surgery by Karthikeyan Subramani in 2022 [13] indicating that flapless surgery resulted in higher accuracy; analysis of the accuracy of edentulous mucosa-supported surgical guides prepared by Stereolithography by Cheongbeom Seo in 2018 [14] and surgical guides for partially edentulous patients analyzed by Ramadhan Hardani Putra in 2022 [3], both exhibiting no significant differences in any specific influencing factors. In addition, R. Eftekhar Ashtiani in 2021 [15] compares the accuracy among different guiding systems and concludes that both the implant and its design software influenced the accuracy, though no final statement could be made on an optimized system. Fernando Bover-Ramos in 2018 [16] compares the accuracy of surgical guides studied in cadaveric, clinical, and in vitro models and finds that in vitro studies showed higher accuracy than clinical and cadaveric studies, and the accuracy of full-guided surgical guides are higher than half-guided ones. A review published by Firas Al Yafi in 2019 [1] summarizes the operational procedures of digital surgical guides in detail, and though lack of in-depth data analyses, provides a comprehensive list of accuracy-affecting factors. A review by Ali Tahmaseb in 2018 [17], based on published research data from 2012–2015, concludes that the accuracy of surgical guide was within the clinically acceptable range in most cases and was higher in partially edentulous patients than edentulous patients.
Considering the lack of a systematic and comprehensive review of the factors influencing the accuracy of surgical guides, this review analyzed and discussed the accuracies of all static digital surgical guides in aspects including guide supporting types, manufacturing methods and design of implant guides (including fixation screws and sleeves), covering different influencing factors to provide a comprehensive guidance for implant design in future. This review aimed to reveal the influence of implant guides on surgical accuracy, and to provide reference for future development of digital dental implantology. It was hypothesized that these aspects influence the accuracies of static digital surgical guides, and the hypothesis was verified by collecting and categorizing the numerical data of surgical accuracy indicator reported in literature of the last five years.
Materials and methods
This review has been registered in PROSPERO, with the registration ID of 416029.
The search was performed using keywords based on the PICO approach. The PICO was formulated as follows: Participants (P) = patients of dental implantation; Intervention (I) = implants placed using digital surgical guides; Comparison or control (C) = different supporting types, design of fixation screws, design of sleeve, manufacturing methods of the surgical guides; Outcome measures (O) = coronal deviation (mm), apical deviation (mm), depth deviation (mm), angular deviation (°).
Based on the above PICO analysis, this review applied the keywords: computer-aided implant surgery (CAIS); static surgical guide; accuracy; deviation; dental implants) and MeSH terms (Surgery, Computer-Assisted) AND (Dental Implants). Advanced searching strategies were established based on the above keywords to perform an extensive search of the literature for papers related to accuracy of digital surgical guides for dental implantation on the databases of Web of Science (WOS) and PubMed as follows:
WOS: TS = (oral OR dental) AND TS = (surgical guide) AND TS = (accuracy OR precision OR rightness) AND TS = (implant OR implantation).
PubMed: (oral OR dental) AND (surgical guide) AND (accuracy OR precision OR rightness) AND (implant OR implantation).
Literatures were screened using predetermined inclusion and exclusion criteria as follows.
Inclusion criteria:
-
1.
Types of literature were limited to research articles, case reports and clinical trials that were peer-reviewed and published in WOS or PubMed cited scientific journals.
-
2.
Titles and abstracts of the articles were related to the accuracy of digital surgical guides for dental implantation.
-
3.
At least one of the following in vivo or in vitro deviation data must be involved: coronal deviation (mm), apical deviation (mm), depth deviation (mm), angular deviation (°).
-
4.
Written in English.
-
5.
The year of publication was restricted in recent five years (2018–2022).
Exclusion criteria:
-
1.
Reviews, meeting abstracts, grey literature or non-peer-reviewed literature were excluded.
-
2.
Written in languages other than English.
-
3.
Published before 2018.
To minimize the potential for reviewer bias, two reviewers (CM and JS) independently conducted literature searches and performed the study selection. Both reviewers strictly followed the inclusion and exclusion criteria, and any disagreement was resolved by discussion.
Data were extracted by one reviewer (JS) and examined by another reviewer (CM). The following data were directly collected from the included articles: literature information (authors, year, and title), research type (clinical/cadaver/in vitro), number of patients/cadavers/models, number of implants, surgical information (full-/half-guided, planning software, implant site, jaw position, bone quality and implant length and diameter), types of surgical guide (bilateral tooth-supported, unilateral tooth-supported, and mucosa-supported guides), guide fabrication method (3D printing/milling), number of fixation pins, deviation data including global/horizontal coronal deviation (mm), global/horizontal apical deviation (mm), angular deviation (°) and vertical deviation (mm). The form of deviation data included mean ± SD and/or median (min, max).
To assess the risk of bias and degree of reliability, clinical studies were scored based on the Newcastle–Ottawa Scale (NOS) adapted by Chambrone et al. [18] including evaluation of four subcategories: sample selection of study groups, comparability, outcome and statistical analysis. Specific scoring items are listed in Additional file 1: Methods. A maximum of 13 points could be obtained for each study, with a score of 10–13 indicating high study quality, a score of 7–9 indicating moderate study quality, and a score of less than 7 indicating low study quality.
Results
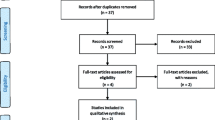
Following the PRISMA guideline (Fig. 2), the search strategy reported 954 records, among which 249 duplicate records were firstly removed. After overviewing the titles, abstracts and keywords, the investigators excluded 54 reviews, 30 articles written in languages other than English, and 580 records with no considerable information about accuracy of surgical guides for dental implantation. The remaining 41 records were sought for full-text retrieval and assessment of data availability, and all 41 articles involved available deviation data. The 41 articles were ultimately included in this review, among which 21 were in vitro studies, 19 were in vivo studies, and 1 was a comparison of in vitro and in vivo accuracy. Among the 19 in vivo studies, 2 were performed on cadaver, and 17 were clinical researches. Among 17 clinical research types, 3 were case–control studies, 11 were clinical trials, and 3 were cohort studies. The comparison of in vitro and in vivo accuracy was a case–control study (Table 1).
Search flowchart according to PRISMA guidelines [19]. (n = number of records)
NOS analysis was performed in the 20 in vivo studies in this paper, of which 3 were of medium quality and 17 were of high quality (Additional file 1: Fig. S1). Specifically, all studies exhibited high scores in sample selection, comparability and statistics, though the outcomes of patient follow-up adequacy were presented in only 3 studies. The NOS results indicated a high degree of credibility of this review.
A descriptive table of the 41 studies included in this review with deviation data including global/horizontal coronal deviation (mm), global/horizontal apical deviation (mm), angular deviation (°) and vertical deviation (mm) in the form of mean ± SD and/or median (min, max) was provided as Table 2, and the data listed and discussed in following part of this review are based on the corresponding information. The comparison criteria (supporting types, design of fixation screws, design of sleeve, manufacturing methods of the surgical guides) and accuracy-related deviation data of the included literatures were summarized as forest plots (Tables 3, 4, 5, Additional file 1: Tables S1–S4) and normal distributions (Figs. 4, 5).
As presented in Table 3 and Additional file 1: Tables S1, S2 forest plots of global coronal deviations, global apical deviations and angular deviations from the reviewed studies are summarized according to different guide supporting types. Tooth-supported guides are the most frequently applied in studies published in recent five years: among the 41 involved literatures, 27 involved bilateral tooth-supported guides, 9 involved mixed tooth-/bone- or tooth-/mucosa-supported guides (unilateral tooth-supported), and only 7 applied mucosa-supported guides. The accuracy-related numerical data (mean value, or median when mean value is not available) of bilateral tooth-supported implant guide ranges within 0.1–1.18 mm (in vitro)/0.46–1.47 mm (in vivo) in global coronal deviation, 0.18 ~ 1.37 mm (in vitro)/0.39–1.07 mm (in vivo) in horizontal coronal deviation, 0.12–1.95 mm (in vitro)/0.28–1.77 mm (in vivo) in global apical deviation, 0.31–1.68 mm (in vitro)/0.64–1.17 mm (in vivo) in horizontal apical deviation, 0.77–7.713° (in vitro)/1.4–4.74° (in vivo) in angular deviation, and 0.11–0.95 mm (in vitro)/0.03–0.84 mm (in vivo) in vertical deviation. Global coronal deviations of unilateral tooth-supported guides (including mixed tooth-/bone- or tooth-/mucosa-supported guides) are between 0.284–1.43 mm (in vitro)/0.21–1.2 mm (in vivo), horizontal coronal deviations between 0.47–0.67 mm (in vitro), global apical deviations between 0.65–2.19 mm (in vitro)/0.67–1.45 mm (in vivo), horizontal apical deviations between 0.91–1.72 mm (in vitro), angular deviations between 2.16–6.81 mm (in vitro)/3.1–5.62 mm (in vivo)°, and vertical deviations between 0.16–0.57 mm (in vitro). Global coronal deviations of mucosa-supported guides are between 0.45–0.82 mm (in vitro)/0.98–1.987 mm (in vivo), horizontal coronal deviations between 0.12–0.67 mm (in vivo), global apical deviations between 0.71–0.99 mm (in vitro)/1.18–2.124 mm (in vivo), horizontal apical deviations between 0.31–0.89 mm (in vivo), angular deviations between 2.39–3.41° (in vitro)/3.12–7.177° (in vivo), and vertical deviations between 0.44–0.52 mm (in vitro)/0.22–0.64 mm (in vivo) (Fig. 4, more data see Table 3).
Only 3 studies involved the comparison of guides with/without fixation screws, among which 2 were in vitro studies, and 1 was in vivo study (Table 4). Only 2 studies involved the comparison of sleeve length of guides.
Among the 41 involved literatures in this review, 29 involved 3D printing-fabricated guides, 3 involved milling-fabricated guides, 3 involved both, and 6 involved no information of fabricating methods. The in vitro accuracy-related numerical data (mean value, or median when mean value is not available) of milling guides ranges within 0.2–1.37 mm in horizontal coronal deviation, 0.62–1.68 mm in horizontal apical deviation, 0.61–3.49° in angular deviation; and the in vitro data of 3d printing guides ranges within 0.1–1.43 mm in global coronal deviation, 0.18–0.95 mm in horizontal coronal deviation, 0.12–2.19 mm in global apical deviation, 0.31–1.72 mm in horizontal apical deviation, 0.78–7.713° in angular deviation., and 0.11–0.64 mm in vertical deviation. The in vivo data of milling guides ranges within 0.12–0.67 mm in horizontal coronal deviation, 0.31–0.89 mm in horizontal apical deviation; and the data of 3d printing guides ranges within 0.21–1.987 mm in global coronal deviation, 0.39–1.12 mm in horizontal coronal deviation, 0.28–2.24 mm in global apical deviation, 0.64–1.57 mm in horizontal apical deviation, 1.4–7.177° in angular deviation, and 0.03–1.26 mm in vertical deviation (Fig. 5, more data see Table 2). Studies of milled guides mainly apply the horizontal deviation indicators following its coordinate system, less than 3 literatures apply the global deviation indicators, therefore the forest plots (Table 5 and Supplementary Table S3-S4) and normal distributions (Fig. 5) concerning different fabrication approaches are based on horizontal coronal deviations and horizontal apical deviations.
As visually shown in Tables 3, 4, 5 and Additional file 1: Tables S1–S4, with current technology of digital implant guides, implant surgery accuracy can be achieved with the mean distance deviation < 2 mm (most < 1 mm) and angular deviation < 8° (most < 5°).
Discussion
Guide supporting type
To verify the hypothesis that supporting types influence the accuracies of surgical guides, we collectively categorized and analyzed guide type and deviation data in existing literature. Implant guides are divided into categories according to its support types, including bone-supported, mucosa-supported, tooth-supported, and any combination (Fig. 3). Theoretically, the anatomical differences among teeth, bone and mucosa may lead to different accuracy of guides with different support types. Bilateral tooth-supported guides provide best retention and biomechanical stability with anchorage on hard tissue, therefore theoretically endow highest accuracy.
Although with advantages in accuracy and operability, bilateral tooth-supported guides are indicated for patients with intact teeth both mesial and distal to the edentulous area. As for distal extension edentulism, one of the most common clinical manifestation, unilateral tooth-supported guides including mixed tooth-/bone- or tooth-/mucosa-supported guides are often used to provide efficient retention.
Bone-supported guides are overlaid on the alveolar crest exposed via full‐thickness mucoperiosteal flap operation and fixed with fixation screws. Its larger surgical wound, upturned tissue flap affects its repositioning, resulting in relatively low theoretical accuracy. Simple bone-supported guides are seldom reported in recent five years [62], and among the 41 researches included in this review, only two studies applied mixed tooth-/bone-supported guides [63, 64].
Mucosa-supported guides are indicated for completely edentulous patients or patients who barely have residual teeth. Without flap operation, it is anchored to the bone through the mucosa with fixation screws. To be noted, a recent research reported that calculation of implant angular deviation of mucosa-supported guides by tissue or implant alignment resulted in different values [41], emphasizing the lack of standard for accuracy measurement, and indicating that comparability of the accuracy indicator values in different literatures should be reviewed dialectically.
Since different guide support types are used in various situations, the existing literature rarely directly compares the accuracy among different guide types. A systematic review in 2012 compares the accuracy of different guide types, and reveals a significant higher accuracy of tooth- and mucosa-supported guides than bone-supported ones, with no significant difference between tooth- and mucosa-supported guides [63]. The review mentions only two researches that directly compare different support types [65, 66]. A systematic review in 2018 showed that the implantation placement in cases using tooth-supported guides is more accurate than bone- and mucosa-supported [16].
In this review, as accuracy-related parameters differ in different studies, three parameters with relatively high frequency of application (global coronal deviations, global apical deviations and angular deviations) are selected for normal distribution analyses (Fig. 4). It is interesting to notice that the normal distribution of deviations varied with research types, especially for mucosa-supported guides, which exhibited the highest deviation peaks in all three parameters in in vivo researches (Fig. 4A–C), and the lowest in deviation peaks and narrowest distribution in vitro studies (Fig. 4D–F). This opposite trend was probably attributed to the different elasticity between in vitro experimental model and in vivo mucosa. In vitro experimental mucosa-imitating models exhibited simpler mechanical properties than living tissues, resulting in higher accuracies.
In addition, the use of anesthetics may affect the accuracy of the procedure due to elasticity of mucosa and its deformation upon penetration [67]. Compared to bilateral tooth-supported guides, unilateral tooth guides exhibited higher deviations in most categories (Fig. 4C–F), except in in vivo global coronal deviation where unilateral tooth-supported guides exhibited slightly lower distribution than bilateral guides (Fig. 4A), and in in vivo global apical deviation where bilateral and unilateral tooth-supported guides showed a similar data range (Fig. 4B).
In summary, bilateral tooth-supported guides exhibited the highest in vitro accuracy and similar in vivo accuracy to unilateral tooth-supported guides; mucosa-supported guides exhibit the lowest in vivo accuracy, while its in vitro data showed low credibility due to the mechanical complexity of living mucosa tissue.
Design of fixation screws
For bone- and mucosa-supported guides, fixation screws can be further introduced to fix the surgical guide and avoid displacement. The accuracy of implantation is reported to be improved by the application of fixation screws and influenced by its distribution [63, 68]. Since fixation screws are regular designs for implant guides, guides without fixation screws are only involved in three studies [21, 43, 57] as included in this review, all compared to groups with fixation screws in the same research. The difference between the two in vitro studies [21, 57] was not significant, and though the in vivo study [43] reported significantly higher accuracy in experimental group using fixation screws, whether the accuracy was influenced by the guide type (full-/half-) or the usage of fixation screws still requires further study due to the small sample size (Table 4).
For mucosa-supported guides used in edentulous patients, the use of fixation screws provide larger surface support and reduce the intraoperative displacement [69], efficiently reduce the angular deviation [68], depth deviation and horizontal deviation [57]. Therefore, in cases demanding a high depth precision and avoiding injury to the mandibular nerve, application of fixation screws contribute to better implant results [57].
For mixed tooth-/mucosa-supported guide in free-end dental implantation, application of fixation screws also results in a significant improvement in the accuracy regarding horizontal apical and depth deviation (direction considered) [21]. Apart from mucosa-supported and mixed tooth-/mucosa-supported guides, fixation screws can also be introduced into tooth-supported guides to achieve improved stability in both maxillary and mandibular anterior implantation [40, 43].
Concerning the number of fixation screws, most design applied three-point fixation [48, 50, 57], though the number can be adjusted [21, 25, 40, 43, 54]. A systematic review in 2012 indicates that when fixation screws are used, the mean deviation for all indicators are reduced, and the deviations decrease as the number of fixation screws increases, but the indicators exhibit no significant difference [63]. Design of two equally distributed fixation screws can also efficiently stabilize the surgical guide, located approximately at the lateral incisor [70]. Ideally, for fully edentulous maxillae, four-point fixation (two in anterior area and distal to the implant site) that covers the entire maxillary arch can efficiently avoid bending of the guide in the distal areas of the surgical field [71].
Design of sleeve
The guidance of drill hole, implant direction, depth, and angle are realized via design of sleeves, which can also reduce surgical time [72,73,74]. Sleeves can be classified as open or closed. Open sleeves with C-shaped buccally opening are applied in posterior areas where mouth opening and interarch space are limited or insufficient [74]. To ensure implant accuracy, the drill should be in the center and parallel to the inner wall of sleeves during hole preparation [75]. As summarized in Table 2, among the 41 involved literatures in this review, only 2 involved the comparison of sleeve design of guides, indicating that smaller distance from sleeve to bone leads to more accurate results [37], and that sleeve design might affect the accuracy [39].
Implant accuracy is affected by the design of height, drilling distance, and sleeve–bone distance, but the sleeve–implant distance and the sleeve axis angle do not affect the accuracy of digital implant guides [20]. By using shorter sleeve heights or shorter implants, decreasing the drilling distance below the guided sleeve can significantly increase the implant accuracy and reduce lateral movement of the drill [76]. However, sleeve heights ≤ 5 mm lead to implant placement deviation and decrease of the accuracy [77]. The increased drilling distance beyond the guiding sleeve results in a significant global and angular deviation at both the implant crest and apex [76]. Decreased sleeve–bone distance results in higher accuracy of the implant surgical guide. With the sleeve–bone distance of 2 or 4 mm, the implant accuracy of closed and open sleeve is similar; whereas with the sleeve–bone distance of 6 mm, lower accuracy is shown in both open and closed sleeves, and open sleeves exhibited a more significant trend [78].
In addition, material of the sleeve also affects implant accuracy. Metal sleeves are common in early surgery guides, and with the development of material science and technology, it is reported that plastic sleeves endow lower angle deviation, depth deviation, placement deviation than metal ones, as well as ensure a faster and easier guided surgery work-flow [79].
Manufacturing accuracy
Currently, digital implant guides can be manufactured using additive manufacturing (3D printing) or subtractive manufacturing (CAD/CAM milling). Typical 3D printing includes stereo lithography appearance (SLA), PolyJet, MultiJet, fused filament fabrication (FFF), digital light processing (DLP), etc. The difference in manufacturing accuracy between these two strategies remains inconclusive. It has been reported that the processing accuracy of milling (0.02–0.25 mm) is higher than printing (0.03–0.44 mm) in the aspect of inner surface, vertical fit discrepancy, guide seating distortion, and error range of anterior and posterior implants [80]. However, some literatures indicated no significant difference in manufacturing accuracy between milling and 3D printing [81]. Difference of manufacturing accuracy among different 3D printing manufacturing technologies has also been reported. For example, DLP printer exhibited a lower manufacturing error compared to FFF printer [80].
Despite of the differences in manufacturing accuracy, clinical researches indicated no significant differences in all surgical accuracy indicators between additive and subtractive manufactured guides [46]. Implant guides manufactured by different additive technologies (SLA, PolyJet and MultiJet) exhibited similar surgical accuracy regarding the angle, coronal and apical deviation [34]. DLP printer showed higher accuracy than SLA printer in coronal and vertical deviation, and no significant difference in apical, horizontal and angle deviation [60].
As shown in Table 5 and Additional file 1: Tables S3–S4, in terms of surgical guide fabrication, 3d printing is of wider application than milling. As indicated in Fig. 5, among the included literatures in this review, 3D printed guides exhibited higher peaks of horizontal coronal and horizontal apical deviations in in vivo researches than milled guides (Fig. 5A, D, in vivo angular deviation data were not sufficient to perform normal distribution analysis herein). While in in vitro researches, different trends were observed in the three deviation parameters (Fig. 5B, C, E).
In summary, though milled guides exhibited higher in vivo accuracy than 3d printed guides, the small sample size of milled guides reviewed in this article resulted in relatively low reliability of this conclusion, and further data support might be needed.
Conclusion and prospect
This review has verified the hypothesis that guide supporting types, manufacturing methods and design of implant guides (including fixation screws and sleeves) could influence the accuracies of static digital surgical guides by collecting and categorizing the numerical data of surgical accuracy indicator reported in literature of the last five years. Bilateral tooth-supported guides exhibited the highest in vitro accuracy and similar in vivo accuracy to unilateral tooth-supported guides; mucosa-supported guides exhibit the lowest in vivo accuracy, while its in vitro data showed low credibility due to the mechanical complexity of living mucosa tissue. Milled guides exhibited higher in vivo accuracy than 3d printed guides, though further data support might be needed. Apart from operator's skill and standardization that may affect the accuracy of implantation, this review has revealed that with current medical technology and the aid of digital implant guides, implant surgery accuracy can be achieved with the distance deviation < 2 mm (most < 1 mm) and angular deviation < 8° (most < 5°). The bottleneck of surgical accuracy improvement resulted from the difficulty of guide fixation in edentulous patients. In addition, the lack of a universal evaluation standard for implantation surgical accuracy remained a major problem in this research field.
As the design of supporting types, fixation screws and sleeves of implant guides can affect the accuracy of implant surgeries, existing studies focus on improving the accuracy via selecting appropriate supporting types, optimizing and customizing the guide design (including fixation screws and sleeves) according to individual demands. Future developing trend of this field may continuously focus on standardization of the evaluation of surgical accuracy and improving minimally invasive surgical methods (such as gradually phasing out bone-support guides that involve flap surgeries). The improvement of implant accuracy for edentulous patients has been a field of intense research in recent years and may remain a research focus in the near future.
The influence of implant guide design on surgical accuracy revealed in this review may shed light on future improvement of digital implant guides. However, this review only analyzed and discussed four influencing factors that affected the implantation accuracy, other factors including the guiding protocol (full/half guide), implant position (maxillary/mandibular, anterior/posterior, etc.), implant size, bone quality, etc., remained undiscussed and required further analysis in future reviews.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- AR:
-
Augmented reality
- CBCT:
-
Cone beam CT
- ISO:
-
International Organization for Standardization
- IOSs:
-
Intraoral scanners
- PICO:
-
Participants, Intervention, Comparison or control and Outcome measures
- 3D:
-
Three-dimensional
- ID:
-
Identity document
- NOS:
-
Newcastle–Ottawa Scale
- WOS:
-
Web of Science
- SD:
-
Standard deviation
- n:
-
Number of records
- CAD:
-
Computer Aided Design
- CAM:
-
Computer Aided Manufacturing
- SLA:
-
Stereo lithography appearance
- FFF:
-
Fused filament fabrication
- DLP:
-
Digital light processing
- Ref.:
-
Reference
- No.:
-
Numero sign/numero symbol
- SUMHS:
-
Shanghai University of Medicine & Health Sciences
References
Al Yafi F, Camenisch B, Al-Sabbagh M. Is digital guided implant surgery accurate and reliable? Dent Clin North Am. 2019;63(3):381–97.
Chen P, Nikoyan L. Guided implant surgery: a technique whose time has come. Dent Clin North Am. 2021;65(1):67–80.
Putra RH, Yoda N, Astuti ER, Sasaki K. The accuracy of implant placement with computer-guided surgery in partially edentulous patients and possible influencing factors: a systematic review and meta-analysis. J Prosthodont Res. 2022;66(1):29–39.
Flügge T, Derksen W, Te Poel J, Hassan B, Nelson K, Wismeijer D. Registration of cone beam computed tomography data and intraoral surface scans—a prerequisite for guided implant surgery with CAD/CAM drilling guides. Clin Oral Implants Res. 2017;28(9):1113–8.
Chandran SK, Jaini JL, Babu AS, Mathew A, Keepanasseril A. Digital versus conventional impressions in dentistry: a systematic review. J Clin Diagn Res. 2019;13(4):1–6.
Cicciu M, Fiorillo L, D’Amico C, Gambino D, Amantia EM, Laino L, et al. 3D digital impression systems compared with traditional techniques in dentistry: a recent data systematic review. Materials. 2020;13(8):1982.
Kihara H, Hatakeyama W, Komine F, Takafuji K, Takahashi T, Yokota J, et al. Accuracy and practicality of intraoral scanner in dentistry: a literature review. J Prosthodont Res. 2020;64(2):109–13.
Giachetti L, Sarti C, Cinelli F, Russo DS. Accuracy of digital impressions in fixed prosthodontics: a systematic review of clinical studies. Int J Prosthodont. 2020;33(2):192–201.
Jacobs R, Salmon B, Codari M, Hassan B, Bornstein MM. Cone beam computed tomography in implant dentistry: recommendations for clinical use. BMC Oral Health. 2018;18(1):88.
Fokas G, Vaughn VM, Scarfe WC, Bornstein MM. Accuracy of linear measurements on CBCT images related to presurgical implant treatment planning: a systematic review. Clin Oral Implants Res. 2018;29:393–415.
Mai HN, Dam VV, Lee DH. Accuracy of augmented reality-assisted navigation in dental implant surgery: systematic review and meta-analysis. J Med Internet Res. 2023;25: e42040.
Naeini EN, Atashkadeh M, De Bruyn H, D’Haese J. Narrative review regarding the applicability, accuracy, and clinical outcome of flapless implant surgery with or without computer guidance. Clin Implant Dent Relat Res. 2020;22(4):454–67.
Subramani K. Is computer-guided implant placement with a flapless approach more accurate than with a flapped surgical approach? Evid Based Dent. 2022;23(3):110–1.
Seo C, Juodzbalys G. Accuracy of guided surgery via stereolithographic mucosa-supported surgical guide in implant surgery for edentulous patient: a systematic review. J Oral Maxillofac Res. 2018;9(1): e1.
EftekharAshtiani R, Ghasemi Z, Nami M, Mighani F, Namdari M. Accuracy of static digital surgical guides for dental implants based on the guide system: a systematic review. J Stomatol Oral Maxillofac Surg. 2021;122(6):600–7.
Bover-Ramos F, Vina-Almunia J, Cervera-Ballester J, Penarrocha-Diago M, Garcia-Mira B. Accuracy of implant placement with computer-guided surgery: a systematic review and meta-analysis comparing cadaver, clinical, and in vitro studies. Int J Oral Maxillofac Implants. 2018;33(1):101–15.
Tahmaseb A, Wu V, Wismeijer D, Coucke W, Evans C. The accuracy of static computer-aided implant surgery: a systematic review and meta-analysis. Clin Oral Implants Res. 2018;29(Suppl 16):416–35.
Raico Gallardo YN, da Silva-Olivio IRT, Mukai E, Morimoto S, Sesma N, Cordaro L. Accuracy comparison of guided surgery for dental implants according to the tissue of support: a systematic review and meta-analysis. Clin Oral Implants Res. 2017;28(5):602–12.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Liu X, Liu J, Feng H, Pan S. Accuracy of a milled digital implant surgical guide: an in vitro study. J Prosthet Dent. 2022;127(3):453–61.
Pessoa R, Siqueira R, Li J, Saleh I, Meneghetti P, Bezerra F, et al. The impact of surgical guide fixation and implant location on accuracy of static computer-assisted implant surgery. J Prosthodont. 2022;31(2):155–64.
D’Haese R, Vrombaut T, Hommez G, De Bruyn H, Vandeweghe S. Accuracy of guided implant surgery using an intraoral scanner and desktop 3D-printed tooth-supported guides. Int J Oral Maxillofac Implants. 2022;37(3):479–84.
Sun Y, Ding Q, Tang L, Zhang L, Sun Y, Xie Q. Accuracy of a chairside fused deposition modeling 3D-printed single-tooth surgical template for implant placement: an in vitro comparison with a light cured template. J Craniomaxillofac Surg. 2019;47(8):1216–21.
Li-Rodriguez JK, Diaz-Durany M, Romeo-Rubio M, Paz Salido M, Pradies G. Accuracy of a guided implant system with milled surgical templates. J Oral Sci. 2022;64(2):145–50.
Baez-Marrero N, Rafel JL, Rodriguez-Cardenas YA, Aliaga-Del Castillo A, Dias-Da Silveira HL, Arriola-Guillen LE. Accuracy of computer-assisted surgery in immediate implant placement: an experimental study. J Indian Soc Periodontol. 2022;26(3):219–23.
Orban K, Varga E Jr, Windisch P, Braunitzer G, Molnar B. Accuracy of half-guided implant placement with machine-driven or manual insertion: a prospective, randomized clinical study. Clin Oral Investig. 2022;26(1):1035–43.
Gargallo-Albiol J, Zilleruelo-Pozo MJ, Lucas-Taule E, Munoz-Penalver J, Paternostro-Betancourt D, Hernandez-Alfaro F. Accuracy of static fully guided implant placement in the posterior area of partially edentulous jaws: a cohort prospective study. Clin Oral Investig. 2022;26(3):2783–91.
Singthong W, Serichetaphongse P, Chengprapakorn W. A randomized clinical trial on the accuracy of guided implant surgery between two implant-planning programs used by inexperienced operators. J Prosthet Dent. 2022.
Feng Y, Su Z, Mo A, Yang X. Comparison of the accuracy of immediate implant placement using static and dynamic computer-assisted implant system in the esthetic zone of the maxilla: a prospective study. Int J Implant Dent. 2022;8(1):65.
Lou F, Rao P, Zhang M, Luo S, Lu S, Xiao J. Accuracy evaluation of partially guided and fully guided templates applied to implant surgery of anterior teeth: a randomized controlled trial. Clin Implant Dent Relat Res. 2021;23(1):117–30.
Schneider D, Sax C, Sancho-Puchades M, Hammerle CHF, Jung RE. Accuracy of computer-assisted, template-guided implant placement compared with conventional implant placement by hand—an in vitro study. Clin Oral Implants Res. 2021;32(9):1052–60.
Song YW, Kim J, Kim JH, Park JM, Jung UW, Cha JK. Accuracy of dental implant placement by a novel in-house model-free and zero-setup fully guided surgical template made of a light-cured composite resin (VARO Guide((R))): a comparative in vitro study. Materials (Basel). 2021;14(14).
Abduo J, Lau D. Accuracy of static computer-assisted implant placement in long span edentulous area by novice implant clinicians: a cross-sectional in vitro study comparing fully-guided, pilot-guided, and freehand implant placement protocols. Clin Implant Dent Relat Res. 2021;23(3):361–72.
Herschdorfer L, Negreiros WM, Gallucci GO, Hamilton A. Comparison of the accuracy of implants placed with CAD-CAM surgical templates manufactured with various 3D printers: an in vitro study. J Prosthet Dent. 2021;125(6):905–10.
Ngamprasertkit C, Aunmeungthong W, Khongkhunthian P. The implant position accuracy between using only surgical drill guide and surgical drill guide with implant guide in fully digital workflow: a randomized clinical trial. Oral Maxillofac Surg. 2022;26(2):229–37.
Spille J, Jin F, Behrens E, Acil Y, Lichtenstein J, Naujokat H, et al. Comparison of implant placement accuracy in two different preoperative digital workflows: navigated vs. pilot-drill-guided surgery. Int J Implant Dent. 2021;7(1):45.
Guentsch A, Sukhtankar L, An H, Luepke PG. Precision and trueness of implant placement with and without static surgical guides: an in vitro study. J Prosthet Dent. 2021;126(3):398–404.
Han YT, Lin WC, Fan FY, Chen CL, Lin CC, Cheng HC. Comparison of dental surface image registration and fiducial marker registration: an in vivo accuracy study of static computer-assisted implant surgery. J Clin Med. 2021;10(18):4183.
Sittikornpaiboon P, Arunjaroensuk S, Kaboosaya B, Subbalekha K, Mattheos N, Pimkhaokham A. Comparison of the accuracy of implant placement using different drilling systems for static computer-assisted implant surgery: a simulation-based experimental study. Clin Implant Dent Relat Res. 2021;23(4):635–43.
Huang L, Zhang X, Mo A. A retrospective study on the transferring accuracy of a fully guided digital template in the anterior zone. Materials (Basel). 2021;14(16):4631.
D’Haese R, Vrombaut T, Hommez G, De Bruyn H, Vandeweghe S. Accuracy of guided implant surgery in the edentulous jaw using desktop 3D-printed mucosal supported guides. J Clin Med. 2021;10(3):391.
Lin CC, Ishikawa M, Maida T, Cheng HC, Ou KL, Nezu T, et al. Stereolithographic surgical guide with a combination of tooth and bone support: accuracy of guided implant surgery in distal extension situation. J Clin Med. 2020;9(3):709.
Chen Y, Zhang X, Wang M, Jiang Q, Mo A. Accuracy of full-guided and half-guided surgical templates in anterior immediate and delayed implantation: a retrospective study. Materials (Basel). 2020;14(1):26.
Wu D, Zhou L, Yang J, Zhang B, Lin Y, Chen J, et al. Accuracy of dynamic navigation compared to static surgical guide for dental implant placement. Int J Implant Dent. 2020;6(1):78.
Cheng KJ, Kan TS, Liu YF, Zhu WD, Zhu FD, Wang WB, et al. Accuracy of dental implant surgery with robotic position feedback and registration algorithm: an in-vitro study. Comput Biol Med. 2021;129: 104153.
Henprasert P, Dawson DV, El-Kerdani T, Song X, Couso-Queiruga E, Holloway JA. Comparison of the accuracy of implant position using surgical guides fabricated by additive and subtractive techniques. J Prosthodont. 2020;29(6):534–41.
Kniha K, Brandt M, Bock A, Modabber A, Prescher A, Holzle F, et al. Accuracy of fully guided orthodontic mini-implant placement evaluated by cone-beam computed tomography: a study involving human cadaver heads. Clin Oral Investig. 2021;25(3):1299–306.
Vinci R, Manacorda M, Abundo R, Lucchina AG, Scarano A, Crocetta C, et al. Accuracy of edentulous computer-aided implant surgery as compared to virtual planning: a retrospective multicenter study. J Clin Med. 2020;9(3):774.
Suksod N, Kunavisarut C, Kitisubkanchana J. Accuracy of computer-guided implantation in the placement of one-piece ceramic dental implants in the anterior region: a prospective clinical study. PLoS ONE. 2020;15(9): e0237229.
Kivovics M, Penzes D, Nemeth O, Mijiritsky E. The influence of surgical experience and bone density on the accuracy of static computer-assisted implant surgery in edentulous jaws using a mucosa-supported surgical template with a half-guided implant placement protocol—a randomized clinical study. Materials (Basel). 2020;13(24):5759.
Smitkarn P, Subbalekha K, Mattheos N, Pimkhaokham A. The accuracy of single-tooth implants placed using fully digital-guided surgery and freehand implant surgery. J Clin Periodontol. 2019;46(9):949–57.
El Kholy K, Lazarin R, Janner SFM, Faerber K, Buser R, Buser D. Influence of surgical guide support and implant site location on accuracy of static computer-assisted implant surgery. Clin Oral Implants Res. 2019;30(11):1067–75.
Skjerven H, Riis UH, Herlofsson BB, Ellingsen JE. In vivo accuracy of implant placement using a full digital planning modality and stereolithographic guides. Int J Oral Maxillofac Implants. 2019;34(1):124–32.
Chang RJ, Chen HL, Huang LG, Wong YK. Accuracy of implant placement with a computer-aided fabricated surgical template with guided parallel pins: a pilot study. J Chin Med Assoc. 2018;81(11):970–6.
Chen Z, Li J, Sinjab K, Mendonca G, Yu H, Wang HL. Accuracy of flapless immediate implant placement in anterior maxilla using computer-assisted versus freehand surgery: a cadaver study. Clin Oral Implants Res. 2018;29(12):1186–94.
Brandt J, Brenner M, Lauer HC, Brandt S. Accuracy of a template-guided implant surgery system with a CAD/CAM-based measurement method: an in vitro study. Int J Oral Maxillofac Implants. 2018;33(2):328–34.
Kauffmann P, Rau A, Engelke W, Troeltzsch M, Brockmeyer P, Dagmar LS, et al. Accuracy of navigation-guided dental implant placement with screw versus hand template fixation in the edentulous mandible. Int J Oral Maxillofac Implants. 2018;33(2):383–8.
Fang Y, An X, Jeong SM, Choi BH. Accuracy of computer-guided implant placement in anterior regions. J Prosthet Dent. 2019;121(5):836–42.
Ma B, Park T, Chun I, Yun K. The accuracy of a 3D printing surgical guide determined by CBCT and model analysis. J Adv Prosthodont. 2018;10(4):279–85.
Gjelvold B, Mahmood DJH, Wennerberg A. Accuracy of surgical guides from 2 different desktop 3D printers for computed tomography-guided surgery. J Prosthet Dent. 2019;121(3):498–503.
Tang W, Liu Q, Zeng X, Yu J, Shu D, Shen G, et al. Accuracy of half-way mucosa-supported implant guides for edentulous jaws: a retrospective study with a median follow-up of 2 years. J Int Med Res. 2021;49(3):300060521999739.
Siqueira R, Chen Z, Galli M, Saleh I, Wang HL, Chan HL. Does a fully digital workflow improve the accuracy of computer-assisted implant surgery in partially edentulous patients? A systematic review of clinical trials. Clin Implant Dent Relat Res. 2020;22(6):660–71.
Van Assche N, Vercruyssen M, Coucke W, Teughels W, Jacobs R, Quirynen M. Accuracy of computer-aided implant placement. Clin Oral Implants Res. 2012;23(Suppl 6):112–23.
Zhou W, Liu Z, Song L, Kuo CL, Shafer DM. Clinical factors affecting the accuracy of guided implant surgery—a systematic review and meta-analysis. J Evid Based Dent Pract. 2018;18(1):28–40.
Ozan O, Turkyilmaz I, Yilmaz B. A preliminary report of patients treated with early loaded implants using computerized tomography-guided surgical stents: flapless versus conventional flapped surgery. J Oral Rehabil. 2007;34(11):835–40.
Ersoy AE, Turkyilmaz I, Ozan O, McGlumphy EA. Reliability of implant placement with stereolithographic surgical guides generated from computed tomography: clinical data from 94 implants. J Periodontol. 2008;79(8):1339–45.
Cunha RM, Souza FA, Hadad H, Poli PP, Maiorana C, Carvalho PSP. Accuracy evaluation of computer-guided implant surgery associated with prototyped surgical guides. J Prosthet Dent. 2021;125(2):266–72.
Cassetta M, Di Mambro A, Giansanti M, Stefanelli LV, Cavallini C. The intrinsic error of a stereolithographic surgical template in implant guided surgery. Int J Oral Maxillofac Surg. 2013;42(2):264–75.
Cassetta M, Giansanti M, Di Mambro A, Stefanelli LV. Accuracy of positioning of implants inserted using a mucosa-supported stereolithographic surgical guide in the edentulous maxilla and mandible. Int J Oral Maxillofac Implants. 2014;29(5):1071–8.
Di Giacomo GA, da Silva JV, da Silva AM, Paschoal GH, Cury PR, Szarf G. Accuracy and complications of computer-designed selective laser sintering surgical guides for flapless dental implant placement and immediate definitive prosthesis installation. J Periodontol. 2012;83(4):410–9.
D’Haese J, Van De Velde T, Elaut L, De Bruyn H. A prospective study on the accuracy of mucosally supported stereolithographic surgical guides in fully edentulous maxillae. Clin Implant Dent Relat Res. 2012;14(2):293–303.
Kuhl S, Payer M, Zitzmann NU, Lambrecht JT, Filippi A. Technical accuracy of printed surgical templates for guided implant surgery with the coDiagnostiX software. Clin Implant Dent Relat Res. 2015;17(Suppl 1):e177–82.
Suriyan N, Sarinnaphakorn L, Deeb GR, Bencharit S. Trephination-based, guided surgical implant placement: a clinical study. J Prosthet Dent. 2019;121(3):411–6.
Oh KC, Shim JS, Park JM. In vitro comparison between metal sleeve-free and metal sleeve-incorporated 3D-printed computer-assisted implant surgical guides. Materials (Basel). 2021;14(3):615.
Lee DH, An SY, Hong MH, Jeon KB, Lee KB. Accuracy of a direct drill-guiding system with minimal tolerance of surgical instruments used for implant surgery: a prospective clinical study. J Adv Prosthodont. 2016;8(3):207–13.
El Kholy K, Janner SFM, Schimmel M, Buser D. The influence of guided sleeve height, drilling distance, and drilling key length on the accuracy of static computer-assisted implant surgery. Clin Implant Dent Relat Res. 2019;21(1):101–7.
Schnutenhaus S, Edelmann C, Rudolph H. Does the macro design of an implant affect the accuracy of template-guided implantation? A prospective clinical study. Int J Implant Dent. 2021;7(1):42.
Guentsch A, An H, Dentino AR. Precision and trueness of computer-assisted implant placement using static surgical guides with open and closed sleeves: an in vitro analysis. Clin Oral Implants Res. 2022;33(4):441–50.
Tallarico M, Czajkowska M, Cicciu M, Giardina F, Minciarelli A, Zadrozny L, et al. Accuracy of surgical templates with and without metallic sleeves in case of partial arch restorations: a systematic review. J Dent. 2021;115: 103852.
Abduo J, Lau D. Effect of manufacturing technique on the accuracy of surgical guides for static computer-aided implant surgery. Int J Oral Maxillofac Implants. 2020;35(5):931–8.
Mukai S, Mukai E, Santos-Junior JA, Shibli JA, Faveri M, Giro G. Assessment of the reproducibility and precision of milling and 3D printing surgical guides. BMC Oral Health. 2021;21(1):1.
Funding
The authors gratefully acknowledge the support of the National Natural Science Foundation of China (No. 32201104, No. 81970973), and the Science and Technology Commission of Shanghai Municipality (No. 22010502600), and the first-class curriculum construction project of SUMHS (2022-14).
Author information
Authors and Affiliations
Contributions
YS, JW, CM and JS contributed to data acquisition, analysis, interpretation, and drafted the manuscript; XD contributed to conception, interpretation, and critically revised the manuscript; DL provided funding support, contributed to conception, design, data analysis, interpretation, and critically revised the manuscript. All authors gave their final approval and agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All of the authors are in agreement with the content of the manuscript.
Competing interests
The authors report no conflicts of interest related to the subject of this review.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Methods. Figure S1. Risk of bias of included observational studies. Table S1. Forest plot showing the global apical deviations of the reviewed studies concerning different guide supporting types in different research types. Table S2. Forest plot showing the angular deviations of the reviewed studies concerning different guide supporting types in different research types. Table S3. Forest plot showing the horizontal coronal deviations of the reviewed studies concerning different guide fabrication in different research types. Table S4. Forest plot showing the horizontal apical deviations of the reviewed studies concerning different guide fabrication in different research types.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shi, Y., Wang, J., Ma, C. et al. A systematic review of the accuracy of digital surgical guides for dental implantation. Int J Implant Dent 9, 38 (2023). https://doi.org/10.1186/s40729-023-00507-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40729-023-00507-w