Abstract
Objectives
The purpose of this umbrella review was to gather and summarize the data from published systematic reviews (SRs) that compared non-surgical mechanical debridement (NSMD) with and without the use of adjunctive treatments on the management of peri-implant mucositis (PIM).
Materials and methods
A protocol was developed and registered in PROSPERO (CRD42021254350) before the systematic search for the SRs. Seven electronic databases, including Cochrane Library, Embase (via Ovid), MEDLINE (via Pubmed), Proquest, Prospero, Scopus and Web of Science, were searched for published reviews. The search for unpublished and informally published reviews was further attempted in the last four databases. The methodological quality of the included reviews was assessed using AMSTAR 2.
Results
Twelve included SRs assessed clinical studies published between 2014 and 2020, including a total of seventeen primary clinical trials. All SRs summarized data from individual studies and provided a narrative conclusion regarding the effectiveness of the adjunctive treatments. Only six SRs performed a meta-analysis (MA) of additional benefits of the adjunctive therapy for PIM, with results indicating no significant difference between the different treatment modalities. The overall confidence was adjudged ranging from critically low to low using AMSTAR 2 and significant additional benefits of any adjunctive treatments in comparison with NSMD were not apparent.
Conclusion
Overall, the reviewed evidence did not support the use of adjunctive treatments for improvement of clinical outcomes in PM management as compared to NSMD alone.
Similar content being viewed by others
Introduction
Peri-implant mucositis (PIM) is defined as inflammation of peri-implant mucosa without evidence of continuing marginal bone loss after initial bone remodeling [1], whereas peri-implantitis is defined as inflammation of peri-implant mucosa and additional marginal bone loss after initial bone healing [2]. Bacterial biofilm is the primary etiological agent in peri-implant mucositis and peri-implantitis [3, 4]. Considering that peri-implant mucositis precedes peri-implantitis, treatment of peri-implant mucositis is considered as the primary preventive modality for peri-implantitis [5]. The resolution of peri-implant mucositis may be achieved effectively by professional non-surgical mechanical debridement (NSMD) and enhanced oral hygiene practice (EOH) [6]. In addition, adjunctive treatments, including air-polishing, laser and photodynamics, antiseptics, and antibiotics, have been proposed to improve the outcomes of this treatment. Several systematic reviews (SRs) and meta-analyses (MA) have analyzed the effect of various adjunctive treatments compared to NSMD alone.
SRs provide a comprehensive synthesis of all available evidence (clinical studies) related to a specific intervention. This type of evidence synthesis focuses on a narrow review question, typically of direct comparison between two therapies and provides the highest level of evidence to designate health care decisions [7]. However, with the increasing number of systematic reviews and meta-analyses, treatment decisions can entail reading several systematic reviews. Therefore, it is appropriate to conduct an overview of reviews or "umbrella review" to compile data from multiple systematic reviews to support health care decision-making [8].
Umbrella reviews use a systematic method similar to systematic reviews, to compile information from systematic reviews instead of individual studies. In addition, umbrella reviews may examine different interventions for a particular disease, while systematic reviews usually focus on a single intervention. The comparison of similar systematic reviews can indicate the consistent or conflicting nature of evidence [9] and addresses the knowledge gap in available evidence for future research [10]. The present umbrella review aimed to gather and summarize the data from published systematic reviews that compared NSMD with adjunctive treatments and NSMD alone for managing PIM. The focus questions of the present umbrella review were:
-
1.
What is the effectiveness of the NSMD with adjunctive measures compared with NSMD alone in managing PIM?
-
2.
What is the quality of the systematic reviews concerning the effectiveness of adjunctive treatment in managing PIM?
Material and methods
The protocol was developed and registered on PROSPERO (CRD42021254350) before the systematic search for the systematic reviews. The SRs included in this umbrella review reported a comparison of the effectiveness of NSMD combined with adjunctive interventions versus NSMD alone. The eligibility criteria and search strategy were constructed with the aid of the following PICOS elements:
-
Population—adult patients with the diagnosis of peri-implant mucositis
-
Intervention—non-surgical mechanical debridement with adjunctive interventions
-
Comparison—non-surgical mechanical debridement alone
-
Outcomes—clinical, microbiological and immunological parameters
-
Study design—systematic reviews with or without meta-analysis.
Search strategy
Seven electronic databases, including Cochrane Library, Embase (via Ovid), MEDLINE (via Pubmed), Proquest, Prospero, Scopus and Web of Science, were identified for published reviews. The search for unpublished and informally published reviews was further attempted in the last four databases. The search term "(peri-implant OR periimplant) AND (mucositis OR disease* OR infect* OR inflammation) AND (treatment OR therapy* OR intervention OR management OR managing OR instrumentation OR "plaque removal" OR intervention)" was used to search for title, abstract and keywords when applicable. Two search strategies were applied for each database. The first strategy was the keyword search with a document-type filter for reviews or systematic reviews. The second strategy was the keyword search without a document-type filter, but the additional "systematic review" term was incorporated into the original search term. Combining two search strategies ensures comprehensiveness of the results since the search with a document-type filter might be of limited sensitivity, and some electronic databases such as Embase could not provide a search filter to identify systematic reviews successfully [11]. In addition, the search was restricted to the English language. All the seven electronic databases were searched for relevant reviews with a publication date until September 15th, 2021. The references, journal title, study title, authors, years of publication and abstract of searched results were exported to an EndNote library (using the management software EndNote X9.3.3, macOS Big Sur). Any duplication was removed before constructing the final list for review selection.
Review selection and additional searches
The review selection included two steps. The first step was screening the reviews by assessing the title and abstract. The second step involved screening by appraising the full text using the table of eligibility criteria (Additional file 1: Table S1). All steps were performed independently, by two reviewers (SC and AA). Any disparity was settled down by consensus and consultation with the third independent person in the team (GP).
The eligible systematic reviews were required to include the primary studies of adult patients diagnosed with peri-implant mucositis. The SRs that exclusively appraised treatment for peri-implantitis were excluded. The included systematic reviews summarized the outcome of primary studies and may also synthesize the data using descriptive analysis or meta-analysis. The primary studies included in the potential SRs were assessed against PICO elements using the same table of eligibility criteria (Additional file 1: Table S1). The irrelevant studies were identified and excluded. The SRs included in this umbrella review contain a minimum of one eligible primary study.
Cohen's k statistic was used to calculate an agreement between two reviewers. The inter-rater agreement for the title and abstract screening was 99.84%, and the Cohen kappa value was 0.97. The inter-rater agreement for full-text selection was 95%, and the Cohen kappa value was 0.89. In addition, hand-searching of the reference lists of the included systematic reviews was carried out to identify additional systematic reviews relevant to the PICO framework of this umbrella review.
Data collection
One of the reviewers (SC) performed the data collection systematically. The data were entered directly into the spreadsheet and checked by the other reviewer (AA). Any disparity in data extraction was resolved by consensus. All included SRs were extracted for the data on the general characteristics of the reviews, clinical and methodological characteristics, synthesized results and conclusion. In addition, the data of the primary studies reported in the selected systematic reviews were also extracted for bibliographic details, clinical and methodology characteristics, result and conclusion, and quality assessment (risk of bias). The data items of the systematic reviews and primary studies are listed in Additional file 1: document 1.
The data were cross-checked with the original articles or the other systematic reviews to correct any reporting errors or completing the required information when the SR report was unclear. In cases where the original reviewers did not provide the overall risk-of-bias of each primary study, the suggested algorithm in the Cochrane handbook for systematic reviews of interventions was applied [12]. The risks were summarized as low, unclear or high based on the presence of the greatest risk in the key domains within the individual studies.
Assessment of methodological quality of systematic reviews
The SRs included in the present umbrella review were assessed for the quality of methodology using AMSTAR 213. AMSTAR 2 is widely used to identify the quality of systematic reviews that include randomized or non-randomized trials of healthcare interventions. The overall confidence of each SR was determined based on the flaws or weaknesses in seven critical and nine non-critical domains [13]. The overall confidence of a systematic review was high when there was none of the critical flaw or only one non-critical flaw. The overall confidence was moderate when there was no critical flow or more than one non-critical flaw. The overall confidence was low when there was one critical flaw, and the overall confidence was critically low when there was more than one critical flaw. The assessment was performed independently by two reviewers (SC and AA). Any disparity in the assessment was settled by consensus.
Data synthesis
Most of the SRs did not provide a definitive conclusion concerning the effect on the outcome measurement (e.g., bleeding on probing or probing depth of peri-implant sulcus) but tabulated the data from the included primary studies, a decision was made to apply the vote-counting method and present the ratio of the primary studies of each outcome parameter to illustrate the outcome of the available evidence in each systematic review.
An additional conclusion was further made based on the ratio of the primary studies [14]. A minimum of three primary studies in each SR was required to conclude the effect of the adjunctive interventions. The adjunctive treatment was considered an additional benefit if more than two-thirds of the primary studies presented significant positive results.
Results
Description of included systematic reviews
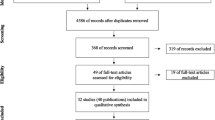
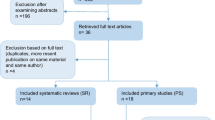
The final list of 701 search results was constructed after de-duplication. The title and abstract screening resulted in the exclusion of 679 references. Out of the potential 22 references for full-text screening, ten references were excluded after assessing the full text. The reasons for the exclusion of each study are presented in Additional file 1: Table S2. Twelve SRs were included in the present umbrella review. All were published outside the Cochrane Database of Systematic Reviews (CDSR) from 2015 to 2020. The flow diagram of the review selection process is illustrated in Fig. 1.
The PICO frameworks in the included SRs differed. Some included populations with PIM and peri-implantitis or compared different adjunctive treatments. Among the included SRs, only one had a specific focus population and adjunctive treatment [15], analyzing adjunctive laser and photodynamic therapy for PIM. Eight SRs reviewed a specific adjunctive intervention for both peri-implant mucositis and peri-implantitis, which encompassed antiseptics [16, 17] probiotics [18,19,20] air-polishing [21], and laser and photodynamic treatment [22, 23]. Two SRs [24, 25] reviewed all adjunctive treatments for PIM, and one [26] reviewed all adjunctive intervention for both PIM and peri-implantitis. The summary of bibliographic information and the PICOS frameworks of included SRs are presented in Table 1.
The assessment of each AMSTAR 2 criteria and the overall confidence of the systematic reviews are presented in Table 2. Affected by critical flaws, seven systematic reviews were scored as "low" in overall confidence, while the other five were scored as "critically low". The most common critical flaw was the lack of interpretation of the risk of bias when discussing the result, in nine systematic reviews [15, 18, 21,22,23,24,25,26]. The second frequent flaw was lack of publication bias assessment, in five systematic reviews [15,16,17,18,19]. Two SRS [19, 22] did not present the list of excluded studies and the reason for exclusion and two [15, 25] did not use appropriate methods for the statistical combination when performing a meta-analysis.
Description of primary studies included in SRs
The included SRs assessed clinical studies published between 2014 and 2020, including 17 clinical trials (CTs):16 randomized controlled clinical trials (RCTs) and one controlled clinical trial (CCT) [27]. Twelve SRs included two to ten relevant CTs. Some of these 17 individual studies were included in more than one systematic review. The overlaps in the CTs among the SRs are presented as a citation network in Fig. 2. The summary of the outcome, meta-analysis and vote-counting is presented in Table 3, grouped by type of the adjunctive treatment. The characteristics of the primary studies and overlapping among the systematic reviews are presented grouped by type of adjunctive treatment in Additional file 1: Table S3–S7.
Network of included systematic reviews and primary studies. The systematic reviews and the primary studies were represented by nodes. Each systematic review was linked to the primary studies that were part of it. Types of adjunctive treatments: open circle antiseptics, filled circle antibiotics, open square air–polishing, filled square probiotics, filled triangle laser and photodynamic therapy
The risk of bias of the clinical trials varied from low to high. The SRs used different tools to assess included CTs. Most used the first version of the Cochrane risk-of-bias tool [28, 29]. One [19] used the updated version 30 and one used its original criteria. Notably, the risk of bias assigned to these individual primary studies differed between systematic reviews even if the grading system was the same.
Interventions and comparators in included primary studies
The included SRs reviewed five RCTs [31,32,33,34,35] regarding adjunctive antiseptic treatment. Chlorhexidine gluconate was used in all trials in the form of gel, solution or spray with different concentrations: 0.12%, 0.2%, 0.5%. The application period differed from 10 days to 12 weeks among those studies.
Six RCTs [36,37,38,39,40,41] of adjunctive probiotic treatment were identified. Most studies use probiotics containing Lactobacillus reuteri in lozenges, which were dissolved in the mouth. Only one study40 used probiotics containing Lactobacillus brevis and Lactobacillus plantarum. This study also provided a probiotic mixture applied in the peri-implant sulcus in the clinic before letting the patients continue with the lozenges. The administration time for the probiotic lozenges varied from 3 weeks to 3 months.
The studies of air-polishing included one randomized controlled clinical trial [42] and one controlled clinical trial [43]. Both trials experimented with a glycine powder air-polishing device by applying at a submucosal level for five seconds on each affected implant site. Two RCTs [44, 45] assessed adjunctive laser and photodynamic treatment. They both used a diode laser in pulse mode by applying for 30 s per surface; however, the wavelength and power settings differed between the two studies. Two adjunctive antibiotic treatment RCTs [46, 47] were reviewed. One study assessed the systemic antibiotic Azithromycin, prescribed for 5 days [46]. Another study evaluated local antibiotic therapy, applying tetracycline HCl fibres in the peri-implant sulcus for ten days [47].
All the controlled trials compared the adjunctive treatment with NSMD (using either hand or ultrasonic instrument), polishing (using polishing paste and rubber cup), or both. However, some studies include adjunctive treatments in their conventional treatment protocol. One of the probiotic treatment studies [40] had photodynamic therapy as part of the control treatment. Some studies included peri-implant sulcus irrigation using 3% hydrogen peroxide [44], 0.12% CHX + 0.05% CPC45, or 0.12% CHX mouth rinsing [47] in their control treatment.
Data analysis in included SRs
All included SRs summarized the data of the individual studies and provided a narrative conclusion regarding the effectiveness of the adjunctive treatments. However, six SRs [16,17,18,19, 23, 24] performed MA of effects for additional benefits of the adjunctive treatment for peri-implant mucositis. These MAs showed no significant difference in probing depth, bleeding on probing, clinical attachment level, or plaque index outcomes between control (conventional treatment or NSMD) and test (conventional treatment with adjunctive therapy) groups. Four SRs [20,21,22, 45] did not conduct MA owing to heterogeneity present in the clinical trials concerning population (i.e., dental implant and restoring unit, peri-implant case definition), adjunctive treatment protocol, conventional treatment protocol, and outcome measurement (i.e., clinical parameters and follow-up period for evaluation). Two SRs [25, 26] conducted the MA of similar clinical outcomes parameters (bleeding on probing, gingival index and probing depth) of different types of adjunctive treatment (antibiotic, antiseptic, air-polishing and probiotic treatment); therefore, the present umbrella review analyzed the MA outcomes of these two SRs [25, 26].
Discussion
This umbrella review included 12 systematic reviews to examine the effect of adjunctive measures on PIM treatment. Considering PICOS framework, not all of the included reviews established definite and narrow PICOS frameworks. There was also variability in PICOS elements (i.e., population, intervention and comparators, outcomes, and study types) among the included systematic reviews.
The population and intervention elements were not well specified in most of the included systematic reviews. The focused populations had both PIM and peri-implantitis and intervention included all types of adjunctive treatments reported in literature. For instance, two systematic reviews [24, 25] included studies of peri-implant mucositis exclusively; however, they did not specify the types of the studied intervention.
Considering case definition of PIM, most systematic reviews did not specify the diagnostic criteria. The inclusion of the studies of peri-implant mucositis was based on the diagnosis assigned in the respective publications. Only one systematic review [45] referred to the 2017 World Workshop classification [48]. Most SRS used more than one parameter to assess the treatment outcome. The most studied outcome was probing depth, followed by bleeding on probing, plaque index and clinical attachment level.
There were differences between the control treatments among the individual studies. While most studies had NSMD as conventional treatment, some also added antiseptic treatment [36, 41, 44, 45, 47] or photodynamic treatment [40] in their control treatments. In addition, there were discrepancies in NSMD protocol, as included studies used curettes or ultrasonic devices, rubber cups and polishing paste, or both.
Some SRs also specified a minimum follow-up time of 1 month [18, 20] or 3 months [15, 22]. The follow-up period of the included primary studies ranged from 1 to 8 months. Most of the studies presented no significant difference between the test and control groups throughout the period of their follow-up. Only two primary studies regarding adjunctive antiseptic treatments [31, 34] showed significant differences that favored the test groups in the short term of the first 3 months; however, the studies did not continue the follow-up to see whether the effect would persist in a longer follow-up period.
Three SRs [24,25,26] (SRs) reviewed different adjunctive treatments and concluded that there was no additional benefit in the adjunctive treatment of PIM when compared to NSMD. Five SRs which reviewed antiseptics [16, 17] air-polishing [21], probiotics [20], and laser and photodynamic treatment [22] also concluded that the adjunctive treatment was not superior to conventional treatment. Four SRs regarding probiotics [18, 19] and laser and photodynamic therapy [15, 23] suggested that the benefit of adjunctive treatment was inconclusive and called for further clinical trials.
Three SRs regarding antiseptic treatment that performed MA indicated no significant difference in probing depth [16, 17, 24] bleeding on probing [17], and clinical attachment level [17, 24] between groups of conventional treatment and adjunctive treatment. MA for effects of adjunctive probiotic treatment was carried out in two SRs [18, 19].and noted no significant difference between conventional and adjunctive treatment groups in probing depth, bleeding on probing, and plaque index outcomes. Only one SR [23] of adjunctive laser and photodynamic treatment conducted MA. Adjunctive laser therapy did not significantly differ in probing depth from conventional treatment.
The effectiveness of the adjunctive treatments presented in the SRs was further determined by vote-counting based on a statistically significant difference in comparison of clinical parameters. Adjunctive antiseptic treatment shows no additional benefit in improving probing depth 16 and bleeding on probing [16, 17]. There was also no additional benefit of adjunctive probiotic treatment in improving probing depth, bleeding on probing, and plaque index [18, 20]. The effectiveness of the other adjunctive treatments could not be synthesized by vote-counting as the SRs included less than three primary studies.
The included SRs' overall confidence (AMSTAR 2) ranged from low to critically low. Overall, the summarized evidence indicated that adjunctive treatments did not significantly improve the clinical outcome parameters compared to NSMD.
Despite a rigorous methodology, this umbrella review has limitations. Firstly, the included systematic reviews and clinical trials were not of high quality and were few in number, including 12 SRs and 17 primary studies. About two-thirds of the primary studies presented with a high risk of bias. The confidence of the SRs was also low to critically low, according to the AMSTAR 2 assessment. Furthermore, the included SRs analyzed overlapping primary studies, which could account for their consistent findings. Finally, the present umbrella review opted for a non-statistical approach in data synthesis by implementing the vote-counting method to identify adjunctive treatment effectiveness for each clinical parameter. However, this approach has limitations [30] as vote-counting does not consider the effect size and the precision of the statistical estimate of the primary studies. Systematic reviews with a narrow scope were lacking and this umbrella review also demonstrated a lack of randomized controlled clinical trials. Sufficient RCTs of good quality need to be available to enable systematic reviews with a clear and narrow scope.
While the conclusion of this umbrella review does not support the general use of adjunctive treatment in managing PIM, patient subsets that may receive benefit from these therapies remain an open question. Clinical trials in patients with a history of periodontitis, diabetes or smoking with increased risk for peri-implant diseases are warranted. In addition, the adjunctive treatment for implants with local risk indicators may be considered [49]. PIM around the deep mucosal tunnel implant presents delayed disease resolution after non-surgical debridement [50]. Implant design with an over-contour prosthetic profile also could pose risks for peri-implant health [51, 52]. The role of adjunctive treatments in such situations needs further investigation. Furthermore, there are several reported adjuncts to NSMD for peri-implant disease including ozone therapy [53, 54], desiccant application [55], electrolytic cleaning procedures [56] and herbal medications [57] for which no evidence was synthesized in the systematic reviews of peri-implant mucositis included in the present study. Therefore, conclusions regarding the efficacy of these measures cannot be drawn from the present study. More clinical trials and subsequent SRs are warranted in order to clarify the effectiveness of emerging therapies.
Conclusion
A small number of primary studies and SRs address outcomes of adjunctive treatment for peri-implant mucositis and the quality of available SRs is generally low. Most of the primary studies have a high risk of bias, with discrepancy in the outcome measurements and follow-up times reported. Within these limitations, the present umbrella review failed to show significant benefit from adjunctive treatments to improve the outcome of NSMD in PIM and no specific adjunctive therapies have emerged as clearly superior to NSMD, so far.
Availability of data and materials
Data will be shared upon reasonable request.
References
Heitz-Mayfield LJA, Salvi GE. Peri-implant mucositis. J Clin Periodontol. 2018;45:S237–45. https://doi.org/10.1111/jcpe.12953.
Schwarz F, Derks J, Monje A, Wang HL. Peri-implantitis. J Clin Periodontol. 2018;45:S246–66. https://doi.org/10.1111/jcpe.12954.
Berglundh T, Zitzmann NU, Donati M. Are peri-implantitis lesions different from periodontitis lesions? J Clin Periodontol. 2011;38:188–202. https://doi.org/10.1111/j.1600-051X.2010.01672.x.
Tonetti MS, Chapple ILC, Jepsen S, Sanz M. Primary and secondary prevention of periodontal and peri-implant diseases. J Clin Periodontol. 2015;42:S1–4. https://doi.org/10.1111/jcpe.12382.
Jepsen S, Berglundh T, Genco R, et al. Primary prevention of peri-implantitis: managing peri-implant mucositis. J Clin Periodontol. 2015;42:S152–7. https://doi.org/10.1111/jcpe.12369.
Salvi GE, Aglietta M, Eick S, Sculean A, Lang NP, Ramseier CA. Reversibility of experimental peri-implant mucositis compared with experimental gingivitis in humans. Clin Oral Implants Res. 2012;23:182–90. https://doi.org/10.1111/j.1600-0501.2011.02220.x.
Aromataris E, Pearson A. The systematic review: an overview. Ame J Nurs. 2014;114:53–8.
Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J. 2009;26:91–108.
Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. JBI Evid Implement. 2015;13:132–40. https://doi.org/10.1097/XEB.0000000000000055.
Hartling L, Vandermeer B, Fernandes RM. Systematic reviews, overviews of reviews and comparative effectiveness reviews: a discussion of approaches to knowledge synthesis. Evid Based Child Health Cochrane Rev J. 2014;9:486–94.
Neilson C, Lê ML. A failed attempt at developing a search filter for systematic review methodology articles in Ovid Embase. J Med Libr Assoc. 2019;107:203.
Higgins JP, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. New Jersey: Wiley; 2019.
Shea BJ, Reeves BC, Wells G, et al. (2017). AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 358.
Squires J, Sullivan K, Eccles M, Worswick J, Grimshaw J. Are multifaceted interventions more effective than single-component interventions in changing health-care professionals’ behaviours? An overview of systematic reviews. Implement Sci. 2014;9:152. https://doi.org/10.1186/s13012-014-0152-6.
Sánchez-Martos R, Samman A, Priami M, Arias-Herrera S. The diode laser as coadjuvant therapy in the non-surgical conventional treatment of peri-implant mucositis: a systematic review and meta-analysis. J Clin Exp Dent. 2020;12: e1171.
Liu S, Limiñana-Cañal J, Yu J. Does chlorhexidine improve outcomes in non-surgical management of peri-implant mucositis or peri-implantitis?: a systematic review and meta-analysis. Med Oral Patol Oral Cir Bucal. 2020;25:e608–15. https://doi.org/10.4317/medoral.23633.
Zhao P, Wang Q, Zhang P, et al. Clinical efficacy of chlorhexidine as an adjunct to mechanical therapy of peri-implant disease: a systematic review and meta-analysis. J Oral Implantol. 2021;47(1):78–87. https://doi.org/10.1563/aaid-joi-D-19-00213.
Albaker AM. The effect of probiotic administration in the treatment of peri-implant diseases: a systematic review and meta-analysis. J Clin Diagn Res. 2019;13:ZE06-ZE13. https://doi.org/10.7860/JCDR/2019/42597.13363.
Gao J, Yu S, Zhu X, Yan Y, Zhang Y, Pei D. Does probiotic lactobacillus have an adjunctive effect in the nonsurgical treatment of peri-implant diseases? a systematic review and meta-analysis. J Evid Based Dent Pract. 2020;20:101398–101398. https://doi.org/10.1016/j.jebdp.2020.101398.
Silva AP, Cordeiro TO, da Costa RA, et al. Effect of adjunctive probiotic therapy on the treatment of peri-implant diseases—a systematic review. J Int Acad Periodontol. 2020;22:137–45.
Schwarz F, Becker K, Renvert S. Efficacy of air polishing for the non-surgical treatment of peri-implant diseases: a systematic review. J Clin Periodontol. 2015;42(10):951–9. https://doi.org/10.1111/jcpe.12454.
Chala M, Anagnostaki E, Mylona V, Chalas A, Parker S, Lynch E. Adjunctive use of lasers in peri-implant mucositis and peri-implantitis treatment: a systematic review. Dentistry. 2020;8:68. https://doi.org/10.3390/dj8030068.
Saneja R, Bhattacharjee B, Bhatnagar A, Kumar P, Verma A. Efficacy of different lasers of various wavelengths in treatment of peri-implantitis and peri-implant mucositis: a systematic review and meta-analysis. J Indian Prosthodont Soc. 2020;20:353–62. https://doi.org/10.4103/jips.jips_144_20.
Barootchi S, Ravidà A, Tavelli L, Wang HL. Nonsurgical treatment for peri-implant mucositis: a systematic review and meta-analysis. Int J Oral Implantol (Berl). 2020;13:123–39.
Schwarz F, Becker K, Sager M. Efficacy of professionally administered plaque removal with or without adjunctive measures for the treatment of peri-implant mucositis. A systematic review and meta-analysis. J Clin Periodontol. 2015;42:S202–13. https://doi.org/10.1111/jcpe.12349.
Schwarz F, Schmucker A, Becker J. Efficacy of alternative or adjunctive measures to conventional treatment of peri-implant mucositis and peri-implantitis: a systematic review and meta-analysis. Int J Implant Dent. 2015;1:1–34. https://doi.org/10.1186/s40729-015-0023-1.
De Siena F, Francetti L, Corbella S, Taschieri S, Del Fabbro M. Topical application of 1% chlorhexidine gel versus 0.2% mouthwash in the treatment of peri-implant mucositis. An observational study. Int J Dent Hygiene. 2013;11:41–7.
Higgins JP, Altman DG. Assessing risk of bias in included studies. In: Cochrane handbook for systematic reviews of interventions. Wiley, New Jersey, 2008;187–241. doi:https://doi.org/10.1002/9780470712184.ch8
Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343: d5928. https://doi.org/10.1136/bmj.d5928.
Higgins J, Savović J, Page M, Elbers R, Sterne J. Chapter 8: Assessing risk of bias in a randomized trial. In: Cochrane handbook for systematic reviews of interventions. Vol version 6.2. Cochrane; Accessed December 9, 2021. www.training.cochrane.org/handbook.
Hallström H, Lindgren S, Twetman S. Effect of a chlorhexidine-containing brush-on gel on peri-implant mucositis. Int J Dent Hyg. 2017;15:149–53. https://doi.org/10.1111/idh.12184.
Heitz-Mayfield LJA, Salvi GE, Botticelli D, Mombelli A, Faddy M, Lang NP. Anti-infective treatment of peri-implant mucositis: a randomised controlled clinical trial. Clin Oral Implants Res. 2011;22:237–41. https://doi.org/10.1111/j.1600-0501.2010.02078.x.
Menezes KM, Fernandes-Costa AN, Silva-Neto RD, Calderon PS, Gurgel BCV. Efficacy of 0.12% chlorhexidine gluconate for non-surgical treatment of peri-implant mucositis. J Periodontol. 2016;87:1305–13. https://doi.org/10.1902/jop.2016.160144.
Porras R, Anderson GB, Caffesse R, Narendran S, Trejo PM. Clinical response to 2 different therapeutic regimens to treat peri-implant mucositis. J Periodontol. 2002;73:1118–25. https://doi.org/10.1902/jop.2002.73.10.1118.
Thöne-Mühling M, Swierkot K, Nonnenmacher C, Mutters R, Flores-de-Jacoby L, Mengel R. Comparison of two full-mouth approaches in the treatment of peri-implant mucositis: a pilot study. Clin Oral Implants Res. 2010;21:504–12. https://doi.org/10.1111/j.1600-0501.2009.01861.x.
Alqahtani F, Alqahtani M, Shafqat SS, Akram Z, Al-Kheraif AA, Javed F. Efficacy of mechanical debridement with adjunctive probiotic therapy in the treatment of peri-implant mucositis in cigarette-smokers and never-smokers. Clin Implant Dent Relat Res. 2019;21:734–40. https://doi.org/10.1111/cid.12795.
Flichy-Fernández AJ, Ata-Ali J, Alegre-Domingo T, et al. The effect of orally administered probiotic Lactobacillus reuteri-containing tablets in peri-implant mucositis: a double-blind randomized controlled trial. J Periodontal Res. 2015;50:775–85. https://doi.org/10.1111/jre.12264.
Galofré M, Palao D, Vicario M, Nart J, Violant D. Clinical and microbiological evaluation of the effect of Lactobacillus reuteri in the treatment of mucositis and peri-implantitis: a triple-blind randomized clinical trial. J Periodontal Res. 2018;53:378–90. https://doi.org/10.1111/jre.12523.
Hallström H, Lindgren S, Widén C, Renvert S, Twetman S. Probiotic supplements and debridement of peri-implant mucositis: a randomized controlled trial. Acta Odontol Scand. 2016;74:60–6. https://doi.org/10.3109/00016357.2015.1040065.
Mongardini C, Pilloni A, Farina R, Di Tanna G, Zeza B. Adjunctive efficacy of probiotics in the treatment of experimental peri-implant mucositis with mechanical and photodynamic therapy: a randomized, cross-over clinical trial. J Clin Periodontol. 2017;44:410–7. https://doi.org/10.1111/jcpe.12689.
Peña M, Barallat L, Vilarrasa J, Vicario M, Violant D, Nart J. Evaluation of the effect of probiotics in the treatment of peri-implant mucositis: a triple-blind randomized clinical trial. Clin Oral Investig. 2019;23:1673–83. https://doi.org/10.1007/s00784-018-2578-8.
Ji YJ, Tang ZH, Wang R, Cao J, Cao CF, Jin LJ. Effect of glycine powder air-polishing as an adjunct in the treatment of peri-implant mucositis: a pilot clinical trial. Clin Oral Implants Res. 2014;25:683–9. https://doi.org/10.1111/clr.12123.
De Siena F, Corbella S, Taschieri S, Del Fabbro M, Francetti L. Adjunctive glycine powder air-polishing for the treatment of peri-implant mucositis: an observational clinical trial. Int J Dent Hyg. 2015;13:170–6. https://doi.org/10.1111/idh.12114.
Aimetti M, Mariani GM, Ferrarotti F, Ercoli E, Liu CC, Romano F. Adjunctive efficacy of diode laser in the treatment of peri-implant mucositis with mechanical therapy: a randomized clinical trial. Clin Oral Implants Res. 2019;30:429–38. https://doi.org/10.1111/clr.13428.
Sánchez-Martos R, Samman A, Bouazza-Juanes K, Díaz-Fernández JM, Arias-Herrera S. Clinical effect of diode laser on peri-implant tissues during non-surgical peri-implant mucositis therapy: randomized controlled clinical study. J Clin Exp Dent. 2020;12:e13–21. https://doi.org/10.4317/medoral.56424.
Hallström H, Persson GR, Lindgren S, Olofsson M, Renvert S. Systemic antibiotics and debridement of peri-implant mucositis. A randomized clinical trial. J Clin Periodontol. 2012;39:574–81. https://doi.org/10.1111/j.1600-051X.2012.01884.x.
Schenk G, Flemmig TF, Betz T, Reuther J, Klaiber B. Controlled local delivery of tetracycline HCl in the treatment of periimplant mucosal hyperplasia and mucositis. A controlled case series. Clin Oral Implants Res. 1997;8:427–33. https://doi.org/10.1034/j.1600-0501.1997.080510.x.
Berglundh T, Armitage G, Araujo MG, et al. Peri-implant diseases and conditions: consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol. 2018;89:S313–8.
Heitz-Mayfield LJA, Heitz F, Lang NP. Implant Disease Risk Assessment IDRA—a tool for preventing peri-implant disease. Clin Oral Implants Res. 2020;31:397–403. https://doi.org/10.1111/clr.13585.
Chan D, Pelekos G, Ho D, Cortellini P, Tonetti MS. The depth of the implant mucosal tunnel modifies the development and resolution of experimental peri-implant mucositis: a case–control study. J Clin Periodontol. 2019;46:248–55. https://doi.org/10.1111/jcpe.13066.
Katafuchi M, Weinstein BF, Leroux BG, Chen YW, Daubert DM. Restoration contour is a risk indicator for peri-implantitis: a cross-sectional radiographic analysis. J Clin Periodontol. 2018;45:225–32. https://doi.org/10.1111/jcpe.12829.
Wada M, Mameno T, Otsuki M, Kani M, Tsujioka Y, Ikebe K. Prevalence and risk indicators for peri-implant diseases: a literature review. Jpn Dent Sci Rev. 2021;57:78–84. https://doi.org/10.1016/j.jdsr.2021.05.002.
Butera A, Gallo S, Pascadopoli M, et al. Ozonized water administration in peri-implant mucositis sites: a randomized clinical trial. Appl Sci. 2021;11:7812. https://doi.org/10.3390/app11177812.
Tonon CC, Panariello BH, Spolidorio DM, et al. Antibiofilm effect of ozonized physiological saline solution on peri-implant–related biofilm. J Periodontol. 2021;92:1151–62. https://doi.org/10.1002/JPER.20-0333.
Lombardo G, Signoriello A, Corrocher G, et al. A topical desiccant agent in association with manual debridement in the initial treatment of peri-implant mucositis: a clinical and microbiological pilot study. Antibiotics. 2019;8:82. https://doi.org/10.3390/antibiotics8020082.
Zipprich H, Weigl P, Di Gianfilippo R, et al. Comparison of decontamination efficacy of two electrolyte cleaning methods to diode laser, plasma, and air-abrasive devices. Clin Oral Invest. 2022. https://doi.org/10.1007/s00784-022-04421-0.
Alzoman H, Alojaym TG, Chalikkandy SN, et al. Comparison of an Herbal-and a 0.12% chlorhexidine-based oral rinse as adjuncts to nonsurgical mechanical debridement in the management of peri-implant mucositis: a randomised controlled trial. Oral Health Prev Dent. 2020;1(18):645–51. https://doi.org/10.3290/j.ohpd.a45069.
Funding
The study was funded by The University of Hong Kong.
Author information
Authors and Affiliations
Contributions
SC collected and analyzed the data and prepared the manuscript, AA assisted in drafting the protocol, data analysis, and revision of the draft. KF aided the development of the research question and protocol LN aided the development of the research question and protocol, and revision of the draft. DH aided data analysis and revisions of the draft. GP conceptualized the study proposal, performed protocol drafting and preparation of the draft. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors. For this type of study, formal consent is not required.
Consent for publication
Not applicable.
Competing interests
No competing interests are declared.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. Eligibility criteria form. Table S2. Excluded reviews at the assessment of full-text and the main reasons for exclusion. Table S3. Characteristic of primary studies and overlap amongst systematic reviews of adjunctive antiseptic treatment. Table S4. Characteristic of primary studies and overlap amongst systematic reviews of adjunctive probiotic treatment. Table S5. Characteristic of primary studies and overlap amongst systematic reviews of adjunctive air-polishing treatment. Table S6. Characteristic of primary studies and overlap amongst systematic reviews of adjunctive laser and photodynamic treatment. Table S7. Characteristic of primary studies and overlap amongst systematic reviews of adjunctive local and systemic antibiotic treatment. Additional document 1. Data items of the systematic reviews and primary studies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chuachamsai, S., Acharya, A., Fischer, K. et al. The effectiveness of adjunctive measures in managing peri-implant mucositis: an umbrella review. Int J Implant Dent 8, 26 (2022). https://doi.org/10.1186/s40729-022-00426-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40729-022-00426-2