Abstract
In this study, Aronia melanocarpa fruit was extracted with ethanol, distilled water (pH 7), acidified distilled waters (at pH 5 and 3) as the solvent, the total polyphenol content, total flavonoid content, and anti-oxidant activity of the extracts were analyzed. Then, we investigated the possibility of producing nanofibers by the electrospinning process by adding the Aronia melanocarpa fruit extract (AE), which showed the greatest antioxidant effect, to a polyurethane (PU). The electrospinning method used a PU concentration of 10 wt% and 12 wt%, an applied voltage of 10 kV, a tip-to-collector distance of 15 cm, and flowrate of 0.3 mL/h. The analysis confirmed that the total polyphenol, total flavonoid content, and antioxidant activity of the distilled water extract were the highest at a pH value of 3. As the AE was added and its concentration increased, both the viscosity and the diameter of the nanofibers also increased. At PU concentrations of 10%, 12%, relatively uniform nanofibers without beads were prepared by adding 2 to 3 wt% and 0.5 to 2 wt% of the AE, respectively. At the above conditions, the diameters of the nanofibers that were produced were in the range of 227 to 420 nm, so it was evident that PU/AE nanofibers with various diameters could be prepared by controlling the concentration of PU and AE. In addition, the FT-IR, XRD, and DSC analyses confirmed that hydrogen bonding occurred between the AE and the PU. Thus, the resulting crystallinity, melting point, and reduction in the heat capacity confirmed that the AE was well mixed with the PU polymer.
Similar content being viewed by others
Introduction
The market for skin care products is expanding due to an increase in income levels, increased interest in appearance, and a shift in the awareness of using cosmetics, especially premium products. Among the skin-care products, mask packs are preferred because hey are readily available and easy to use. Mask packs have various functions, such as hydration, whitening, anti-aging, lifting, and soothing (Lee et al. 2017; Yi & Lee, 2019; Youn et al. 2014). The mask sheet is made of a variety of materials, the ability to absorb moisture and oil is important because it acts as a medium to deliver active ingredients in cosmetics, such as essence (Jang, 2017). The role of the sheet is to deliver nutrients, as this role is emphasized, nanofibers are attracting attention as a next-generation material (Byun, 2019; Fathi-Azarbayjani et al. 2010). Nanofibers have been reported to be effective in removing wastes by adsorbing fine dust, sebum, and other substances to the fibrous tissue due to their excellent ability adhere to the skin and to transmit essence (Korea Patent No. 10–2017-0,077,658, 2017).
Electrospinning technology is a very efficient method for manufacturing nanofibers, it is used extensively due to its simple process. The nanofibers that are formed have high surface areas and porosity, so they can be used in various ways, including the delivery of drugs (Kim & Lee, 2018, 2019; Park et al. 2018). Nanofibers prepared by adding natural extracts to polymers have the advantage of simultaneously expressing the properties of the natural extracts and polymers (Kim et al. 2016). Therefore, if a natural extract that has a high antioxidant effect is mixed with a PU polymer to produce nanofibers, it will be applicable as a mask pack to beautify the skin.
Fruits are rich in physiologically-active substances, berries are representative of this composition. Berries contain polyphenols, phenolic acids, tannins, and other organic compounds. They especially contain large amounts of anthocyanins (Bagchi et al. 2004; Miller & Shukitt-Hale, 2012). Aronia melanocarpa is a berry in the Rosaceae family. It also is known as black chokeberry or King's berry, it is native to North America. It has white flowers that bloom from late April to early May, and it is black when it ripens in August. The Indians and the early settlers in the United States used it as a medicinal agent to cure various diseases (Jeong 2008; Kim & Park 2014). Aronia melanocarpa fruit contains a large amount of polyphenol components, such as anthocyanin, flavonoid, and tannin. It has various physiological effects, such as antioxidant, anti-cancer, anti-diabetic, anti-mutation, anti-vascular disease, and anti-wrinkles (Kim 2019). Aronia melanocarpa has a dark purple color because of its high content of anthocyanin. Aronia's content of anthocyanin is 80 times that of grapes, 23 times that of strawberries, 11 times that of cranberries, 4 times that of blueberries, and 3.6 times that of Asian berries (Kraemer-Schafhalter et al. 1998). As a result of comparing the antioxidant effects of Aronia melanocarpa fruit and leaf extracts, it was reported that the fruit extracts have higher antioxidant activity than the leaves, they also have excellent protective effects against cell damage (Lee et al. 2013; Shin & Choe 2015).
Therefore, in this study, because of its excellent antioxidant effect, Aronia melanocarpa fruit was extracted with different solvents to examine the antioxidant effect of the extracts and to explore the possibility of fabricating AE/PU nanofibers.
Method
Materials
Dry Aronia melanocarpa fruit from Geumsan, Chungcheongnam-do was obtained from an herbal medicine dealer. Tertiary distilled water (H2O), 99.9% ethanol (C2H5OH), and 99.0% of acetic acid (CH3COOH) were used. Polyurethane (PU, pellethane 2103-80AE, MW = 80,000) was obtained from Lubrizol (USA), and N-dimethylformamide (DMF, > 98%) was purchased from Daejung Chemicals (Korea). Folin & Ciocalteu's phenol reagent, tannic acid (ACS reagent), potassium acetate (≥ 99.0%) and quercertin (≥ 95%) were purchased from Sigma Aldrich (USA). Sodium carbonate(99.0%) and aluminum nitrate(99.0%) were purchased from Samchun Chemicals (Korea).
Aronia melanocarpa fruit extraction
Ethanol and distilled water (pH 7), acidified distilled waters (at pH 5 and 3) were used as the extraction solvent. As an ethanol extraction method, dry Aronia melanocarpa fruit and a solvent were immersed in a beaker at a liquid ratio of 1:10 for 48 h at room temperature, the process was repeated 3 times. For the distilled water extraction method, the ratio of the dry Aronia melanocarpa fruit to the quantity of distilled water was 1:5. The extraction was repeated three times in a beaker at 60 °C for 60 min. The extract was filtered three times with filter paper until there was no precipitate left in the liquid, the liquid was concentrated to a gum state via reduced pressure, after which it was diluted and used. The concentration using reduced pressure was done in a rotary evaporator (RV10, IKA®, Germany) at 60 °C with a rotation speed of 55 rpm.
Determination of total polyphenol and flavonoid contents
The total polyphenol content was measured by modifying the Folin-Denis method (Swain & Hillis 1959). After mixing 0.2 mL of AE diluted to 1 mg/mL with 0.2 mL of Folin & Ciocalteu's phenol reagent, the mixture was reacted at room temperature for 3 min. Then, 3 mL of a 10% sodium carbonate solution were added to the mixture, mixed throroughly, reacted in a dark room for 1 h. The resulting solution was measured for absorbance at 745 nm using a spectrophotometer. The total polyphenolic content was obtained through extrapolation of obtained absorbance from standard curve of serially diluted tannic acid of concentrations 15.625, 31.25, 62.5, 125 and 250 µg/mL against corresponding absorbance. The total flavonoid content was measured by modifying the method of Nieva Moreno (Moreno et al. 2000). After mixing 0.4 mL of 80% ethanol to 0.1 ml of AE diluted to a concentration of 10 mg/mL, 0.1 mL of 10% aluminum nitrate solution, 0.1 mL of a 1 M potassium acetate solution, 4.7 mL of 80% ethanol were added and reacted for 40 min. A spectrophotometer was used to measure absorbance of reacted solution at wavelength 415 nm. The total flavonoid content was obtained through extrapolation of obtained absorbance from standard curve of serially diluted quercetin of concentrations 62.5, 125, 250, and 500 μg/mL against corresponding absorbance.
DPPH (2,2-diphenyl-1-picrylhydrazyl) and ABTS (2,2′-azino-bis (3-ethylben zothiazoline-6-sulfonic acid) diammonium salt) radical scavenging activity measurement
DPPH radical scavenging activity was measured by modifying the method of Brand-Willams et al. (Kim et al. 2018). After the AE was diluted to a concentration in the range of 1∼4 mg/mL, 0.1 mL of the AE was mixed with 1 mL of a 0.4 mM DPPH solution, 1.4 mL of ethanol were added, the mixture was reacted for 30 min in a dark room. A spectrophotometer was used to determine the absorbance of the reacted solution at 517 nm. Equation (1) was used to calculate the DPPH radical scavenging ability.
The ABTS radical scavenging ability was measured by partially modifying the methods of Re (Kim et al. 2018). Thus, 14 mM ABTS and 4.9 mM potassium persulfate solution were mixed at the ratio of 1:1 (v/v) and reacted in the dark room for 12 to 16 h, after which the mixture was diluted with distilled water to have an absorbance value of 0.7 to 0.8 at734 nm. We added 0.05 mL of AE diluted at a concentration of 1 to 4 mg/mL to 0.95 mL of the diluted ABTS + solution, the change in absorbance was measured after 10 min. Equation (1) was used to calculate the ABTS radical scavenging activity:
As: Absorbance of the solution with the extract, Ac: Absorbance of the solution without the extract.
Fabrication of AE/PU
PU polymer concentration was prepared by referring to a previous study of PU electrospinning fabrication with natural extract (Kim & Lee 2016). A 10 wt% and 12 wt% PU solution was prepared using DMF as the solvent. After stirring PU solution for 12 h, ultrasonic treatment was performed for 1 h. 0.5, 1, 2, and 3 wt% of the AE extracted from the distilled water at pH 3 were added to the PU solution and stirred for 12 h to prepare a PU composite spinning solution containing the AE. The viscosity of the solution was measured five times using a viscometer (Viscometer, DV-I Prime, Brookfield, USA), the average value was calculated. Electrospinning was done at the following conditions, i.e., a voltage of 20 kV, a tip-to-collector distance of 5 cm, and a flowrate of 0.3 mL/h.
Characterization
Measurement of the morphological study of the AE/PU nanofibers
To observe the shape of the nanofibers, the surface properties of the AE/PU nanofibers were analyzed using a scanning electron microscope (Scanning Electron Microscope, JSM-6010LA, JEOL, Japan). The diameters of the nanofibers were measured, the formation of the beads and their smoothness were observed.
Infrared absorption spectra of the AE/PU nanofibers
In order to confirm the changes in the chemical structure of the AE/PU nanofibers and the interaction between PU and AE, the surfaces of the AE/PU nanofibers were examined with an infrared spectrometer (FT-IR Spectrophotometer, ALPHA-P, Bruker, Germany).
X-ray diffractograms of AE/PU nanofibers
The influence of the change in the crystal structure between the two molecules when the AE and PU were mixed was measured using a High Resolution X-ray Diffractometer (Bruker AXS, Germany). The measurements were investigated under the conditions of 40 kV and 40 mA using Cu Kα as an X-ray tube. Diffractogram measured 2θ in the range of 5—80° at a stepsize rate of 0.5°/sec.
Thermal properties of the AE/PU nanofibers
The thermal properties of the AE/PU nanofibers were measured using a thermal analyzer (Thermal Analyzer, TGA/DSC 1, Mettler-Toledo, Korea) to confirm their thermal behavior. The measurement conditions were measured in a nitrogen gas atmosphere at a heating rate of 10 °C/min and a change in heat capacity from 0 to 600 °C.
Results and discussion
Total polyphenol and flavonoid contents of AE
Table 1 shows the total polyphenol and total flavonoid contents of the AE according to the extraction solvent. Among the AE, the total polyphenol content of the pH 3 distilled water extract was the highest at 364.03 mg/g. The ethanol extract was 362.05 mg/g, the pH 7 distilled water extract was 306.99 mg/g, the pH 5 distilled water extract was 298.83 mg/g. The total flavonoid content was 183.91 mg/g for the pH 3 distilled water extract, followed by 155.60 mg/g for the ethanol extract, 124.74 mg/g for the pH 7 distilled water extract, 120.92 mg/g for the pH 5 distilled water extract. The total polyphenol and total flavonoid contents of the pH 3 distilled water extract were the highest. It was found that Aronia melanocarpa's total polyphenol and total flavonoid contents were higher than those of the same berries, such as acai, blueberries, blackcurrants, and cranberries (Li & Jeong 2015). Since polyphenols have a property that the hydroxyl groups (-OH) combine easily with various chemicals, flavonoids are components of the polyphenol family. They are known to have antioxidant, antibacterial, anti-inflammatory, and antiviral effects (Hertog et al. 1993). Therefore, it is expected that AE extracted with pH 3 distilled water can be applied to fiber materials, such as mask packs for skin beauty by using it for nanofiber fabrication.
DPPH and ABTS radical scavenging ability of AE
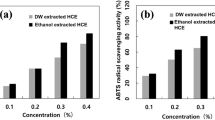
DPPH radical scavenging ability is measured by using the principle that DPPH free radicals, which are dark purple, decolorize yellow when they receive electrons or hydrogen from antioxidants. ABTS radical scavenging ability is a method that is measured using the principle that the ABTS free radical generated by the reaction of ABTS solution and potassium persulfate is removed by the antioxidants present in the extract, the radical-specific blue-green color is disappeared (Chung, 2016). Figure 1 shows the results of DPPH and ABTS radical scavenging activity of AE. The DPPH radical scavenging ability of the ethanol extract was 36.73–57.24%, the pH 3 distilled water extract was 72.99–84.59%, the pH 5 distilled water extract was 76.52–83.47%, the pH 7 distilled water extract was 49.35–68.81%. The ABTS radical scavenging activity of ethanol extract was 11.16–40.21%, the pH 3 distilled water extract was 40.40–97.12%, the pH 5 distilled water extract was 34.47–95.20%, the pH 7 distilled water extract was 35.00–93.31%. All of them showed high antioxidant activity. As the concentration of the aronia extract increased, the activity increased, and the physiological activity of the distilled water extract was higher than that of the ethanol extract. The antioxidant activity decreased as the pH increased.
Viscosity of the PU/AE spinning solution and morphology of the PU/AE nanofibers
Table 2 shows the viscosity of the spinning solution and the diameter of the nanofibers according to the concentrations of PU and AE to confirm the conditions for forming uniform and stable fibers when preparing nanofibers that contain aronia extract. In addition, Figs. 2 and 3 show SEM images of the electrospun nanofibers.
The viscosity of the 10% PU spinning solution was very low, i.e., 1263 cP, which gradually increased as the concentration of the AE increased. When 3% of AE was added, the viscosity was 2126 cP. When the AE was 0.5 wt %, the diameter of the nanofibers was187.96 nm, when 1 wt% was added, the average diameter of the nanofibers was 210.31 nm, when 2 wt% was added, the average diameter of the nanofibers was 227.03 nm. When 3 wt% was added, the average diameter of the nanofibers was 227.50 nm. However, when an AE concentration of 0.5 wt% was added, many beads were generated due to the low viscosity. When the concentration of AE that was added exceeded 1 wt%, the beads disappeared, fine-diameter nanofibers were formed relatively well.
The viscosity of the 12% PU spinning solution was 2160 cP, as the amount of AE increased, the viscosity gradually increased, when 3% of the Aaronia extract was added, the viscosity increased to 4,800 cP. The nanofibers that were spun alone showed a mixture of beads and fibrous forms, when an AE was added, the viscosity increased and the beads disappeared. The average diameter of the nanofibers increased as the added amount of aronia extract increased. When the contents of aronia extract were 0.5, 1.0, 2.0, and 3.0 wt%, the diameters of the nanofibers were 655.2, 801.8, 817.2, and 1077.4 nm, respectively. The average diameter of the nanofibers was 817.2 nm, when 3 wt% of the AE was added, the average diameter of the nanofibers was 1077.4 nm, the average diameter increased as the amount of the AE increased. When the concentration of the Aronia extract was 0.5 to 2 wt%, it was uniformly fabricated without the occurrence of beads, however at 3 wt%, the viscosity increased, resulting in a bulging-like shape distortion on the surface of the spun nanofibers, showing that they were unevenly fused. This occurred because the fluidity of the polymer solution in the spinning solution decreased as the viscosity increased, so the formation of tailor cones became difficult, decreasing the probability that fiber splitting would occur during spinning, indicating the form of the fused fibers (Satapathy et al. 2011). Therefore, the concentration of PU had to be decreased in order to increase the amount of AE. It was confirmed that PU/AE nanofibers that have the desired diameter size can be fabricated by adjusting the concentration of the AE that is added in the production of PU/AE nanofibers by setting the concentration of PU so that it constitutes 10–12% of the total.
Infrared absorption spectrum of the PU/AE nanofibers
By analyzing the chemical composition of PU nanofibers containing Aronia melanocarpa fruit distilled water extract via an infrared absorption spectrum, we tried to confirm the compatibility of the AE and PU polymers in the fibers. Figure 4 shows the results of comparing the FT-IR spectrum of the PU nanofibers with and without the AE.
The absorption band of the nanofibers that were spun with PU alone has an NH stretching vibration at a peak of 3334 cm−1, a CH group stretching vibrations at 2953 cm−1 (asymmetric stretching vibration) and 2895 cm−1 (symmetric stretching vibration). The elastic vibrations caused by the -(C = O)- group present in the urethane, urea, ester group were 1724 cm−1 and 1706 cm−1, the vibration observed for the -(CONH) group was 1529 cm−1. The bending by the CH3 group bending vibration at 1414 cm−1, bending vibration by the -(CNH) group at 1359 cm−1, stretching vibrations due to ester linkages at 1218 cm−1, 1167 cm−1, and 1071 cm−1 were showed. The absorption band of CH was observed at 769 cm−1. This showed that the PU spinning solution was produced as a nano-fiber through electrospinning without changing the PU component (Da Silva et al. 2011; Jiang et al. 2006Paul et al. 2013).
The absorption band of the AE showed a relatively wide and strong absorption peak in the vicinity of 3283 cm−1, which is characteristically showing the high concentration of phenolic hydroxyl groups due to the stretching vibration of O–H. In addition, strong elastic vibrations of C-H, an aliphatic spectrum, were observed around 2939 cm−1, strong C-O absorption bands were observed around 1029 cm−1 were observed. Looking at the absorption spectrum of the PU nanofibers containing the AE, it can be seen that the spectra at 3330 cm−1, 2957 cm−1, and 1029 cm−1 were due to the AE overlap in the band of the composite nanofiber. In addition, looking at (b) to (e), the FT-IR spectrum of PU nanofibers containing AE, the characteristic peak of PU is maintained as it is, it can be seen that as the concentration of AE increases, the spectrum at 3330 cm−1increases. This is the influence of the peak due to the stretching vibration of the hydroxyl group, which is a specific peak of the AE.
In this way, the presence of the overlapping absorption bands in the absorption spectrum of the PU/AE nanofibers and the increase in the peak of the specific spectrum in the PU/AE nanofibers indicated that the AE was bound successfully in the PU matrix. This implies that the main chemical aspect of the AE added during the fabricating process was preserved (Charernsriwilaiwat et al. 2013; Da Silva et al. 2011; Kim et al. 2014).
X-ray diffractograms of AE/PU nanofibers
X-ray Diffractometer (XRD) analysis is a method of determining the crystal structure and crystallinity of a polymer, it is used to examine the crystal properties of PU nanofibers without Aaronia extract and nanofibers with Aaronia distilled water extract. Figure 5 shows the comparison of the spectra.
It was confirmed that certain peaks of the PU appeared around 20° and 30°. The wide diffraction peak of the PU nanofibers was due to the low crystallinity of the PU. No diffraction peaks caused by AE were observed. This seems to be because the concentration of the AE contained in the nanoweb was too low (Sabitha and Sheeja 2015). In addition, as the content of the aronia extract increased, the specific peak of PU decreased somewhat. This is because the molecular weight of the PU, which contributes to crystallization, was reduced by the phenomenon in which internal and external molecules of hydrogen bonds are formed between the PU and the AE (Kim and Yeum 2011).
Thermal properties of PU/AE nanofibers
The results of differential scanning calorimetry (DSC) of PU nanofibers without and with AE are shown in Fig. 6 and Table 3. The melting point (Tm) of the nanofibers fabricated alone was 407.46 °C, when the AE was added, it was found to be lowered to 363.85 °C, 358.54 °C, 353.72 °C, and 348.57 °C. In addition, the heat required for thermal decomposition of the nanofibers (△Hm) was found to be 371.24 J/g in pure PU nanofibers. With the addition of 0.5%, 1%, and 2% of AE, the ΔHm of PU nanofibers gradually decreased to 226.59 J/g, 210.38 J/g, and 191.27 J/g. This means that as the AE is added to the PU nanofibers, the amount of heat required for thermal decomposition of the nanofibers decreases. The nanofibers that contained 3% AE did not decrease continuously to 199.29 J/g. It was assumed that, when the concentrtaion of the AE distilled water extract was 3 wt%, the dispersion of the AE was impeded, beads were generated in the nanofibers, a lot of energy was required to melt them. In addition, TGA (thermogravimetric analysis) was used to investigate the stability of the PU/AE nanofibers against thermal decomposition. Figure 7 shows the results. The thermal decomposition of the PU nanofibers without the addition of AE and the nanofibers with the addition of 0.5, 1, 2, and 3 wt% AE began at 280, 250, 200, 170, and 140 °C, respectively. The temperature representing a weight reduction of 10% was shown at 380 °C for PU nanofibers without AE, whereas PU nanofibers with 0.5 wt% and 1 wt% of AE had weight reductions of 10% at 370 °C and 360 °C, respectively. When the 2 wt% and 3 wt% PU nanofibers combined, two-step pyrolysis occurred before and after 260 °C. It seemed apparent that the thermal stability decreases as the amount of AE added increases.
Conclusions
In this study, Aronia melanocarpa fruit was extracted using ethanol and distilled water ( pH 7), acidified distilled waters (at pH 5 and 3) as solvents, the antioxidant components and antioxidant activities of the extract were analyzed. After that AE, which has the best antioxidant effect, was added to PU, the shape and properties of AE/PU nanofibers prepared through electrospinning were analyzed.
Among the various extraction solvents, the total polyphenol and total flavonoid content of Aronia melanocarpa fruit extract obtained from acidified aqueous medium (at pH 3) as extracting solvent were the highest, DPPH radical scavenging activity and ABTS radical scavenging activity were also the highest. Nanofibers that had diameters in the range of about 227 to 420 nm were produced without generating beads at 2 to 3 wt% at 10% PU concentration and 0.5 to 2 wt% at 12% PU concentration. PU/AE nanofibers were produced with the desired fineness by controlling the concentrations of PU and AE. As a result of FT-IR, XRD, DSC, and TGA analyses, it was confirmed that AE was bonded successfully in the PU matrix, thereby reducing the crystallinity, melting point, and heat capacity. This means that PU/AE nanofibers were fabricated in a state in which AE and PU were bonded between molecules.
Through the above studies, it was confirmed that PU/AE nanofibers can be produced by electrospinning. The AE has high antioxidant activity, so it is expected that PU/AE nanofibers can be applied to various fiber materials, such as facial sheet mask for skin care. The PU/AE nanofibers fabricated in this study will require a follow-up study to confirm the applicability of the facial sheet mask through various studies, such as studies to determine the absorption, moisture, adhesion, antioxidant activity, and whitening effect.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Change history
12 April 2021
This article has been updated to correct affiliation 1.
Abbreviations
- AE:
-
Aronia melanocarpa Fruit extract
- PU:
-
Polyureathane
- DMF:
-
N-dimethylformamide
- DW:
-
Distilled water
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- ABTS:
-
2,2′-Azino-bis (3-ethylben zothiazoline-6-sulfonic acid) diammonium salt
References
Bagchi, D., Sen, C. K., Bagchi, M., & Atalay, M. (2004). Anti-angiogenic, antioxidant, and anti-carcinogenic properties of a novel anthocyanin-rich berry extract formula. Biochemistry (Moscow), 69(1), 75–80. https://doi.org/10.1023/B:BIRY.0000016355.19999.93.
Byun, S. Y. (2019). Fabrication and performance evaluation of layered nanofibers containing alginate and spirulina extract [Unpublished master’s thesis]. InHa University, Korea.
Charernsriwilaiwat, N., Rojanarata, T., Ngawhirunpat, T., Sukma, M., & Opanasopit, P. (2013). Electrospun chitosan-based nanofiber mats loaded with Garcinia mangostana extracts. International Journal of Pharmaceutics, 452(12), 333–343. https://doi.org/10.1016/j.ijpharm.2013.05.012.
Chung, H. J. (2016). Comparison of bioactive constituents and biological activities of Aronia, Blackcurrant, and Maquiberry. Journal of the Korean Society Food Science and Nutrition, 45(8), 1122–1129. https://doi.org/10.3746/jkfn.2016.45.8.1122.
Da Silva, G. R., Da Silva-Cunha Jr, A., Behar-Cohen, F., Ayres, E., & Oréfice, R. L. (2011). Biodegradable polyurethane nanocomposites containing dexamethasone for ocular route. Materials Sci Engineering: C, 31(2), 414–422. https://doi.org/10.1016/j.msec.2010.10.019.
Fathi-Azarbayjani, A., Qun, L., Chan, Y. W., & Chan, S. Y. (2010). Novel vitamin and gold-loaded nanofiber facial mask for topical delivery. AAPS Pharm Sci Tech, 11(3), 1164–1170. https://doi.org/10.1208/s12249-010-9475-z.
Hertog, M. G. L., Hollman, P. C. H., & Putte, B. (1993). Content of potentially anti-carcinogenic flavonoids of tea infusions, wines, and fruit juices. Journal of Agricultural and Food Chemistry, 41(8), 1242–1246. https://doi.org/10.1021/jf00032a015.
Jang, H-I. (2017). A study on the characteristic of mask sheets. Journal of the Korean Applied Science and Technology, 34(4), 787–798. https://doi.org/10.12925/jkocs.2017.34.4.787.
Jeong, J. M. (2008). Antioxidative and antiallergic effects of Aronia (Aronia melanocarpa) extract. Journal of the Korean Society of Food Science and Nutrition, 37(9), 1109–1113. https://doi.org/10.3746/jkfn.2008.37.9.1109.
Jiang, Z., Yuan, K. J., Li, S. F., & Chow, W. K. (2006). Study of FTIR spectra and thermal analysis of polyurethane. Network Security Technology Application, 26(4), 624–628.
Kim, H., & Lee, S. (2018). Characterization of carbon nanofiber (CNF)/polymer composite coated on cotton fabrics prepared with various circuit patterns. Fashion and Textiles. https://doi.org/10.1186/s4069.
Kim, I. K., & Yeum J. H. (2011). Electrospinning fabrication and characterization of Poly(vinyl alcohol)/Waterborne Polyurethane/Montmorillonitr nanocomposite nanofibers. Polymer(Korean), 35(6), 553–557. https://doi.org/10.7317/pk.2011.35.6.553.
Kim, J. H., Lee, H., Jatoi, A. W., Im, S. S., Lee, J. S., & Kim, I. (2016). Juniperus chinensis extracts loaded PVA nanofiber: Enhanced antibacterial activity. Materials Letters, 181(15), 367–370. https://doi.org/10.1016/j.matlet.2016.05.164.
Kim, J. H., & Lee, J. S. (2016). Electrospinning fabrication of Juniperus Chinensis extracts loaded PU Nanoweb. Science of Emotion and Sensibility, 19(3), 43–50. https://doi.org/10.14695/KJSOS.2016.19.3.43.
Kim, J. I., Pant, H. R., Sim, H. J., Lee, K. M., & Kim, C. S. (2014). Electrospun propolis/polyurethane composite nanofibers for biomedical applications. Materials Scien Engineering: C, 44, 52–57. https://doi.org/10.1016/j.msec.2014.07.062.
Kim, J. W., Um, M., & Lee, J. W. (2018). Antioxidant activities of hot water extracts from different parts of Rugosa rose (Rosa rugosa Thunb). Journal of Korean Wood Science and Technology, 46(1), 38–47. https://doi.org/10.5658/WOOD.2018.46.1.38.
Kim, J. Y., & Park, C. H. (2014). Hair dyeing properties using extracts of Aronia melancocarpa. Asian Journal of Beauty & Cosmetology, 12(5), 663–668.
Kim, M. O., & Lee, J. S. (2019). The effect of 10,12-pentacosadiynoic acid on the morphology and characteristics of electrospun PDA/PU nanofibers. Fashion and Textiles, 6, 27. https://doi.org/10.1186/s40691-019-0183-3.
Kim, S. W. (2019). Major constituents content and bioactivities by different parts of Aronia (Aronia melanocarpa) [Unpublished master’s thesis]. Chonnam National University, Korea.
Kim, Y., Lee, D., Seong, H., Kim, H., Jang, S., Shin, J., Park, S. & Hur, W. (2017). Soft nano mask pack using complex nano fiber sheet and the method for preparing thereof (Korea Patent No. 10–2017–0077658). Daejeon, Korean Intellectual Property Office
Kraemer-Schafhalter, A., Fuchs, H., & Pfannhauser, W. (1998). Solid-Phase extraction comparison of (SPE)-a16 materials for the puriücation of anthocyanins from Aronia melanocarpa var Nero. Journal of the Science of Food and Agriculture, 78(3), 435–440.
Lee, A. R., Kim, H. O., Song, W. J., Min, Y. H., & Roh, S. S. (2017). Effects of mask pack containing Yam Bean (Pachyrhizus erosus) extracts on skin improvement. Asian Journal of Beauty & Cosmetology, 15(2), 180–190. https://doi.org/10.20402/ajbc.2016.0123.
Lee, H. M., Kong, B. J., Kwon, S. S., Kim, K. J., Kim, H. S., Jeon, S. H., et al. (2013). Antioxidative activities of Aronia melanocarpa fruit and leaf extracts. Journal of the Society of Cosmetic Scientists of Korea., 39(4), 337–345. https://doi.org/10.15230/SCSK.2013.39.4.337.
Li, H., & Jeong, J. M. (2015). Antioxidant activities of various berries ethanolic extract. Korean J Med. Crop Sci, 23(1), 49–56. https://doi.org/10.7783/KJMCS.2015.23.1.49.
Moreno, M. I. N., Isla, M. I., Sampietro, A. R., & Vattuone, M. A. (2000). Comparison of the free radical-scavenging activity of propolis from several regions of Argentina. Journal of Ethnopharmacology, 71(1), 109–114. https://doi.org/10.1016/S0378-8741(99)00189-0.
Miller, M. G., & Shukitt-Hale, B. (2012). Berry fruit enhances beneficial signaling in the brain. Journal of Agricultural and Food Chemistry, 60(23), 5709–5715. https://doi.org/10.1021/jf2036033.
Park, S. C., Kim, M. J., Kim, J., & Choi, S. (2018). Influence of shell compositions of solution blown PVP/PCL core–shell fibers on drug release and cell growth. RSC Advances, 8, 32470–32480. https://doi.org/10.1039/c8ra05485a.
Sabitha, M., & Sheeja, R. (2015). Preparation and characterization of ampicillin-incorporated electrospun polyurethane scaffolds for wound healing and infection control. Polymer Engineering and Science, 55(3), 541–548. https://doi.org/10.1002/pen.23917.
Satapathy, S., Pawar, S., Gupta, P. K., & Varma, K. B. R. (2011). Effect of annealing on phase transition in poly(vinylidene fluoride) films prepared using polar solvent. Bulletin of Materials Science, 34(4), 727–733. https://doi.org/10.1007/s12034-011-0187-0.
Shin, D. H., & Choe, T. B. (2015). Study on the bioactive characteristics of Aronia extract as a cosmetic raw material. Asian Journal of Beauty & Cosmetology, 13(2), 275–283.
Swain, T., & Hillis, W. E. (1959). The phenolic constituents of Prunus domestica. I.- The quantitative analysis of phenolic constituents. Journal of the Science of Food and Agriculture, 10(1), 63–68. https://doi.org/10.1002/jsfa.2740100110.
Yi, J., & Lee, S. (2019). Effect of paraffin wax mask pack on skin improvement. Journal of the Korea Convergence Society, 10(3), 237–242. https://doi.org/10.15207/JKCS.2019.10.3.237.
Youn, Y. H., Kim, S. H., & Kim, K. Y. (2014). Optical temperature of paraffin wax and improvement effect of facial skin parameter in paraffin wax mask application. Journal of the Korean Society of Cosmetology, 20(5), 878–884.
Acknowledgements
This work was supported by the National Research Foundation of Korea(NRF) grant funded by Ministry of Science, ICT & Future Planning of the Korea government (NRF-2017R1A2B4009315).
Funding
This work was supported by the National Research Foundation of Korea(NRF) grant funded by Ministry of Science, ICT & Future Planning of the Korea government (NRF-2017R1A2B4009315).
Author information
Authors and Affiliations
Contributions
WHW and LJS were contributed to the conception of the study, designed the experiment, conducted the work and drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The author declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Woo, H.W., Lee, J.S. Characterization of electrospun Aronia melanocarpa fruit extracts loaded polyurethane nanoweb. Fash Text 8, 12 (2021). https://doi.org/10.1186/s40691-021-00250-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40691-021-00250-z