Abstract
Bridelia ferruginea B dye was extracted from the bark of the tree using aqueous extraction method. Extracted dye was used to dye cellulosic (cotton) fabric in presence of 5% calcium chloride (CaCl2) or 5% alum (KAl(SO4)2·12H2O) of weight of fabric (o.w.f) as mordant. Fabric dyed without mordant was lighter in hue than metal ion mordanted dyed fabrics. The fabrics dyed in presence of calcium chloride as mordant are of deeper hue than those dyed with alum as mordant. Hence, their dye-uptake and color strength (K/S) are in the same order. K/S value of fabric dyed with alum is 43.71% higher than that without mordant and fabric dyed with calcium chloride has K/S value 51.09% higher than dyed with alum as mordant. CIEL*a*b* coordinate indicator and color space quadrant showed that those dyed fabrics without mordant and with alum as mordant are closer to yellow than red color. Those cellulosic fabrics dyed with calcium chloride as mordant are closer to red than yellow color as confirmed in colour space quadrant. Pre-mordanted dyed fabrics are of deeper color than post-mordanted dyed fabrics than meta-mordanted dyed fabrics than unmordant dyed fabric. Fastness properties of B. ferruginea B dyed cellulosic fabrics ranged from good (3) to excellent (5).

Similar content being viewed by others
Introduction
Appealing of textile apparels to the consumers is strongly aided by the color(s) on them (Bhuiyan et al. 2017; Opoku-Asare et al. 2013). These colors are obtainable from either dyes or pigments (Yusuf et al. 2016). Most dyes are water soluble in nature, therefore used in aqueous dyeing of textile materials (Yusuf et al. 2015). Most pigments are water insoluble in nature and are used in printing fabrics or production of paints (Islam et al. 2014). Natural and synthetic dyes are types of dyes used in coloring textile materials (Khan et al. 2012). Synthetic dyes are products of laboratory synthesis that have their precursors mostly from petrochemicals (Bechtold et al. 2003). Natural dyes are gotten from plants, animals and minerals (Yusuf et al. 2015). Natural dyes are first made to color textile materials, history dated back to ancient time of pre civilized day of Egyptians (Koren 1995). The applications of these dyes on textile apparels witness set back due to their limitations, such as lack of hue uniformity, low yield, poor adhesion and poor fastness properties (Micheal et al. 2003). These limitations made colorists to venture into replacement of natural dyes with synthetic dyes (El-Nagar et al. 2005). Non biodegradable, toxic and environmental challenges of synthetic dyes on textile materials and their consumers made colorists and researchers to turn back to the use of natural dyes that are free from these challenges (Shahid et al. 2012). Poor adhesion, color uniformity and fastness properties of natural dyed textile materials are enhanced with the application of mordant in natural dyeing process (Khan et al. 2006). In mordant dyeing process, mordant reacts with textile materials, opens up their structures to improve their chemical affinity for dye (Cristea and Vilarem 2006). Dye molecules from solution then diffuse into the fabric/fibre matrix; this is followed by adsorption of dye molecules at surface of fabric/fibre. The adsorbed dye molecules are then absorbed into the polymer matrix of the textile materials, follow by chemical fixation of dye molecules unto the mordanted fabric (Bukhari et al. 2017). Due to environmental awareness, a reasonable number of researchers have worked on coloration of textile materials using natural dyes. Some of these researchers are Shahid et al. (2012), (2016), Rather et al. (2016), Uddin (2015) and Yusuf et al. (2016). They all reported enhancement of hue and fastness properties in naturally dyed textile materials, when mordanted in presence of metallic or bio-metallic mordant.
In this research work, Bridelia ferruginea B tree (Euphorbiaceae family) was chosen as a representative source of natural dye. It is a tropical straggly tree of height between 15–18 m and width between 0.9–1.2 m at maturity stage. It is a native of West Africa (Nigeria, Ghana, Niger, Sierria Leone, Togo and Ivory Coast). In south west Nigeria, its leaves, root and bark are used in herbal medicine, for treatment of pile, diarrhea, dysentery, gonorrhea, cardiac disease, diabetes and expelling round worm (Oduro and Addo-Yobo 2015). It is equally well known for its antipyretic and antibacterial action (Ngueyem et al. 2009). Flavonoids are chromophoric compounds in B. ferruginea B, residing majorly at the bark of tree as secondary metabolites (Brusotti et al. 2010). Brusotti et al. (2010) isolated these flavonoids as quercetin and its derivatives (Fig. 1) from Bridelia specie stem bark.
Some southwest Nigerians (Yorubas) use CaCl2(aq) as anti-bleeding agent during first laundry operation on their indigenous Africa print (Kampala) textile to reduce washing out of dye from locally dyed clothing materials during usage. The aim of this work was to extract, purify B. ferruginea B dye from bark of the plant, use it for coloring cellulosic (cotton) fabrics, investigate Ca2+ mordanting capacity, fastness properties of the mordanted dyed fabrics and compare their quality with those obtained from frequently use alum mordant.
Methods
Materials
Bridelia ferruginea B tree bark was obtained from Itaoniyan farm plantation, off Ondo road, Akure. The plant material was identified at Forestry and Wood Technology Department, the Federal University of Technology, Akure, Nigeria.
Grey cotton (100%) used was obtained from Oja Oba market, at Akure south local government, Akure, Ondo state, Nigeria.
Calcium chloride (CaCl2), alum (KAl(SO4)2.12H2O), n-hexane and ethanol used are Analytical grade obtained from Sigma-Aldrich Chemie, Steiaheim, Germany.
Dye extraction
The obtained B. ferruginea B tree bark was washed, sun dried, crushed and ground using wood grinding mill. Aqueous extraction method was used in extracting dye from the ground plant material as described by Ali et al. (2016). Mixture of distilled water and ethanol (85:15 v/v) was poured into the beaker containing ground plant material at liquor ratio 1:10 and allowed to soak for 30 min at room temperature. Beaker containing soaked plant material was heated on water bath at 95 °C for 3 h. After extraction period of 3 h, extracted dye solution was filtered off and filtrate concentrated through evaporation.
The extracted crude dye was then purified by refluxing with n-hexane at 60 °C for 1 h. Hexane containing dissolved impurity was distilled off from dye solution in rotary evaporator and purified dye was dried to a constant weight in the oven at temperature of 60 °C within 2 h.
Preparation of dye solution
Preparation of dye stock solution was done as described by Bukhari et al. (2017). Purified B. ferruginea B dye (500 mg) was weighed into conical flask (100 mL) and distilled water (10 mL) was added to make a paste. Distilled water (20 mL) was added and stirred to make a solution. The dye solution was poured into standard flask (1000 mL) and little quantity of water was poured into the conical flask to wash the remnant of the dye solution into the standard flask. The dye solution in the standard flask was then made up to the marked point with distilled water.
Characterization of extracted dye
The extracted dye was characterized by Fourier transform infrared spectroscopic (FTIR) method as described by Yusuf et al. (2016). The mixture (extracted dye powder and potassium bromide in ratio 1:100) was made to a dice of specific dimension to form pellet. Pellet was formed by pressing with a hand press machine and placed on the sample holder of the FTIR spectrometer (Perkin Elmer) operated at spectral range 4000–500 cm−1. The infrared spectrum of the dye was recorded and printed out from FT-IR spectrometer.
Uv–visible spectrophotometer (752 N) was used to obtain absorption spectrum of the extracted dye solution as described by Bukhari et al. (2017). This is done by varying wavelength of the spectrophotometer from 400 to 780 nm and corresponding absorbance recorded. The wavelength at highest absorbance was noted as wavelength of maximum absorption (λmax).
Standardization of extracted dye solution
Prepared dye solution (500 mg/L) was standardized on Uv–visible spectrophotometer (752 N). Wavelength of maximum absorption obtained was used in measuring absorbance of the dye solution of various concentrations (100, 200, 300, 400 and 500 mg/L) by applying Beer–Lambert’s law. Absorbance of effluent from dye bath was equally measured for evaluating quantity of dye absorbed by fabric (Geelani et al. 2016).
Dyeing
Dyeing method reported by Hong (2018) was adopted with slight modification: grey cotton fabrics used were first cleansed through desizing, scouring, bleaching and mercerization (Bhuiyan et al. 2017). Prepared dye solution (100 mL of 500 mg/L) was measured into each of the six cups of Roeaches dyeing machine (Model IIMB). Four bleached fabrics (2 g each) were introduced into first two pairs of dyeing cups. One calcium chloride and one alum pre-mordanted fabrics (2 g each) were introduced into third pair of dyeing cups. The temperature of dyeing bath was raised to 60 °C within 30 min of dyeing and dyeing was continued for the next 30 min with rotating speed of dyeing cups at 100 rpm. The last pair of cotton fabrics (2 g each) introduced into two different dyeing cups. One of the two cups contained 100 mL of 500 mg/L dye solution with dissolved 5% calcium chloride on weight of fabric (o.w.f) and the other contained 100 mL of 500 mg/L dye solution with dissolved 5% alum (o.w.f). The pair of dyeing cups containing fabrics and mordant dissolved dye solution was equilibrated in the dyeing machine at dyeing conditions stated earlier. The B. ferruginea B dyed cotton fabrics were then washed with water to get rid of excess dye molecules at surface of cotton fabrics (Ding and Freeman 2017).
Mordanting
Mordanting solution was prepared by dissolving metal salt (0.1 g) of calcium chloride (CaCl2) or alum (KAl(SO4)2·12H2O) in standard flask containing little quantity of distilled water and made up to 100 mL marked point with distilled water.
Pre-mordanting was done by treatment of cellulosic fabric (2 g) with metal solution (5% o.w.f) for 1 h with continuous stirring (100 rpm) at temperature of 60 °C and liquor ratio 1:50 before dyeing process.
Meta-mordanting was done by dissolving metal salt (0.1 g) in little quantity of dye solution in standard flask (100 mL) and made up to marked point with dye solution before introduction of cellulosic fabric (2 g) to the solution in dyeing cup for dyeing at liquor ratio 1:50 for simultaneous mordanting and dyeing.
Post-mordanting was done by treatment of the dyed cellulosic fabric (2 g) with metal solution (5% o.w.f) at temperature 60 °C and liquor ratio 1:50 for 1 h with continuous stirring (100 rpm) (Bhattacharya and Shah 2000).
The structures of unmordanted, calcium chloride and alum mordanted B. ferruginea B dyed cellulosic fabrics are shown in Figs. 2, 3 and 4 respectively.
Measurement of colour and colour strength
Spectrophotometer (Datacolor 500 USA) was used to generate values of range of colour obtained on both unmordanted and mordanted natural dyed cellulosic fabrics automatically under D65 10 light for colour (CIELab coordinates for L*, a*, b*, h* and c*) and colour strength (K/S) as described by Rather et al. (2016). The colour strength (K/S) was calculated from measured reflectance (R) using the Kubelka–Munk equation (Eq. 1).
where K is the absorption coefficient, S is the scattering coefficient and R is the reflectance of dyed fabricsL* is the lightness, the lower the L*, the deeper the color on dyed fabric.
Hue angle is calculated using Eq. 2.
where a* and b* are color axices, +a* = red, +b* = yellow, −a* = green and −b* = blue color.
Determination of colour fastness properties
The colour fastness to washing and light of dyed cotton fabrics were determined according to American Association of Textile Chemists and Colourists (AATCC) Test Method 61 (2009) and AATCC Test Method 16 (2004) respectively. Colour fastness to crocking of dyed cotton fabrics (dry and wet rubbing, alkaline and acidic perspiration) was determined according to AATCC Test Method 8 (2001).
Results and discussion
Dye extraction
The yield of the extract of B. ferruginea B tree back was calculated from weight of ground material before and after extraction/purification operations (Table 1). Yield of crude dye was 28.96% higher than that of purified dye. The reduction in yield of extracted dye after purification operation was due to removal of wax, fat and other related impurities from the crude dye. This observation agreed with Uddin (2015), in purification of the natural dyestuff extracted from mango back for application on protein fibres. The yield obtained in extraction of B. ferruginea B dye showed that the dye from the plant can be employed for commercial purpose.
Standardization and characterization of extracted dye stuff
The FT-IR spectrum of B. ferruginea B dye displays bands in the regions of 3402, 2925–2377, 1731, 1618, 1450, 1256 and 1046 cm−1 corresponding to –OH, –CH, –C=O, –C=C, –H–O–H, –C–O, and –C–O–C functional groups respectively (Fig. 5). These functional groups indicate presence of phenolic, aromatic, carbonyl and alkoxyl fragments in the dye structure. The presence of these fragments in the dye structure predicts possibility of chromophoric compounds in the dye to be quercetin and its derivatives (Fig. 1). This observation agreed with the findings made by Bechtold et al. (2006), in natural dyes for textile dyeing: a comparison of methods to assess the quality of Canadian golden rod plant material.
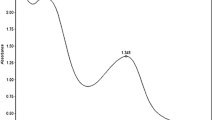
The absorption spectrum of B. ferruginea B dye solution shows two intense bands in the regions 460 and 530 nm (Fig. 6). The absorption and emission of radiant energy from Uv–visible spectrophotometer light source lead to π → π* transition in aromatic rings (double bond to single bond alternating conjugate) of dye molecule (auxochrome). Equally, absorption and emission of radiant energy lead to n → π* electronic transition in heteroatom of dye molecule (chromophore). Hence, combination of π → π* and n → π* electronic transitions in dye molecule resulted in color emission at 460 and 530 nm. The higher wave length of absorption is called wavelength of maximum absorption, which is seen at 530 nm (λmax). The presence of substituent (R1–4) in the dye confers water solubility and enhances colour formation on B. ferruginea B dye (Yusuf et al. 2016).
Absorbance of serially diluted B. ferruginea B dye solutions was read in Uv–visible spectrophotometer at wavelength 530 nm (λmax) and used to plot calibration graph (Additional file 1) used for this study (Fig. 7). The linearity and ability of calibration graph to pass through the origin made it agree with Beer-Lambert law as described by Uddin (2015).
Dyeing and dye-uptake of dyed cellulosic fabrics
The absorbance of dye in effluent of dyeing solutions was read from Uv–visible spectrophotometer at 530 nm and concentration interpolated from the calibration graph (Fig. 7). The quantity of dye absorbed and dye-uptake were calculated from those of initial dye concentration, interpolated effluent concentrations and mass of the fabric (Table 2). It can be seen from Table 2 that mordanted (with exception to post-mordanted) dyed fabrics have higher dye-uptake than unmordanted dyed fabric. This is due to enhancement in affinity of dye for the cellulosic fabric by mordant used in mordanted dyed fabric (Uddin 2015). Dye-uptake of post-mordanted dyed fabric equal to that of unmordanted dyed fabric, because post-mordanted mordanting process was done after dyeing process. The meta-mordanted dyed cellulosic fabrics have lower dye-uptake than pre-mordanted dyed fabrics. This might be as a result of mordant/dye interaction that reduced quantity of dye molecules diffused in and absorbed by cotton matrix during simultaneous mordanting-dyeing process. This observation agreed with findings made by Yusuf et al. (2016) in eco-dyeing of wool with Rubia cordifolia root extract: assessment of the effect of Acacia catechu as biomordant on color and fastness properties.
Characteristics of dyed cellulosic fabrics and colour strength
The appearances of unmordanted and mordanted B. ferruginea B dyed cotton fabrics are shown in Fig. 8. The mordanted B. ferruginea B dyed cotton fabrics are of deeper shade than unmordanted dyed fabric (Bhattacharya and Shah 2000). This might be as a result of ability of metal salts to enhance hue of natural dye in cellulosic fabric. This observation agreed with the finding of Bukhari et al. (2017), in dyeing studies and fastness properties of brown naphtoquinone colourant extracted from Juglans regia L. on natural protein fibre using different metal salt mordants. It was also observed that pre-mordanted dyed fabrics have deepest shade among all dyed cellulosic fabric. Deeper shade was seen in post-mordanted dyed fabrics and meta-mordanted dyed fabrics have lightest shade among all mordanted dyed cotton fabrics. This is in line with Yusuf et al. (2016). In general, all the calcium chloride mordanted dyed fabrics are deeper in shade than their corresponding alum mordanted dyed cellulosic fabrics (Fig. 8). This might be as a result of higher reactivity of Ca2+ towards hydroxyl functional group (OH−) of cellulosic fabric than Al3+. The higher reactivity of Ca2+ enhances faster diffusion of dye molecule into the cellulosic fabric matrix than that of Al3+ as mordant.
Unmordanted, calcium chloride and alum mordanted B. ferruginea dyed cotton fabric. a CaCl2 pre-mordanted dyed fabric, b CaCl2 post-mordanted dyed fabric, c CaCl2 meta- mordanted dyed fabric, d unmordanted dyed fabric, e alum pre-mordanted dyed fabric, f alum post-mordanted dyed fabric, g alum meta-mordanted dyed fabric
The colour developed on dyed cotton fabrics ranged between light and deep (Fig. 8). Unmordanted dyed cotton has the highest L* value and lightest colour. Calcium chloride mordanted dyed cotton fabrics have deeper colour than alum mordanted dyed cotton fabrics. This further confirms that mordant enhances dye hue on cellulosic fabric. The L* values of the post-mordanted dyed fabrics are higher than those of meta-mordanted and lower than those of pre-mordanted dyed fabrics (Table 3).
The values of b* > a* and both positive in unmordanted and alum mordanted dyed fabrics indicated their appearance colour closer to yellow more than red colour. The values of their hue angles (h*) > 70° is a strong indication of the appearance colour closer to yellow more than red in colour space quadrant (Fig. 9). Alternatively, the values of b* < a* (both are positive) in calcium chloride mordanted dyed fabrics showed that their appearance colour is closer to red more than yellow. On space colour quadrant, the values of their h* < 40° is a further indication of their colour closer to red more than yellow (Fig. 9). This is in agreement with Shabbir et al. (2016) in application of Terminalia chebula natural dye on wool fibre-evaluation of colour and fastness properties. Table 3 shows that the deeper the colour of dyed fabric, the higher the colour strength value (K/S). The K/S value of unmordanted dyed fabric is less than those of metal salt mordanted dyed fabrics. Calcium chloride mordanted dyed fabrics have higher colour strength values than alum mordanted dyed fabrics. This observation may be as a result of deeper colour of calcium chloride mordanted dyed fabrics than alum mordanted dyed fabrics. It was equally observed that pre-mordanted dyed fabric has highest K/S value, post-mordanted dyed fabric has higher K/S value and meta-mordanted dyed fabric has the lowest K/S value among all the mordanted dyed fabric. Rather et al. (2016) also made the same findings.
Fastness properties of dyed cellulosic fabrics
Fastness properties rating of B. ferruginea B dyed cellulosic fabrics are shown in Table 4. Wash fastness property ranged from good (3) to excellent (5), light fastness property ranged from very good (5) to excellent (7) and rubbing fastness property ranged from good/very good (3.5) to excellent (5). Other fastness properties determined on dyed cellulosic fabrics are perspiration fastness property, which ranged from good (3) to very good/excellent (4.5) and hot ironing fastness property that was seen to be excellent (5) in all dyed cotton fabrics. These observations agreed with Sufian et al. 2016.
Generally, fastness properties of dyed cellulosic fabrics were enhanced through mordanting (Table 4). The ability of mordant metal ion to chelate dye molecules forming complex in dyed fabric makes mordanted dyed fabrics of higher fastness properties than unmordanted fabric. The presence of covalent, coordinate and hydrogen bonds, in addition to complex dye formation (Fig. 3) make calcium chloride mordanted dyed cellulosic fabrics to have best fastness properties. The alum mordanted dyed fabrics that contain only covalent and coordinate bonds in addition to complex dye-metal-fabric formations (Fig. 4) have better fastness properties. Dye molecules aggregation, together with covalent and hydrogen bonds conferred good fastness properties on unmordanted dyed fabric (Fig. 2). These observations agreed with Geelani et al. (2016) in Dyeing and fastness properties of Quercus robur with natural mordants on natural fibre.
Conclusions
Mordanting enhances hue and fastness properties of natural dyed cotton fabric. Pre-mordanting dyeing technique gave dyed cellulosic fabric with deepest hue and post-mordanting technique gave dyed fabrics with best fastness properties. Calcium chloride was seen to be a mordant with deeper hue development on B. ferruginea B dyed cellulosic fabric. B. ferruginea B mordanted dyed cellulosic fabrics are of good to excellent fastness properties. Therefore, B. ferruginea B dye has a good potential of complementing synthetic dyes in dye house.
Availability of data and materials
All the necesssary data and materials supporting our findings can be found within the article.
References
Ali, M. K., Islam, S., & Mohammed, F. (2016). Extraction of natural dye from walnut bark and its dyeing properties on wool yarn. Journal of Natural Fibres, 13, 458–469.
Bechtold, T., Mahmud-Ali, A., & Mussak, R. (2006). Natural dyes for textile dyeing: A comparison of methods to assess the quality of Canadian golden rod plant material. Dyes and Pigments, 75, 287–293.
Bechtold, T., Turcanu, A., Ganglberger, E., & Geissler, S. (2003). Natural dyes in modern textile dye house—now to combine experiences of two centuries to meet demands of the future? Journal of Cleaner Production, 11, 499–509.
Bhattacharya, S. D., & Shah, A. K. (2000). Metal ion effect on dyeing of wool fabric with catechu. Coloration Technology, 116, 10–12.
Bhuiyan, M. A. R., Islam, A., Islam, S., Hossain, A., & Nahar, K. (2017). Improving dyeability and antibacterial activity of Lawsonia inermis L. on jute fabrics by chitosan pretreatment. Textile and Clothing Sustainability, 3(1), 1–10.
Brusotti, G., Ngueyem, T. A., Biesuz, R., & Caccialanza, G. (2010). Optimum extraction process of polyphenols from Bridelia grandis stem bark using experimental design. Journal of Separation Science, 33(11), 1692–1697.
Bukhari, M. N., Islam, S., Shabbir, M., Rather, L. J., Shahid, M., Singh, U., et al. (2017). Dyeing studies and fastness properties of brown naphtoquinone colorant extractfrom Juglans regia L. on natural protein fibre using different metal salt mordants. Textiles and Clothing Sustainability, 3(3), 1–9.
Cristea, D., & Vilarem, G. (2006). Improving light fastness of natural dyes on cotton yarn. Dyes and Pigments, 70, 238–245.
Ding, Y. I., & Freeman, H. S. (2017). Mordant dye application on cotton; optimization and combination with natural dyes. Coloration Technology, 133(5), 369–375.
El-Nagar, K., Sanad, S. H., Mohamed, A. S., & Ramadan, A. (2005). Mechanical properties and stability to light exposure for dyed Egyptian cotton fabric with natural and synthetic dyes. Polymer-Plastics Technology and Engineering, 44, 1269–1279.
Geelani, S. M., Ara, S., Mir, N. A., Bhat, S. J. A., & Mishra, P. K. (2016). Dyeing and fastness properties of Quercus robur with natural mordants on natural fibre. Textile and Clothing Sustainability, 2(8), 1–10.
Hong, K. H. (2018). Effects of tannin mordanting on coloring and functionalities of wool fabrics dyed with spent coffee grounds. Fashion and Textiles, 5, 33.
Islam, S., Rather, L. J., Khan, M. A., & Mohammed, F. (2014). Study the effect of ammonia post-treatment on colour characteristics of annatto-dyed textile substrate using reflectance spectrophotometry. Industrial Crops and Products, 59, 337–342.
Khan, S. A., Ahmad, A., Khan, M. I., Yusuf, M., Shhahid, M., Manzoor, N., et al. (2012). Antimcrobial activity of wool yarn dyed with Rheum emodi L. (Indian Rhubarb). Dyes and Pigments, 95, 206–214.
Khan, M. A., Khan, M., Srivastava, P. K., & Mohammed, F. (2006). Extraction of natural dyes from cutch, ratanjot and madder, and their application on wool. Colorage, 53, 61–68.
Koren, Z. C. (1995). Historico–chemical analysis of plant dye stuffs used in textiles from ancient Israel (pp. 269–310). Washington, D.C: American Chemical Society.
Micheal, M. N., Tera, F. M., & Aboelanwar, S. A. (2003). Color measurementz and colourant estimation of natural red dyes on natural fabrics using different mordants. Colorage, 1, 31–42.
Ngueyem, T. A., Brusotti, G., Caccialanza, G., & Vita, F. P. (2009). A phytochemical and enthnopharmacological review. Journal of Enthnopharmacologicology, 124(3), 339–349.
Oduro, W. O., & Addo-Yobo, F. (2015). Optimising the extraction of a natural dye from Bridelia ferruginea. South Africa Journal of Chemical Engineering, 20(3), 27–40.
Opoku-Asare, N. A., Korankye, O., & Baah, A. K. (2013). Assessing the efficacy of dyes extracted from some local plants for colouring cotton fabrics. Journal of Science and Technology, 33(3), 51–59.
Rather, L., Islam, S., Shabbir, M., Bukhari, M. N., Shahid, M., Khan, M. A., et al. (2016). Ecological dyeing of woolen yarn with Adhatoda vasica natural dye in the presence of biomordants as an alternative copartner to metal mordants. Journal of Environmental Chemical Engineering, 4, 3041–3049.
Shabbir, M., Islam, S., Bukhari, M. N., Rather, L. J., Khan, M. A., & Mohammed, F. (2016). Application of Terminalia chebula natural dye on wool fibre-evaluation of colour and fastness properties. Textile and Clothing Sustanability, 2, 1–9.
Shahid, M., Ahmed, A., Yusuf, M., Khan, M. I., Khan, S. A., Manzoor, N., et al. (2012). Dyeing, fastness and antimicrobial properties of woolen yarns dyed with gallnut (Quercus infectoria Oliv.) extract. Dyes and Pigments, 95, 53–61.
Sufian, A., Hannan, A., Rana, M., & Huq, M. Z. (2016). Comparative study of fastness properties and colorabsorbance criteria of conventional and avitera reactive dyeing on cotton knit fabric. European Scientific Journal, 12(15), 352–364.
Uddin, M. G. (2015). Extraction of eco-friendly natural dyes from mango leaves and their application on silk fabric. Textile and Clothing Sustainability, 1, 7.
Yusuf, M., Mohammad, F., Shabbir, M., & Khan, M. A. (2016). Eco-dyeing of wool with Rubia cordifolia root extract: Assessment of the effect of Acacia catechu as biomordant on color and fastness properties. Textile and Clothing Sustainability, 2(10), 1–9.
Yusuf, M., Shahid, M., Khan, S. A., Khan, M. A., & Mohammad, F. (2015). Dyeing studies with henna and madder: A research on effect of tin (II) chloride mordant. Journal of Saudi Chemical Society, 19, 64–72.
Acknowledgements
The authors are highly thankful to the Federal Government of Nigeria for awarding Tertiary Education Trust Fund (2016) grants to equip Textile and Polymer Research Laboratory, Department of Chemistry, the Federal University of Technology, Akure (FUTA) where laboratory facilities for the success of this research work were obtained.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
AIO carried out all the experimental work under the supervision of the JMJ who provided guidance for carrying out the experiments, helped in reviewing the manuscript. TAAT performed the collection of the samples and helped in drafting the manuscript in conjunction with AIO. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Table A.
Results data for absorption spectrum and calibration curve.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jabar, J.M., Ogunmokun, A.I. & Taleat, T.A.A. Color and fastness properties of mordanted Bridelia ferruginea B dyed cellulosic fabric. Fash Text 7, 1 (2020). https://doi.org/10.1186/s40691-019-0195-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40691-019-0195-z