Abstract
Background
To investigate the optical coherence tomography angiography (OCTA) characteristics of diabetic macular edema (DME) at different stages.
Methods
This study was a cross-sectional study. Patients diagnosed with DME were recruited. DME was classified into early, advanced, and severe DME. The vessel density (VD) in the superficial vascular plexus (SVP), deep vascular plexus (DVP) and foveal avascular zone (FAZ) parameters, including FAZ area, FAZ perimeter, acircularity index and foveal VD in a 300-μm-wide region around the FAZ (FD-300), were calculated by the AngioVue software. A multivariate generalized estimating equation was used to evaluate the associations between visual acuity and OCTA metrics.
Results
Ninety-two eyes from 74 patients with DME were included in this study. Compared to early (P = 0.006) and advanced DME (P = 0.003), the acircularity index was higher in severe DME. Both whole and parafoveal VD in the DVP decreased in eyes with severe DME compared to early DME (P = 0.018, P = 0.005, respectively) and advanced DME (P = 0.035, P = 0.012, respectively). In the multivariate generalized estimating equation, DME severity, FAZ area and foveal thickness were positively associated with worse visual acuity (P = 0.001, P = 0.007 and P = 0.001, respectively).
Conclusion
Compared to early and advanced DME, severe DME showed increased irregularity in the FAZ and more extensive vessel damage in the DVP. Greater severity level of DME, larger FAZ area, and increased foveal thickness could be risk factors for poor visual acuity.
Trial registration The protocol was published in the Chinese Clinical Trial Registry (ChiCTR2000033082).
Similar content being viewed by others
Background
Diabetic retinopathy (DR) is rising dramatically due to the steep increase in global diabetes incidence. It has been estimated that the number of patients with DR is 103 million in 2020 and could increase to 161 million in 2045 [1]. As one of the leading causes of visual impairment and blindness in the working-age population and the elderly [2, 3], diabetic macular edema (DME), which can occur at any stage of DR, has attracted great attention.
Optical coherence tomography angiography (OCTA) visualizes different retinal vascular layers noninvasively and has been utilized to study the associations between vessel density (VD) and foveal avascular zone (FAZ) parameters and DME [4, 5]. However, these studies have focused on either the association between intravitreal injection and OCTA parameters or the comparison of OCTA parameters in DR with or without DME. The association between the stages of DME and OCTA parameters has rarely been reported.
In the past, DME severity was assessed via ophthalmoscopy or color fundus photography [6]. Currently, DME grading classifications are also based on spectral domain optical coherence tomography (SD-OCT) and fluorescein angiography [7,8,9]. Despite attempts to classify DME through a variety of methods, grading DME with more comprehensive details visible on SD-OCT could be more conducive to improving the understanding of DME pathophysiology. An international SD-OCT-based classification of DME called “TCED-HFV”, which considers the specific morphologic features of OCT, including foveal thickness, intraretinal cysts, the state of the ellipsoid zone (EZ) and the external limiting membrane (ELM), the presence of disorganization of the inner retinal layers (DRIL) , the quantity of hyperreflective foci, the occurrence of subfoveal fluid, and the vitreoretinal relationship has been proposed recently [10]. Thus, it is important to utilize the newly proposed grading [10] to evaluate DME severity and to further analyze the OCTA characteristics among different grades of DME.
The objective of this study was to evaluate DME severity and to analyze the association between DME severity and OCTA parameters. The association between OCTA metrics and visual acuity in DME was also studied.
Methods
Subjects
Between June 2019 and September 2021, patients with DME were recruited consecutively at the Eye and ENT Hospital of Fudan University. The protocol was published in the Chinese Clinical Trial Registry (ChiCTR2000033082). This cross-sectional observational study was approved by the Ethics Committee of the Eye and ENT Hospital of Fudan University and conformed to the tenets of the Declaration of Helsinki. All participants provided written informed consent.
Criteria for inclusion included a central retinal thickness (CRT) greater than 320 μm for males or 305 μm for females [11], a diagnosis of DME, and age ≥ 18 years old. In this study, SD-OCT (Spectralis, Heidelberg, Germany) was used to calculate CRT and evaluate DME, and DME was equivalent to center-involved DME, which was defined as intraretinal cystoid changes and/or retinal thickening and/or neurosensory retinal detachment involving the central ring of the ETDRS macular map [10]. Participants were excluded due to (1) OCTA images scan quality score less than 6/10, motion artifacts, inaccurate segmentation, blurry images, and poor centration; (2) macular atrophy, high myopia, uveitis, glaucoma, severe media opacities, and previous ocular trauma; or (3) laser photocoagulations, intravitreal drug application and ocular surgery in the 6-month period before the study.
Ophthalmic examinations including best-corrected visual acuity (BCVA; logMAR visual acuity), intraocular pressure (non-contact tonometer), slit-lamp biomicroscopy, color fundus photography, OCT and OCTA were performed. The evaluation of DME severity was based on an OCT-based grading protocol [10], and DME was classified into early, advanced, and severe DME. Early DME was characterized by the presence of small intraretinal cysts, well-recognizable and detectable inner retinal layers, EZ, and ELM, and increase in CRT less than 30% of upper normal values. Advanced and severe DME were both defined by a CRT above 30% of upper normal values, macrocysts and/or multiple intraretinal cystoid spaces, and/or the loss of clear demarcation in inner retinal layers. The EZ/ELM may be impaired but still partially visible in the fovea in advanced DME, while the EZ/ELM are mostly undetectable in severe DME. DR was classified as non-proliferative (NPDR) or proliferative DR (PDR) according to the ETDRS Retinopathy Severity Scale [12]. Glycated hemoglobin A1c, serum lipids, and blood pressure were collected within two weeks of the start of the study. Hypertension was defined as a self-reported history of hypertension and/or a clinic blood pressure ≥ 140/90 mmHg [13]. Increased total cholesterol (≥ 5.2 mmol/L), low-density lipoprotein cholesterol (≥ 3.35 mmol/L), and triglyceride levels (≥ 2.25 mmol/L) were regarded as hyperlipidemia [14, 15]. Other relevant data, including age, sex, body mass index, duration of diabetes, type of diabetes, renal impairment, history of medication, and smoking and drinking status, were also recorded.
OCTA
OCTA images were acquired after pupillary dilation using AngioVue OCTA (RTVue XR Avanti 2017.1 version, Optovue Inc., Fremont, CA, USA). Centered at the fovea, the OCTA scanning area was 3 × 3 mm with a resolution of 304 × 304 pixels. The superficial vascular plexus (SVP) and deep vascular plexus (DVP) were divided automatically by the AngioVue software. Cases with segmentation errors were corrected manually. The SVP extends from the inner limiting membrane (ILM) to 9 μm above the inner plexiform layer (IPL)-inner nuclear layer (INL) junction, while the DVP lies between 9 μm above the IPL-INL junction and 9 μm beneath the outer plexiform layer (OPL)-outer nuclear layer (ONL) junction. Two observers (R.G. and R.H.) examined image quality independently and ruled out inferior quality images leading to possible segmentation errors. The whole and parafoveal VD in the SVP and DVP were calculated automatically by the AngioVue software (algorithm version: A2017, 1, 0, 155). Vessel density was defined as the proportion of vessel area with flowing blood over the total measurement area. The whole area was defined as a circle area centered at the fovea with a diameter of 3 mm. The parafoveal area was defined as the whole area minus the central circle area that centered at the fovea with a diameter of 1 mm. Furthermore, FAZ parameters, including FAZ area, FAZ perimeter, acircularity index, and foveal VD in a 300-μm-wide region around the FAZ (FD-300), were measured automatically based on the retina slab (between the ILM and 9 μm beneath the OPL-ONL junction). FAZ perimeter was defined as the length that encompasses the FAZ. The acircularity index was defined as the ratio of the measured FAZ perimeter to the perimeter of a circle with the same area. In addition, foveal thickness was also measured automatically via the built-in software.
Statistical analysis
SPSS software (version 25.0, IBM Corporation, Chicago, IL, USA) was used for statistical analysis. Before and after adjusting for DR severity and systemic risk factors including sex, age, type of diabetes mellitus, and a history of glycated hemoglobin A1c, hyperlipidemia, hypertension, renal impairment, and smoking [16], the one-way analysis of variance, least significant difference test or Kruskal-Wallis H test were utilized to compare the OCTA metrics among three DME groups with different grades. The univariate and multivariate generalized estimating equations were used to evaluate potential associations between visual acuity and OCTA metrics and other risk factors. A two-tailed P value less than 0.05 indicated statistical significance.
Results
A total of 74 patients (92 eyes) with a mean age of 56.23 ± 12.35 years were included. This cohort was type 2 diabetes-predominant and male-predominant with type 2 diabetes making up 93.24% and males making up 68.92% of the group. The mean level of glycated hemoglobin A1c was 8.12 ± 1.82%, and the mean duration of DM was 13.13 ± 7.67 years. More than half of the participants had hypertension (58.11%), 44.59% of the participants were diagnosed with hyperlipidemia, and 37.84% of the individuals had a history of renal impairment. For DR severity level, 52 eyes had NPDR, while 40 eyes had PDR. This study consisted of 24 eyes with early DME, 53 eyes with advanced DME, and 15 eyes with severe DME. The mean BCVA was 0.2 ± 0.15, 0.48 ± 0.23, and 1.06 ± 0.27 logMAR for early, advanced, and severe DME. LogMAR visual acuity was significantly different among different DME grades (P < 0.001).
OCTA data among different stages of DME are displayed in Table 1. After adjusting for DR severity and systemic risk factors, the acircularity index was significantly different among different DME grades (P = 0.016), and the increase in the acircularity index was shown in severe DME compared to early (P = 0.006) and advanced DME (P = 0.003). The whole and parafoveal VD in the DVP in severe DME showed more diffuse vascular rarefaction than early DME (P = 0.018, P = 0.005, respectively) and advanced DME (P = 0.035, P = 0.012, respectively). No significant trend in VD in the SVP was found among these groups. Furthermore, foveal thickness was positively associated with DME severity (P < 0.001).
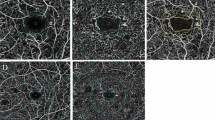
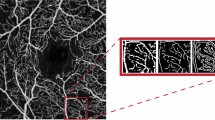
Figure 1 presents OCTA images in the SVP and DVP among different DME grades. Figure 2 shows FAZ parameters among different DME grades. Morphologically, severe DME had lower VD in the DVP and higher acircularity index.
Optical coherence tomography angiography images of superficial vascular plexus (SVP) and deep vascular plexus (DVP) in different stages of diabetic macular edema (DME). As DME progressed, whole vessel density (VD) in the DVP decreased dramatically, while the change in whole VD in the SVP was marginal. In this figure, the eye with early DME has a VD of 38.4% in the SVP and a VD of 44.1% in the DVP, the eye with advanced DME has a VD of 39% in the SVP and a VD of 44.7% in the DVP, and the eye with severe DME has a VD of 36.1% in the SVP and a VD of 35.6% in the DVP
Optical coherence tomography angiography images of foveal avascular zone (FAZ) parameters among different diabetic macular edema (DME) grades. The acircularity index was significantly increased as DME progresses. The eye with early DME has an acircularity index of 1.14, the eye with advanced DME has an acircularity index of 1.36, and the eye with severe DME has an acircularity index of 1.67
Table 2 presents the univariate and multivariate generalized estimating equations of the associations between logMAR visual acuity and OCTA parameters as well as other associated risk factors. For the multivariate generalized estimating equation, a 1 mm2 increase in FAZ area was associated with + 0.24 increase in logMAR visual acuity (P = 0.007), and a 100 μm increase in foveal thickness was associated with + 0.109 increase in logMAR visual acuity (P = 0.001). Moreover, DME severity was associated with logMAR visual acuity (compared to early DME, advanced or severe DME was associated with + 0.223 increase in logMAR visual acuity, P = 0.001).
Discussion
Recently, an OCT-based grading system called “TCED-HFV” was proposed to assess DME severity. OCTA allows the presentation of different retinal vascular layers. Therefore, it may help to uncover the potential pathology of DME progression. In this cross-sectional observational study, OCTA characteristics among different stages of DME were investigated, and the results suggested that VD in the DVP rather than in the SVP decreased significantly as DME progressed. Furthermore, DME severity, FAZ acircularity index, and foveal thickness were associated with visual acuity. To our knowledge, this is the first study to evaluate associations between OCTA characteristics and DME stages by an OCT-based grading system.
Compared to eyes without DME, macular ischemia was more profound in the DVP of eyes with DME [17, 18]. Congruently, our data also demonstrated that DME severity was associated with decreased VD in the DVP but not with that in the SVP. On the one hand, the decreased VD in the DVP might result in the progression of DME. Fluid production may originate from the SVP, whereas Müller cells and the DVP play roles in fluid removal [19]. We hypothesize that the decreased or absent flow in the DVP places the burden of fluid removal on the Müller cells, consequently contributing to edema at that site due to the imbalance between fluid entry and efflux [20].
On the other hand, it could be that microvascular changes due to the progression of DME occur earlier in the DVP than in the SVP. First, according to the grading of DME severity in the current study [10], a larger intraretinal cyst size partially represents increased DME severity. The capillary segments in the DVP are all interlocked and are approximately 150 μm in length [21], whereas the diameter of the intraretinal cysts could be hundreds of microns [19]. We speculate that the capillaries in the DVP are stretched and interrupted by the increased size of the intraretinal cysts. In addition, inflammation plays an indispensable role in the pathogenesis of DME and results in leukostasis, which is involved in vascular remodeling and capillary nonperfusion [22,23,24,25]. Very small vessels, which exist in the entire DVP but only a small part of the SVP, could be plugged by leukocytes [19]. Consequently, the occurrence of leukostasis in the DVP could be more frequent. Finally, the elevated level of vascular endothelial growth factor (VEGF) leads to the development of DME [26]. VEGF has been shown to cause intravascular endothelial proliferation in vivo [27]. Similar to the pathophysiological mechanism of leukostasis, the DVP is more vulnerable to VEGF-induced intravascular endothelial proliferation than the SVP. Hence, the decreased flow of DVP could be positively associated with DME severity.
The association between FAZ area and DME remains to be elucidated. A study showed that the FAZ area was increased in eyes with DME compared to eyes without DME [18]. In contrast, Tarassoly and colleagues confirmed that the FAZ area was equal in diabetic eyes with and without cystoid edema [4]. In this study, both the FAZ area and perimeter were not significantly changed among different DME grades. However, similar to studies suggesting that worsening stages of DR was correlated with higher irregularity in the FAZ [28,29,30], the acircularity index significantly increased as DME progressed. The possible explanations are as follows. First, there are considerable variations of FAZ metrics in healthy individuals. The FAZ area and perimeter increased with age, and the FAZ area was associated with sex, while the acircularity index, an indicator of irregularity in the FAZ, was independent of age and sex [31]. Second, unlike the FAZ area and perimeter that could be imprecisely measured owing to the alterations in retinal magnification after lens implantation and the difference in axial length, the acircularity index is a quotient that does not require the correction of axial length [28]. Additionally, vascular layer segmentation and the method of calculating FAZ parameters could be responsible for the discrepancy. More importantly, the potential pathophysiology of the increased acircularity index as DME progressed was multifactorial. Mechanical stretch and damage to capillaries due to DME in the foveal region may lead to irregular FAZ development [19]. Foveal neovascularization and capillary abnormalities could also cause irregularity in the FAZ [28, 32]. Therefore, we think the acircularity index could be more sensitive to the progression of DME than the FAZ area and perimeter.
It has been shown previously that the FAZ area, foveal thickness, DRIL, and ELM and EZ disruption are associated with worse visual acuity in DME [18, 33,34,35,36,37]. In our study, advanced and severe DME were characterized by greater foveal thickness, the occurrence of DRIL and/or the disruption or absence of ELM/EZ compared to early DME. These studies mentioned above are in line with our data demonstrating that the FAZ area, foveal thickness, and DME severity were associated with visual acuity in DME.
There are several limitations in this study. First, the presence of DME interferes with OCTA signal intensity [38] and quantitative vascular measurements [39]. Second, the distribution of different DME grades was uneven in this study, and the cross-sectional design of this small-scale study restrained us from assessing the temporal patterns in retinal blood vessels during the progression of DME. Our findings need to be fully validated by a larger, evenly distributed population of DME of a prospective design. In addition, the 3 × 3 mm OCTA scanning field can present only a small area of the retinal posterior pole. Evaluating the OCTA parameters of the perifoveal and peripheral retina is also beneficial for understanding microvasculature variations with the development of DME.
Conclusion
The progression of DME causes vascular rarefaction in the DVP. Increased DME severity, greater foveal thickness, and larger FAZ area result in worse visual acuity. OCTA could be a promising device for evaluating DME severity and visual acuity.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- BCVA:
-
Best-corrected visual acuity
- CRT:
-
Central retinal thickness
- DME:
-
Diabetic macular edema
- DR:
-
Diabetic retinopathy
- DRIL:
-
Disorganization of the inner retinal layers
- DVP:
-
Deep vascular plexus
- ELM:
-
External limiting membrane
- EZ:
-
Ellipsoid zone
- FAZ:
-
Foveal avascular zone
- FD-300:
-
Foveal VD in a 300-μm-wide region around the FAZ
- ILM:
-
Limiting membrane
- INL:
-
Inner nuclear layer
- IPL:
-
Inner plexiform layer
- NPDR:
-
Non-proliferative diabetic retinopathy
- OCTA:
-
Optical coherence tomography angiography
- ONL:
-
Outer nuclear layer
- OPL:
-
Outer plexiform layer
- PDR:
-
Proliferative diabetic retinopathy
- SD-OCT:
-
Spectral domain optical coherence tomography
- SVP:
-
Superficial vascular plexus
- VD:
-
Vessel density
References
Teo ZL, Tham YC, Yu M, Chee ML, Rim TH, Cheung N, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology. 2021;128(11):1580–91.
Ciulla TA, Amador AG, Zinman B. Diabetic retinopathy and diabetic macular edema: pathophysiology, screening, and novel therapies. Diabetes Care. 2003;26(9):2653–64.
Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, Berg K, Chakravarthy U, Gerendas BS, et al. Guidelines for the management of diabetic macular edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica. 2017;237(4):185–222.
Tarassoly K, Miraftabi A, Soltan Sanjari M, Parvaresh MM. The relationship between foveal avascular zone area, vessel density, and cystoid changes in diabetic retinopathy: an optical coherence tomography angiography study. Retina. 2018;38(8):1613–9.
Sun Z, Tang F, Wong R, Lok J, Szeto SKH, Chan JCK, et al. OCT angiography metrics predict progression of diabetic retinopathy and development of diabetic macular edema: a prospective study. Ophthalmology. 2019;126(12):1675–84.
Wilkinson CP, Ferris FL 3rd, Klein RE, Lee PP, Agardh CD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110(9):1677–82.
Bolz M, Lammer J, Deak G, Pollreisz A, Mitsch C, Scholda C, et al. SAVE: a grading protocol for clinically significant diabetic macular oedema based on optical coherence tomography and fluorescein angiography. Br J Ophthalmol. 2014;98(12):1612–7.
Flaxel CJ, Adelman RA, Bailey ST, Fawzi A, Lim JI, Vemulakonda GA, et al. Diabetic Retinopathy Preferred Practice Pattern®. Ophthalmology. 2020;127(1):P66-145.
Parodi Battaglia M, Iacono P, Cascavilla M, Zucchiatti I, Bandello F. A pathogenetic classification of diabetic macular edema. Ophthalmic Res. 2018;60(1):23–8.
Panozzo G, Cicinelli MV, Augustin AJ, Battaglia Parodi M, Cunha-Vaz J, Guarnaccia G, et al. An optical coherence tomography-based grading of diabetic maculopathy proposed by an international expert panel: the European School for Advanced Studies in Ophthalmology classification. Eur J Ophthalmol. 2020;30(1):8–18.
Chalam KV, Bressler SB, Edwards AR, Berger BB, Bressler NM, Glassman AR, et al. Retinal thickness in people with diabetes and minimal or no diabetic retinopathy: Heidelberg spectralis optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53(13):8154–61.
Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 Suppl):823–33.
Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127-248.
Kopin L, Lowenstein C. Dyslipidemia. Ann Intern Med. 2017;167(11):ITC81-96.
Rhee EJ, Kim HC, Kim JH, Lee EY, Kim BJ, Kim EM, et al. 2018 Guidelines for the management of dyslipidemia. Korean J Intern Med. 2019;34(4):723–71.
Ting DSW, Tan GSW, Agrawal R, Yanagi Y, Sie NM, Wong CW, et al. Optical coherence tomographic angiography in type 2 diabetes and diabetic retinopathy. JAMA Ophthalmol. 2017;135(4):306–12.
Lee J, Moon BG, Cho AR, Yoon YH. Optical coherence tomography angiography of DME and its association with anti-VEGF treatment response. Ophthalmology. 2016;123(11):2368–75.
AttaAllah HR, Mohamed AAM, Ali MA. Macular vessels density in diabetic retinopathy: quantitative assessment using optical coherence tomography angiography. Int Ophthalmol. 2019;39(8):1845–59.
Spaide RF. Retinal vascular cystoid macular edema: review and new theory. Retina. 2016;36(10):1823–42.
Spaide RF. Volume-rendered optical coherence tomography of diabetic retinopathy pilot study. Am J Ophthalmol. 2015;160(6):1200–10.
Shimizu K, Ujiie K. Structure of ocular vessels. Tokyo: Igaku-Shion Ltd; 1978. p. 8–49.
Miyamoto K, Khosrof S, Bursell SE, Rohan R, Murata T, Clermont AC, et al. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci U S A. 1999;96(19):10836–41.
Wang J, Xu X, Elliott MH, Zhu M, Le YZ. Muller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes. 2010;59(9):2297–305.
Rangasamy S, McGuire PG, Das A. Diabetic retinopathy and inflammation: novel therapeutic targets. Middle East Afr J Ophthalmol. 2012;19(1):52–9.
Fu S, Dong S, Zhu M, Sherry DM, Wang C, You Z, et al. Müller glia are a major cellular source of survival signals for retinal neurons in diabetes. Diabetes. 2015;64(10):3554–63.
Das A, McGuire PG, Rangasamy S. Diabetic macular edema: pathophysiology and novel therapeutic targets. Ophthalmology. 2015;122(7):1375–94.
Tolentino MJ, Miller JW, Gragoudas ES, Jakobiec FA, Flynn E, Chatzistefanou K, et al. Intravitreous injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology. 1996;103(11):1820–8.
Krawitz BD, Mo S, Geyman LS, Agemy SA, Scripsema NK, Garcia PM, et al. Acircularity index and axis ratio of the foveal avascular zone in diabetic eyes and healthy controls measured by optical coherence tomography angiography. Vision Res. 2017;139:177–86.
Li H, Yu X, Zheng B, Ding S, Mu Z, Guo L. Early neurovascular changes in the retina in preclinical diabetic retinopathy and its relation with blood glucose. BMC Ophthalmol. 2021;21(1):220.
Choi JM, Kim SM, Bae YH, Ma DJ. A study of the association between retinal vessel geometry and optical coherence tomography angiography metrics in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2021;62(13):14.
Lavia C, Bonnin S, Maule M, Erginay A, Tadayoni R, Gaudric A. Vessel density of superficial, intermediate, and deep capillary plexuses using optical coherence tomography angiography. Retina. 2019;39(2):247–58.
Joondeph BC, Joondeph HC, Flood TP. Foveal neovascularization in diabetic retinopathy. Arch Ophthalmol. 1987;105(12):1672–5.
DaCosta J, Bhatia D, Talks J. The use of optical coherence tomography angiography and optical coherence tomography to predict visual acuity in diabetic retinopathy. Eye (Lond). 2020;34(5):942–7.
Das R, Spence G, Hogg RE, Stevenson M, Chakravarthy U. Disorganization of inner retina and outer retinal morphology in diabetic macular edema. JAMA Ophthalmol. 2018;136(2):202–8.
Ou WC, Brown DM, Payne JF, Wykoff CC. Relationship between visual acuity and retinal thickness during anti-vascular endothelial growth factor therapy for retinal diseases. Am J Ophthalmol. 2017;180:8–17.
Diabetic Retinopathy Clinical Research Network, Browning DJ, Glassman AR, Aiello LP, Beck RW, Brown DM, et al. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 2007;114(3):525–36.
Borrelli E, Grosso D, Barresi C, Lari G, Sacconi R, Senni C, et al. Long-term visual outcomes and morphologic biomarkers of vision loss in eyes with diabetic macular edema treated with anti-VEGF therapy. Am J Ophthalmol. 2021;235:80–9.
Toto L, D’Aloisio R, Di Nicola M, Di Martino G, Di Staso S, Ciancaglini M, et al. Qualitative and quantitative assessment of vascular changes in diabetic macular edema after dexamethasone implant using optical coherence tomography angiography. Int J Mol Sci. 2017;18(6):1181.
Borrelli E, Sacconi R, Parravano M, Costanzo E, Querques L, Battista M, et al. Optical coherence tomography angiography assessment of the diabetic macula: a comparison study among different algorithms. Retina. 2021;41(9):1799–808.
Acknowledgements
Not applicable.
Funding
The present work was supported by the Clinical Research Plan of SHDC (SHDC2020CR2041B) and the Science and Technology Commission of Shanghai Municipality (20Y11911100).
Author information
Authors and Affiliations
Contributions
WL and GX designed the study, RH and RG conducted the study, RH and RG drafted the manuscript, and WL and RH revised the manuscript. All authors reviewed the final version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol was published in the Chinese Clinical Trial Registry (registration number: ChiCTR2000033082). The study was approved by the Ethics Committee of the Eye and ENT Hospital of Fudan University (No. 2020043) and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all participants included in the study.
Consent for publication
Not applicable.
Competing interests
All authors of this publication have no conflict of interest to declare.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Han, R., Gong, R., Liu, W. et al. Optical coherence tomography angiography metrics in different stages of diabetic macular edema. Eye and Vis 9, 14 (2022). https://doi.org/10.1186/s40662-022-00286-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40662-022-00286-2