Abstract
Purpose
To assess clinical results of the 9 mW/5.4 J/cm2 accelerated crosslinking (ACXL) in the treatment of progressive keratoconus (KC) over a span of 5 years.
Methods
The prospective open non-randomized interventional study (Siena Eye-Cross Study 2) included 156 eyes of 112 patients with early progressive KC undergoing the Epi-Off 9 mW/5.4 J/cm2 ACXL at the Siena Crosslinking Centre, Italy. The mean age was 18.05 ± 5.6 years. The 20-min treatments were performed using the New KXL I (Avedro, Waltham, USA), 10 min of 0.1% HPMC Riboflavin soaking (VibeX Rapid, Avedro, Waltham, USA) and 10 min of continuous-light UV-A irradiation. Uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA), Kmax, coma, minimum corneal thickness (MCT), surface asymmetry index (SAI), endothelial cell count (ECC) were measured, and corneal OCT performed.
Results
UDVA and CDVA improved significantly at the 3rd (P = 0.028), Δ + 0.17 Snellen lines and 6th postoperative month, respectively (P < 0.001), Δ + 0.23 Snellen lines. Kmax improved at the 6th postoperative month (P = 0.03), Δ − 1.49 diopters from the baseline value. Also, coma aberration value improved significantly (P = 0.004). A mild temporary haze was recorded in 14.77% of patients without affecting visual acuity and without persistent complications. Corneal OCT revealed a mean demarcation line depth at 332.6 ± 33.6 μm.
Conclusion
The 5-year results of Epi-Off 9 mW/5.4 J/cm2 ACXL demonstrated statistically significant improvements in UCVA and CDVA, corneal curvature and corneal higher-order aberrations which confers a long-term stability for progressive ectasia. Based on the results of the Siena Eye-Cross Study 2, the 9 mW/5.4 J/cm2 ACXL is a candidate to be the natural evolution of Epi-Off CXL treatment for the management of early progressive corneal ectasia, and thus optimize clinic workflow.
Similar content being viewed by others
Background
Conventional riboflavin UV-A induced corneal crosslinking (CXL) with epithelium removal (Epi-Off) [1, 2] represents a cost-effective treatment [3] with documented long-term efficacy in stabilizing progressive keratoconus [4, 5] and secondary ectasia [6, 7] in randomized clinical trials [8] and open non-randomized studies both in young adult [9] and pediatric populations [10], which thus reduce the need of corneal transplants for keratoconus by 25% [11] to 50% [12].
The standard energy dose of 5.4 J/cm2 delivered in 30 min at 3 mW/cm2 UV-A irradiance after 30 min of isotonic 0.1% Riboflavin-Dextran 20% stromal soaking (the Dresden protocol) [13], was approved in the United States by the Food and Drug Administration (U.S. FDA) in April 2016 [14], and requires a long treatment time of 1 h [15]. In order to shorten the duration of CXL, improve patients’ comfort and reduce wound-related stimuli of the corneal stroma, different Epi-Off accelerated crosslinking protocols (ACXL) [16,17,18,19,20,21,22,23,24,25,26] were tested based on the equal-dose principle stated in the Bunsen Roscoe’s law of reciprocity [27] and on preclinical lab studies [28] addressing the biomechanical equivalence between conventional Epi-Off CXL and ACXL with continuous and pulsed UV-light exposure [29,30,31]. The most commonly used settings in Epi-Off ACXL treatments were the 9 mW/5.4 J/cm2 × 10 min of continuous UV-light exposure [17], the 18 mW/5.4 J/cm2 × 5 min22, the 15 mW/5.4 J/cm2 × 12 min with pulsed-UV-light exposure [25] and the 30 mW/5.4 J/cm2 × 3 min continuous [23] and pulsed-light [16]. Richoz et al. [32] demonstrated that the Bunsen-Roscoes’ law does not apply in full for ACXL because the biomechanical effect of CXL decreases significantly when using high-UV irradiance with short irradiation times due to the reduced stromal oxygen diffusion capacity which may be a limiting factor that reduces overall treatment efficiency. Clinical studies [33,34,35] demonstrated that the 9 mW/5.4 J/cm2 ACXL gained comparable visual outcomes with conventional 3 mW/cm2 Dresden protocol with keratoconus stabilization [36, 37].
We report the long-term (5-years) clinical results of the 9 mW/5.4 J/cm2 ACXL protocol also named “Dresden Accelerated Protocol” in a large cohort of patients performed in Italy at the Siena Crosslinking Centre named “Siena Eye-Cross Study 2”.
Patients and methods
Dataset and study design
The prospective long-term open non-randomized, non-comparative interventional Siena Eye-Cross Study 2 was approved by the institutional review board (IRB) of the Siena Crosslinking Center following the tenets of the Declaration of Helsinki and included 156 eyes of 112 patients who underwent an Epi-Off 9 mW/5.4 J/cm2 ACXL procedure for progressive KC and completed the 5-year follow-up. All patients were affected by progressive (stage I and II) KC and were enrolled in the ACXL treatment protocol from January 2014 to April 2015. The prospective open non-randomized interventional Siena Eye-Cross Study 2 evaluating the 9mW Dresden accelerated CXL protocol included all patients who completed the 5-year follow-up. Bilateral treatments included 88 of 156 eyes (56.4%) that showed a preoperative bilateral KC progression. All patients 18 years old and under (N = 20), including 40 eyes, belong to bilateral treatments (25.6%). Patients 18 years old and younger represent 45.5% of the total number of bilateral treatments. The remaining 24 patients who underwent a bilateral treatment, including 48 eyes (54.5%), were between 19 and 25 years old. The unilateral treatments were performed in 68 of the 156 eyes (43.6%) and included 19 eyes of 19 patients with unilateral Keratoconus (12.1%), while the remaining unilateral ACXL treatments included 49 eyes of 49 patients ranging from 26 to 31 years old, showing no progression in the fellow eye during the follow-up. Bilateral treatments were performed after a minimum time interval of 30 days to a maximum of 60 days (mean 40 days). Seventy patients were male (87.5%). The mean age at the time of enrolment in the treatment protocol was 18.05 ± 5.6 years (range: 8–31 years).
Inclusion criteria
Progressive stage II KC was identified according to Krumeich’s staging system [38]. Progression of KC was defined as an increase in apical keratometry (AK) ≥ 1 diopter (D) on the anterior corneal topography using a Scheimpflug-Placido corneal tomography system Sirius, Costruzione Strumenti Oftalmici (C.S.O.), Florence, Italy; minimum corneal thickness (MCT) reduction ≥ 10 μm; worsening of uncorrected distance visual acuity (UDVA) and corrected distance visual acuity (CDVA) ≥ 0.1 decimal equivalent or change of ≥ 0.5 in mean refractive spherical equivalent (MRSE) in the last 6 months of clinical and instrumental observation [39]. MCT was at least 400 μm (epithelium included), measured with no-contact optical pachymetry. Corneas were clear with no sub-apical opacities or scars and no markedly visible Vogt’s striae, no previous infectious keratitis or autoimmune diseases and no severe dry eye. All patients included in the study gave their specific written informed consent.
Surgical procedure
All treatments were performed at the Siena Crosslinking Centre, Italy, by the same surgeon (CM). The 9 mW/5.4 J/cm2 ACXL treatment was performed using the KXL I system (Avedro, Waltham, MS, USA) under topical anesthesia (4% Oxibuprocaine chlorydrate 1.6 mg/0.4 mL drops) that was applied 10 min before the treatment after a premedication with 2% pilocarpine instilled 30 min before the operation. After applying a closed valves eyelid speculum, a 9-mm diameter epithelium was removed with a blunt metal spatula. After epithelial removal, a dextran-free plus hydroxyl-propyl methylcellulose (HPMC) disposable 0.1% riboflavin isotonic solution (VibeX Rapid, Avedro, Waltham, MA, USA) was instilled for one drop every minute for 10 min of corneal soaking, before starting continuous light UV-A irradiation. During UV-A-irradiation, 2 drops of riboflavin solution were administered every 2.5 min for a total of 10 min of UV-A exposure at 9 mW/cm2 of UV-A power and standard Fluence of 5.4 J/cm2. At the end of the UV-A irradiation, the cornea was washed with balanced saline solution (BSS) and medicated with preservative-free netilmicin plus dexamethasone, cyclopentolate eyedrops and dressed with a therapeutic bandage soft contact lens for 4 days. After therapeutic contact lens removal, fluorometholone 0.2% eye-drops (tapered 3 times/day) and sodium hyaluronate 0.2% lacrimal substitutes were administered for 6 to 8 weeks.
Measurements and devices
Ophthalmic evaluations were performed before CXL and at all follow-up visits (1, 3, 6, 12, 24, 36, 48 and 60 months). The evaluation included UDVA, CDVA and a slit-lamp clinical examination. Scheimpflug based corneal tomography (Sirius, CSO, Florence, Italy) was used to measure maximum curvature simulated k reading (Kmax), coma high-order aberration, MCT, tomography derived SAI and topographic cylinder (CYL). Anterior segment optical coherence tomography (AS-OCT) with the I-Vue (Optovue, Freemont CA, USA) was performed to assess the demarcation line depth at the first post-operative month. Endothelial cell count was measured by the I-Konan Non-Co Robot (Konan Inc., Hyogo, Japan) preoperatively and every year until the end of the study.
Statistical analysis
A two-tailed paired samples t test was used to compare each baseline measurement with the respective follow-up measurements. Differences with P < 0.05 were considered statistically significant. Data were collected and analyzed with PRISM 6.0 GraphPad Software (La Jolla, California, USA).
Results
Baseline data
Visual acuity was represented in Snellen lines and decimal equivalents (d.e.). UDVA was 0.37 ± 0.11 and mean CDVA was 0.63 ± 0.09. Mean steepest corneal curvature (Kmax) was 55.70 ± 3.10 D. MCT was 443.76 ± 37.17 μm. Vertical coma high-order aberrations value was 1.01 ± 0.016 μm. SAI values were 4.77 ± 2.43. Mean CYL values were 2.94 ± 1.74 D. Endothelial cell count (ECC) was 2437.75 ± 287.6 cell/mm2. Baseline data are displayed in Table 1.
UDVA improved from baseline, becoming statistically significant at the 3rd postoperative month (P = 0.028) and remained significant until the end of follow-up (Fig. 1).
CDVA showed a statistically significant improvement at the 3rd (P < 0.001) postoperative month, remaining significant until the end of follow-up, (Fig. 2).
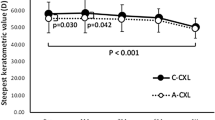
The baseline and follow-up measurements of Kmax documented that the value improved significantly at the 6th postoperative month, from 55.7 D to 54.36 D (P = 0.03) and this improvement remained statistically significant up to the 5th year follow-up (P = 0.001; delta − 1.49 D, Fig. 3).
The coma high order aberration mean values improved during the follow-up, becoming statistically significant at the 1st postoperative month (P = 0.004, Fig. 4).
Tomographic mean MCT value decreased significantly at the 1st (P = 0.0001) and 3rd postoperative month (P = 0.03), returning to baseline at the 6th month and maintaining a substantial stability up to 5-years follow-up (Fig. 5).
The topographic SAI values showed a significant change at the 1st month (P = 0.04), (Fig. 6).
The CYL average values remained stable along the entire follow-up in the whole study cohort, despite a positive trend. In the pediatric group, the CYL values showed a temporary significant improvement at the 1st month (P = 0.034) but became insignificant after the 3rd postoperative month (Fig. 7).
ECC showed no significant postoperative variations during any follow-up point (P > 0.05).
AS-OCT evaluation of the demarcation line depth at the 1st postoperative month showed a depth of 332.6 ± 23.6 μm in the overall study cohorts (Fig. 8).
The overall follow-up results are reported in Table 2.
Adverse events
No postoperative infections, persistent haze or endothelial cell failure were encountered during the entire follow-up period in the whole study cohort. In 16 eyes (10.25%) of 13 patients, a mild temporary haze at the 1st postoperative month at slit lamp examination was observed. However, this did not affect visual acuity and disappeared between the 3rd and the 6th month visit once topical steroids therapy was started (preservative-free 1 mg/mL dexamethasone eye-drops, tapered 4 times a day for 4 to 8 weeks). Thirteen eyes (8.33%) of 8 patients had a Kmax progression of 1 D within the 2nd-3rd year follow-up visits, returning to baseline value after 30 ± 6 months. All patients with such progression were affected with severe allergic papillary conjunctivitis. No retreatment was performed in the entire 5-year follow-up period. Data for adverse events are reported in Table 3.
Discussion
The 5-years follow-up results after accelerated epithelium-off CXL (9 mW/cm2 for 10 min) in the broadest case series of 156 KC eyes of 88 consecutive patients and with the longest follow-up currently reported internationally, showed clinically significant improvements in UDVA and CDVA, corneal curvature reduction with typical central flattening of the apical region of the KC with paracentral compensatory steepening of the flattest areas, inducing an improved corneal symmetry and corneal higher-order aberrations reduction (Fig. 9). In addition, the procedure was well tolerated in all patients, conferring a long-term (over 5-years) stability for progressive KC. No significant adverse events such as postoperative infections, persistent haze or endothelial cell failure were encountered during the entire follow-up period. A mild temporary haze that did not affect visual acuity was observed at the 1st postoperative month in around 15% of patients, disappearing after the 3rd month of clinical observation after topical steroid therapy.
Maximum corneal curvature progression of 1 D, which returned to baseline, was recorded between the 24th and 36th month follow-up in 13 eyes of 8 patients (8.33%). Patients that returned to baseline after initial functional improvement were all affected with allergies and were eye-rubbers. Interestingly, despite no retreatments being performed in the 5-years follow-up, none of these patients showed worsening in either UDVA or CDVA. Severe allergic conjunctivitis, eye-rubbing, and blepharitis-associated ocular surface chronic inflammation, however, were recognized as possible causes of poorer response and corneal topography instability [40].
The 5-years progression, intended as Kmax deterioration of 1 D or more occurred in 8.33% of patients, however, the improvement in CDVA did remain significant. AS-OCT evaluation of the demarcation line depth at the 1st postoperative month showed a depth of 332.6 ± 33.6 μm in the overall study cohort. To ensure a long-lasting stability of keratoconus and secondary ectasia, UV-A power settings and exposure time were targeted to allow a treatment penetration at least at 250 μm. Such depth of treatment penetration would allow a large portion of the anterior stroma to be cross-linked, which would be desirable especially since the anterior 40% of the central corneal stroma is the stiffest region of the cornea. Moreover, according to Kohlhaas et al. [41], the CXL treatment significantly stiffens the cornea only in the anterior 200 μm. The depth-dependent stiffening effect can be explained by the absorption behavior for UV-A on a riboflavin-soaked cornea, where about 70% of UVA irradiation is absorbed within the anterior 200 μm and only 20% in the next 200 μm [42]. This aspect may affect long-term ectasia stabilization according to different crosslinking penetration rate [43]. While maintaining a standard 5.4 J/cm2 dose delivery, it was demonstrated by Mazzotta et al. in the "M nomogram" for the standardized treatment of all thickness ectatic corneas including the thin and ultrathin KC maintaining the standardized fluence of the original Dresden protocol (5.4J/cm2) in all cases, that UV-A power can be calibrated between 9 and 15 mW/cm2 with continuous or pulsed-light mode of exposure [44]. Indeed, the stress-strain testing data from an experimental study performed by Krueger et al. [29] demonstrated a substantial biomechanical equivalence between 3 mW/5.4 J/cm2 CXL for 30 min of continuous UVA light exposure, 9 mW/5.4 J/cm2 ACXL for 10 min of continuous UVA light exposure and 15 mW/5.4 J/cm2 ACXL with continuous or pulsed-light exposure [25].
Despite laboratory data from Richoz et al. [32] showing lower stiffening effect due to oxygen availability in ACXL procedures, the 9 mW/5.4 J/cm2 was estimated to have the best oxygen diffusion profile in the ACXL procedures panorama. Several clinical studies also demonstrated that the 9 mW/5.4/cm2 Epi-Off ACXL obtained comparable visual outcomes and KC stabilization with conventional 3 mW/5.4 J/cm2 CXL (Dresden protocol). Moreover, the AS OCT and in vivo confocal microscopy (IVCM) studies by Mazzotta et al. [45] showed that the mean depth of the demarcation line after the 9 mW/5.4 J/cm2 ACXL was at 332 ± 20 μm, the nearest to the conventional 3 mW/5.4 J/cm2 CXL Dresden protocol 320 μm (range 270–350 μm) [1, 4].
The 9 mW/5.4 J/cm2 ACXL for 10 min of continuous light UVA exposure was first demonstrated to be effective in stabilizing topographic parameters after 12-months of follow-up in mild-moderate KC affected corneas by Elbaz et al [17]. An improvement in the UDVA and stabilization of all tested corneal parameters were noted after the treatment. Moreover, the 9 mW/5.4 J/cm2 ACXL was safe for corneal endothelium, stabilizing the progression of iatrogenic ectasia with a significant reduction in topographic keratometric values and a significant increase in CDVA, comparable with conventional 3 mW/cm2 CXL in a mid-term (two-years) follow-up as recently documented by Turhan et al [37]. A recent study performed by Sadoughi et al. [33] comparing the conventional 3 mW/5.4 J/cm2 CXL to 9 mW/5.4 J/cm2 ACXL in patients with bilateral progressive KC where the fellow eyes were randomly assigned to conventional 3 mW/5.4 J/cm2 CXL or to 9 mW/5.4 J/cm2 ACXL, revealed similar refractive, visual, keratometric and aberrometric outcomes after 12 months of follow-up. The study confirmed that the one and two-years functional outcomes of the 9 mW/5.4 J/cm2 ACXL protocol were similar and comparable with the conventional 3 mW/5.4 J/cm2 CXL.
In a comparative study of 29 eyes treated with 9 mW/cm2 for 10 min, Kirgiz A et al. [35] reported that ACXL using 10 min of UVA irradiance at 9 mW/cm2 showed better topographic and coma values improvements vs. 5 min of UVA at 18 mW/cm2 irradiance, independent of keratoconus severity. Furthermore, Lang et al. [34] in a comparative study using standard CXL protocol and accelerated protocols in patients with progressive keratoconus reported that Epi-Off 3 mW/5.4 J/cm2 CXL for 30 min, 5.4 J/cm2 and 9 mW/5.4 J/cm2 ACXL for 10 min showed similar improvements in Kmax and CDVA.
Kobashi et al. [36] in a recent meta-analysis compared the clinical results of ACXL and standard corneal collagen cross-linking (SCXL) in progressive keratoconus of randomized controlled trials, which showed a comparable efficacy and safety profile at the 1-year follow-up between the two procedures.
Besides performing stress-strain experiments, CXL efficacy has also been shown to drop from SCXL to short ACXL protocols using inflation tests or air-puff tonometry. SCXL and ACXL have shown similar results [46, 47].
Although 9 mW/5.4 J/cm2 ACXL for 10 min provides less biomechanical increase under laboratory conditions [32], it is not known exactly how much stiffening each keratoconic cornea would need for the progressive character of the disease to be stabilized.
Our study data suggest that 9 mW/cm2 for 10 min may be sufficient to efficiently prevent progression. Nevertheless, we are currently evaluating in the laboratory whether a high-fluence ACXL Epi-Off setting using 9 mW/7.2 J/cm2 for 13 min and 20 s would further increase the biomechanical stability. If this were the case, then an increased fluence might provide an additional level of efficacy to the treatment.
Conclusion
The 5-years results of the 9 mW/cm2 Epi-Off ACXL with 5.4 J/cm2 energy, evaluated in the Siena Eye-Cross Study 2, demonstrated statistically significant improvements of UCVA, CDVA, corneal curvature and corneal higher-order aberrations, and thus confers a long-term stability for progressive KC. In our series, bilateral treatments were performed in 56.4% of patients and the second eye was operated after a minimum time interval of 30 days to a maximum of 60 days (mean 40 days). If keratoconus progression is documented in both eyes, the treatment of the second eye should be performed in a timely fashion, especially in pediatric patients to avoid further risk of progression and higher economic burden [48]. The 9 mW/5.4 J/cm2 for 10 min ACXL protocol – supported by photochemistry, microstructural and long-term clinical data – became our Epi-Off CXL treatment of choice, which reduces CXL treatment time from 1 h to 20 min allowing better patient comfort while maintaining overall CXL safety and efficacy. According to the Siena Eye-Cross Study 2, this accelerated crosslinking protocol is a candidate to be the natural evolution of the original Dresden Epi-Off CXL treatment for the management of early progressive corneal ectasia, thus optimizing clinic workflow, and patient compliance being really efficiacious, less time-consuming and more cost-effective.
Availability of data and materials
Not applicable.
References
Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135(5):620–7.
Caporossi A, Baiocchi S, Mazzotta C, Traversi C, Caporossi T. Parasurgical therapy for keratoconus by riboflavin-ultraviolet type A-induced cross-linking of corneal collagen: preliminary refractive results in an Italian study. J Cataract Refract Surg. 2006;32(5):837–45.
Godefrooij DA, Mangen MJ, Chan E, O'Brart DPS, Imhof SM, de Wit GA, et al. Cost-effectiveness analysis of corneal collagen crosslinking for progressive keratoconus. Ophthalmology. 2017;124(10):1485–95.
Caporossi A, Mazzotta C, Baiocchi S, Caporossi T. Long-term results of riboflavin ultraviolet a corneal collagen cross-linking for keratoconus in Italy: the Siena eye cross study. Am J Ophthalmol. 2010;149(4):585–93.
O'Brart DP, Patel P, Lascaratos G, Wagh VK, Tam C, Lee J, et al. Corneal cross-linking to halt the progression of keratoconus and corneal ectasia: seven-year follow-up. Am J Ophthalmol. 2015;160(6):1154–63.
Hafezi F, Kanellopoulos J, Wiltfang R, Seiler T. Corneal collagen crosslinking with riboflavin and ultraviolet a to treat induced keratectasia after laser in situ keratomileusis. J Cataract Refract Surg. 2007;33(12):2035–40.
Richoz O, Mavrakanas N, Pajic B, Hafezi F. Corneal collagen cross-linking for ectasia after lasik and photorefractive keratectomy: long-term results. Ophthalmology. 2013;120(7):1354–9.
Wittig-Silva C, Chan E, Islam FM, Wu T, Whiting M, Snibson GR. A randomized, controlled trial of corneal collagen cross-linking in progressive keratoconus: three-year results. Ophthalmology. 2014;121(4):812–21.
Raiskup F, Theuring A, Pillunat LE, Spoerl E. Corneal collagen crosslinking with riboflavin and ultraviolet-a light in progressive keratoconus: ten-year results. J Cataract Refract Surg. 2015;41(1):41–6.
Mazzotta C, Traversi C, Baiocchi S, Bagaglia S, Caporossi O, Villano A, et al. Corneal collagen cross-linking with riboflavin and ultraviolet A light for pediatric keratoconus: ten-year results. Cornea. 2018;37(5):560–6.
Godefrooij DA, Gans R, Imhof SM, Wisse RP. Nationwide reduction in the number of corneal transplantations for keratoconus following the implementation of cross-linking. Acta Ophthalmol. 2016;94(7):675–8.
Sandvik GF, Thorsrud A, Råen M, Østern AE, Sæthre M, Drolsum L. Does corneal collagen cross-linking reduce the need for keratoplasties in patients with keratoconus? Cornea. 2015;34(9):991–5.
Raiskup F, Spoerl E. Corneal crosslinking with riboflavin and ultraviolet A. I. Principles. Ocul Surf. 2013;11(2):65–74.
Hersh PS, Stulting RD, Muller D, Durrie DS. Rajpal RK; U.S. Crosslinking Study Group. U.S. multicenter clinical trial of corneal collagen crosslinking for treatment of corneal ectasia after refractive surgery. Ophthalmology. 2017;124(10):1475–84.
Spoerl E, Mrochen M, Sliney D, Trokel S, Seiler T. Safety of UVA-riboflavin cross-linking of the cornea. Cornea. 2007;26(4):385–9.
Mazzotta C, Traversi C, Paradiso AL, Latronico ME, Rechichi M. Pulsed light accelerated crosslinking versus continuous light accelerated crosslinking: one-year results. J Ophthalmol. 2014;2014:604731.
Elbaz U, Shen C, Lichtinger A, Zauberman NA, Goldich Y, Chan CC, et al. Accelerated (9-mW/cm2) corneal collagen crosslinking for keratoconus-A 1-year follow-up. Cornea. 2014;33(8):769–73.
Hashemian H, Jabbarvand M, Khodaparast M, Ameli K. Evaluation of corneal changes after conventional versus accelerated corneal cross-linking: a randomized controlled trial. J Refract Surg. 2014;30(12):837–42.
Marino GK, Torricelli AA, Giacomin N, Santhiago MR, Espindola R, Netto MV. Accelerated corneal collagen cross-linking for postoperative LASIK ectasia: two-year outcomes. J Refract Surg. 2015;31(6):380–4.
Shetty R, Pahuja NK, Nuijts RM, Ajani A, Jayadev C, Sharma C, et al. Current protocols of corneal collagen cross-linking: visual, refractive and tomographic outcomes. Am J Ophthalmol. 2015;160(2):243–9.
Chow VW, Chan TC, Yu M, Wong VW, Jhanji V. One year outcomes of conventional and accelerated collagen crosslinking in progressive keratoconus. Sci Rep. 2015;5:14425.
Hashemi H, Miraftab M, Seyedian MA, Hafezi F, Bahrmandy H, Heidarian S, et al. Long-term results of an accelerated corneal cross-linking protocol (18mW/cm2) for the treatment of progressive keratoconus. Am J Ophthalmol. 2015;160(6):1164–70.
Tomita M, Mita M, Huseynova T. Accelerated versus conventional corneal collagen crosslinking. J Cataract Refract Surg. 2014;40(6):1013–20.
Ulusoy DM, Göktaş E, Duru N, Özköse A, Ataş M, Yuvacı İ, et al. Accelerated corneal crosslinking for treatment of progressive keratoconus in pediatric patients. Eur J Ophthalmol. 2017;27(3):319–25.
Mazzotta C, Baiocchi S, Bagaglia SA, Fruschelli M, Meduri A, Rechichi M. Accelerated 15 mW pulsed-light crosslinking to treat progressive keratoconus: two-year clinical results. J Cataract Refract Surg. 2017;43(8):1081–8.
Hagem AM, Thorsrud A, Sandvik GF, Drolsum L. Randomized study of collagen cross-linking with conventional versus accelerated UVA irradiation using riboflavin with hydroxypropyl methylcellulose: two-year results. Cornea. 2019;38(2):203–9.
Brindley GS. The Bunsen-Roscoe law for the human eye at very short durations. J Physiol. 1952;118(1):135–9.
Schumacher S, Oeftiger L, Mrochen M. Equivalence of biomechanical changes induced by rapid and standard corneal cross-linking, using riboflavin and ultraviolet radiation. Invest Ophthalmol Vis Sci. 2011;52(12):9048–52.
Krueger RR, Herekar S, Spoerl E. First proposed efficacy study of high versus standard irradiance and fractionated riboflavin/ultraviolet A cross-linking with equivalent energy exposure. Eye Contact Lens. 2014;40(6):353–7.
Kamaev P, Friedman MD, Sherr E, Muller D. Photochemical kinetics of corneal cross-linking with riboflavin. Invest Ophthalmol Vis Sci. 2012;53(4):2360–7.
Mazzotta C, Traversi C, Caragiuli S, Rechichi M. Pulsed vs continuous light accelerated corneal collagen crosslinking: in vivo qualitative investigation by confocal microscopy and corneal OCT. Eye (Lond). 2014;28(10):1179–83.
Richoz O, Hammer A, Tabibian D, Gatzioufas Z, Hafezi F. The biomechanical effect of corneal collagen cross-linking (CXL) with riboflavin and UV-A is oxygen dependent. Transl Vis Sci Technol. 2013;2(7):6.
Sadoughi MM, Einollahi B, Baradaran-Rafii A, Roshandel D, Hasani H, Nazeri M. Accelerated versus conventional corneal collagen cross-linking in patients with keratoconus: an intrapatient comparative study. Int Ophthalmol. 2018;38(1):67–74.
Lang PZ, Hafezi NL, Khandelwal SS, Torres-Netto EA, Hafezi F, Randleman JB. Comparative functional outcomes after corneal crosslinking using standard, accelerated, and accelerated with higher total fluence protocols. Cornea. 2019;38(4):433–41.
Kirgiz A, Eliacik M, Yildirim Y. Different accelerated corneal collagen cross-linking treatment modalities in progressive keratoconus. Eye Vis (Lond). 2019;6:16.
Kobashi H, Tsubota K. Accelerated versus standard corneal cross-linking for progressive keratoconus: a meta-analysis of randomized controlled trials. Cornea. 2020;39(2):172–80.
Turhan SA, Yargi B, Toker E. Efficacy of conventional versus accelerated corneal cross-linking in pediatric keratoconus: two-year outcomes. J Refract Surg. 2020;36(4):265–9.
Krumeich JH, Daniel J, Knülle A. Live-epikeratophakia for keratoconus. J Cataract Refract Surg. 1998;24(4):456–63.
Mazzotta C, Sgheri A, Bagaglia SA, Rechichi M, Di Maggio A. Customized corneal crosslinking for treatment of progressive keratoconus: clinical and OCT outcomes using a transepithelial approach with supplemental oxygen. J Cataract Refract Surg. 2020;46(12):1582–7.
Mazzotta C, Traversi C, Mellace P, Bagaglia SA, Zuccarini S, Mencucci R, et al. Keratoconus progression in patients with allergy and elevated surface matrix metalloproteinase 9 point-of-care test. Eye Contact Lens. 2018;44(Suppl 2):S48–53.
Kohlhaas M, Spoerl E, Schilde T, Unger G, Wittig C, Pillunat LE. Biomechanical evidence of the distribution of cross-links in corneas treated with riboflavin and ultraviolet A light. J Cataract Refract Surg. 2006;32(2):279–83.
Hafezi F, Kling S, Gilardoni F, Hafezi N, Hillen M, Abrishamchi R, et al. Individualized corneal cross-linking with riboflavin and UV-A in ultrathin corneas: the sub400 protocol. Am J Ophthalmol. 2021;224:133–42.
Mazzotta C, Wollensak G, Raiskup F, Pandolfi AM, Spoerl E. The meaning of the demarcation line after riboflavin-UVA corneal collagen crosslinking. Expert Rev Ophthalmol. 2019;14(2):115–31.
Mazzotta C, Romani A, Burroni A. Pachymetry-based accelerated cross-linking: the “M Nomogram” for standardized treatment of all-thickness progressive ectatic corneas. Int J Keratoconus Ectatic Corneal Dis. 2019;7(2):137–44.
Mazzotta C, Hafezi F, Kymionis G, Caragiuli S, Jacob S, Traversi C, et al. In vivo confocal microscopy after corneal collagen crosslinking. Ocul Surf. 2015;13(4):298–314.
Bao F, Zheng Y, Liu C, Zheng X, Zhao Y, Wang Y, et al. Changes in corneal biomechanical properties with different corneal cross-linking irradiances. J Refract Surg. 2018;34(1):51–8.
Herber R, Francis M, Spoerl E, Pillunat LE, Raiskup F, Sinha Roy A. Comparison of waveform-derived corneal stiffness and stress-strain extensometry-derived corneal stiffness using different cross-linking irradiances: an experimental study with air-puff applanation of ex vivo porcine eyes. Graefes Arch Clin Exp Ophthalmol. 2020;258(10):2173–8.
Pagano L, Gadhvi KA, Borroni D, Iselin KC, Vinciguerra R, Tzamalis A, et al. Bilateral keratoconus progression: immediate versus delayed sequential bilateral corneal cross-linking. J Refract Surg. 2020;36(8):552–6.
Acknowledgements
Not applicable.
Disclosures
None.
Funding
The authors declare that they have no funding and support for the research.
Author information
Authors and Affiliations
Contributions
MC: Study Conception, Design, Execution, Treatments and Data Collection, Analysis and Article Writing; RF: Data Analysis and Interpretation. HF: Data Interpretation, Study Supervision; TNEA: Statistical Expertise and Data Collection; ABA: Statistical Analysis and Data Interpretation; GG: Final Supervision; BSA: Data Collection, Analysis and Article Writing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was unanimously approved by the Institutional Review Board of the Siena Crosslinking Center, Italy and named “Siena Eye-Cross Study 2”.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mazzotta, C., Raiskup, F., Hafezi, F. et al. Long term results of accelerated 9 mW corneal crosslinking for early progressive keratoconus: the Siena Eye-Cross Study 2. Eye and Vis 8, 16 (2021). https://doi.org/10.1186/s40662-021-00240-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40662-021-00240-8