Abstract
Background
CD4+CD25highFOXP3+ regulatory T (Treg) cells, which include thymus-derived and peripherally induced cells, play a central role in immune regulation, and are therefore crucial to prevent graft-versus-host disease (GVHD). The increasing use of allogeneic hematopoietic stem cell transplantation (allo-HSCT) for elderly patients with thymus regression, and our case of allo-HSCT shortly after total thymectomy, raised questions about the activity of thymus-derived Treg cells and peripherally induced Treg cells, which are otherwise indistinguishable.
Results
We found that despite pre-transplant thymectomy or older age, both naïve and effector Treg cells, as well as naïve and effector conventional T cells, proliferated in allo-HSCT recipients. Higher proportions of total Treg cells 1 month post allo-HSCT, and naïve Treg cells 1 year post allo-HSCT, appeared in patients achieving complete chimera without developing significant chronic GVHD, including our thymectomized patient, compared with patients who developed chronic GVHD.
Conclusions
Treg cells that modulate human allogeneic immunity may arise peripherally as well as in the thymus of allo-HSCT recipients.
Similar content being viewed by others
Background
Allogeneic hematopoietic stem cell transplantation (allo-HSCT), including bone marrow (BM) transplantation (BMT), peripheral blood (PB) stem cell transplantation (PBSCT), and cord blood transplantation, is widely used for the treatment of malignant and nonmalignant hematologic diseases [1]. However, allo-HSCT often shows comorbidity or mortality due to graft-versus-host disease (GVHD), opportunistic infections, regimen-related toxicity and relapse of the original hematologic disease. Donor immune cells provide graft-versus-leukemia effects and protect against opportunistic infections. However, in GVHD, donor immune cells respond inappropriately to host antigens in a pro-inflammatory background of tissue damage and cytokine dysregulation [2].
Patients with acute GVHD (aGVHD) and chronic GVHD (cGVHD) show deficits of CD4+CD25high regulatory T (Treg) cells, which express the transcription factor, fork-head box P3 (FOXP3), and play a crucial role in control of autoimmunity [2, 3]. Treg cells usually derive from the thymus with upregulation of FOXP3. Thymus also provides naïve conventional T (Tcon) cells, which are considered to be crucial to maintain proper immune responses [4]. Therefore, therapeutic strategies that facilitate recovery of thymopoiesis have been attempted [5]. In addition, there is a second subset of Treg cells, periphery-derived induced Treg cells (iTreg cells), which can be transformed from Tcon cells through upregulation of FOXP3 in the context of TGF-β and IL-2 [6–8]. It has also been reported that alloantigen can induce Treg cells via a thymus-independent pathway in mice [9]. However, the role of iTreg cells in allo-HSCT is unknown because there is no known marker to discriminate iTreg cells from thymus-derived Treg cells.
After the introduction of reduced-intensity conditioning regimens, allo-HSCT increased for elderly patients, for whom the thymus is naturally regressed. In fact, delayed recovery of thymopoiesis, which can be inferred by T cell receptor rearrangement excision circle levels and naïve T cells, has been reported among elderly allo-HSCT recipients [4]. Other investigators showed a more rapid reconstitution of effector T cells in allo-HSCT recipients older than 42 years compared with younger recipients [10]. In addition, even in patients with delayed recovery of thymopoiesis, clinical consequences of allo-HSCT vary. Moreover, we recently encountered a case in which allo-HSCT for acute lymphoblastic leukemia shortly after thymectomy did not provoke significant GVHD. These facts prompted us to investigate the role of thymopoiesis after allo-HSCT, especially in relation to Treg cells. Hence, in this study, we investigated Treg and Tcon cells, including naïve and effector in allo-HSCT recipients in the context of patient age and cGVHD.
Results
Allo-HSCT in young and old patients
We investigated 22 patients (Table 1), comparing clinical findings and T cell populations between 14 young (<50 years) and 8 old (≥50 years) recipients. Old recipients more frequently received reduced-intensity conditionings compared with young recipients [6/8 (75%) vs. 2/14 (15%), P = 0.038]. There was a trend that PBSCT was more common among old recipients (BMT vs. PBSCT, 3 vs. 5) compared with young recipients (11 vs. 3) (P = 0.054). The thymectomized patient received PBSCT from an HLA allele fully matched related donor (30 year-old daughter) after reduced-intensity conditioning for acute lymphoblastic leukemia at complete remission. Her leukemia remains in complete remission at the time of evaluation, with 100% donor-derived cells in PB mononuclear cells (MNCs) and CD3+ cells, and BM MNCs. PB MNCs of all other patients were also in complete chimera (>90% cells were donor-derived).
CD4+ conventional and regulatory T cells in young and old allo-HSCT recipients early after transplantation
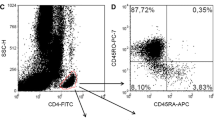
At day 30 after allo-HSCT, we performed 3-color flow cytometry, in which CD4+CD25highFoxp3+ lymphocytes and all other CD4+Foxp3− lymphocytes were defined as Treg cells and Tcon cells, respectively (Fig. 1a) [11]. Proportions of Tcon cells, rather than Treg cells, were significantly greater in young recipients compared with old recipients 1 month after allo-HSCT (Fig. 1b). Proportions of Treg cells were not correlated with ages of either recipients or donors (Fig. 1c), whereas there was a trend (P = 0.073) that proportions of Tcon cells were negatively correlated with recipient’s age, but not donor’s (Fig. 1d). On the other hand, we found that proportions of Treg cells were already lower at 1 month after allo-HSCT in patients who eventually developed clinically significant cGVHD compared with those without (Fig. 2). There were no significant differences in proportions of Treg cells (0.24 ± 0.20 vs. 0.36 ± 0.42%) and Tcon cells (38 ± 19 vs. 23 ± 13%) between BMT recipients and PBSCT recipients. At this point, there were not any differences in the absolute lymphocyte counts between young recipients and old recipients (0.59 ± 0.47 vs. 0.56 ± 0.26 × 109/L), and both Treg cells and Tcon cells were already detectable in the thymectomized patient (Fig. 1a), who also had already achieved complete chimera with 100% donor-derived PB MNCs and BM MNCs at this point.
CD4+ regulatory T (Treg) and conventional T (Tcon) cells at 1 month after allo-HSCT. a Flow cytometric analysis of the patient who had undergone thymectomy prior to PBSCT. Both CD4+CD25high Treg cells and CD4+ Tcon cells were among the CD4+ cells detected by flow cytometry of peripheral blood mononuclear cells at 1 month after PBSCT. Treg cells expressed Foxp3 more abundantly than Tcon cells. b Comparisons in the proportions of CD4+CD25highFoxp3+ Treg cells and CD4+Foxp3− Tcon cells between young and old allo-HSCT recipients. Correlations between proportions of Treg cells (c) or Tcon cells (d) and ages of recipients or donors. The close circles indicate data of the thymectomized patient.
Naïve and effector T cells in allo-HSCT recipients 1 year after transplantation
We studied proportions of naïve and effector fractions of Treg cells and Tcon cells (Fig. 3) [12], in young and old recipients at approximately 1 year after allo-HSCT. At this point, both in Treg cells and Tcon cells, CD45RA+ naïve cells remained at significantly low proportions in allo-HSCT recipients, regardless of age (Fig. 4). However, these naïve cells, as well as CD45RA− effector cells, were certainly detectable in all of these patients examined, even in the thymectomized patient (Fig. 3c), whose complete chimera still persisted with 100% donor-derived PB MNCs and CD3+ lymphocytes, and BM MNCs at this point. Proportions of both naïve Treg cells and Tcon cells were not different between young and old recipients. We also compared proportions of Treg cells and Tcon cells with respect to cGVHD. In patients with clinically significant cGVHD, we found significantly lower proportions of Treg cells, especially in the naïve fraction (0.015 ± 0.011 vs. 0.049 ± 0.022%, P = 0.024), compared with those without. In contrast, proportions of effector Tcon cells were significantly greater in patients with clinically significant cGVHD compared with other patients (34.0 ± 6.9 vs. 20.2 ± 4.2%, P = 0.022).
Detection of naïve and effector CD4+ cells in peripheral blood mononuclear cells. According to Ref. [14], naïve Tcon cells (fraction I), effector Tcon cells (II), naïve Treg cells (III), and effector Treg cells (IV) were CD4+ by flow cytometry. CD25 expression in each subpopulation is shown in the histogram. Representative data from a healthy donor, a patient with chronic GVHD (cGVHD), and our thymectomized patient are shown.
Comparisons in the proportions of naïve and effector Treg cells and Tcon cells between allo-HSCT recipients and healthy controls. Proportions of indicated cells in allo-HSCT recipients (R), determined by flow cytometry as shown in Fig. 3, were compared with age-matched controls (C). The close circles indicate data of the thymectomized patient.
Discussion
After allo-HSCT, the T-cell compartment is slowly reconstituted with both thymus-independent and -dependent pathways [4]. Early after transplantation, the thymus-independent pathway by either adoptively transferred donor-derived T cells or recipient-derived T cells that survive conditioning treatment predominates. The transferred T cells expand in response to early post-transplant circumstances with lymphopenia and high cytokine levels, and oligoclonal proliferation associated with cognate antigens. Another pathway, which is a more prolonged process of reconstitution of functional T cells with sufficient and broad antigenic specificity, depends on the de novo production of naïve T cells by the thymus. Thus, thymic regeneration may be crucial to supply new Tcon cells and Treg cells that contribute to prevention of relapsing hematologic malignancies, opportunistic infections, and cGVHD [5, 13]. We found a lower frequency of Tcon cells rather than Treg cells early after allo-HSCT in the elderly recipients (Fig. 1). Our present study, however, revealed that naïve and effector Treg cells, as well as naïve and effector Tcon cells, exist even in allo-HSCT recipients more than 50 years old, including our surgically athymic patient, at 1 year after allo-HSCT (Fig. 3). The detailed kinetics of Treg cells is unclear in allo-HSCT recipients, but proportions of naïve Treg cells and Tcon cells were lower in recipients compared with healthy controls, independent of recipient or donor age, at both 1 month and 1 year in the present study. Next, we observed lower frequencies of Treg cells at both 1 month and 1 year after allo-HSCT in patients who eventually developed clinically significant cGVHD, consistent with previous studies [14, 15]. Imanguli et al. [16] recently compared proportions of naïve and effector Treg cells between patients with cGVHD and normal controls with the same method used in our present study [12]. They found significantly lower frequency of naïve Treg cells, but not effector Treg cells, in allo-HSCT recipients compared with controls. In our present study, regardless of the presence of cGVHD, allo-HSCT recipients consistently showed decreased naïve Tregs. Nevertheless, we found that proportions of Treg cells, especially in the naïve fraction, were significantly lower in patients with cGVHD compared with patients without cGVHD, despite a small cohort with heterogeneities including donor sources and conditioning protocols in the present study. Another concern of this study is that the thymectomized patient belongs to the older group. These facts limit the confidence that can be placed in our findings. However, it has been recently reported that impaired reconstitution of naïve Treg cells was also associated with aGVHD [17] as well as cGVHD, lending support to the importance of naïve Tregs in the modulation of allogeneic immune phenomena that cause GVHD.
In patients who were coincidentally thymectomized through cardiac surgery, atopic and autoimmune-like symptoms with elevated anti-dsDNA antibodies were frequently found [18]. Such autoimmune-like diseases were associated not with total numbers of Treg cells, but Treg homeostasis preserved by naïve Treg cells in these post-surgery individuals with impaired thymopoiesis. In an established mouse model, female B6AF1 mice thymectomized on day 3 develop autoimmune ovarian disease and dacryoadenitis [19, 20]. These mice possess disease-relevant Treg cells of neonatal origin, which accumulate in the regional lymphoid tissues and inhibit autoimmune disease [20]. Although they cannot fully prevent development of autoimmune diseases, the autoimmune diseases are dramatically enhanced by in vivo depletion of Treg cells in these mice. On the other hand, the role of thymic ablation in allogeneic immunity largely unknown, although we could speculate about the poor clinical consequences of allo-HSCT recipients with delayed thymopoiesis in recipients with reduced pre-transplant thymic function and in patients suffering from acute or chronic GVHD [21–24]. There has been only a single case report of thymectomy followed by allo-HSCT for a patient with systemic lupus erythematosus, thymoma, and pure red cell aplasia [25]. Although this patient achieved clinical remission of red cell aplasia and lupus, autoantibodies did not disappear after allo-HSCT. It has also been reported that a murine model showed an extrathymic de novo generation of donor-derived T cells against murine cytomegalovirus, although these cells were numerically and functionally reduced compared with thymus-derived T cells [26, 27]. These facts and the existence of naïve Treg cells and Tcon cells in our present study may together suggest that some T cells derived independent of the thymus contribute to immune reconstitution after allo-HSCT. In fact, our thymectomized patient, who had a 100% donor-derived CD3+ cell population including Treg cells, has not required systemic steroids for cGVHD without relapse of leukemia.
There are two types of Treg cells, thymus-derived natural Treg cells, and peripherally generated adaptive Treg cells (iTreg cells) [6–8]. Tcon cells can be converted to Foxp3+ iTreg cells, under the influence of IL-2, but not thymus. In addition, alloantigens can induce Tregs via a thymus-independent pathway in mice [9]. Successful allo-HSCT in our thymectomized patient offers strong evidence that peripherally derived adoptive Treg cells can proliferate, a phenomenon also supported by observation of Treg cells in elderly allo-HSCT recipients in the present study and another previously reported thymectomized patient [25]. This might also partly explain the efficacy of low-dose IL-2 administration [28], and support adoptive Treg cell infusion [29], although the functional difference of Treg cells among young, old and/or thymectomized patients remain unclear. So far, there have been not any specific markers to distinguish natural Treg cells and iTreg cells, but naïve Treg cells observed in our thymectomized patient were considered to include iTreg cells. Indeed, it has been reported that Foxp3+ iTreg cells showed the characteristics of T-cell receptor repertoire of naïve Tcon cells [6]. The role of iTreg cells, in addition to strategies aiming thymic restoration [5, 13, 30], in allo-HSCT should be further investigated.
Conclusions
Even with pre-transplant thymectomy or older age, both naïve and effector Treg cells certainly proliferated in allo-HSCT recipients. This finding suggests that Treg cells, as well as Tcon cells, can arise peripherally to modulate allogeneic immune responses in humans.
Methods
Patients and controls
Twenty-two patients who underwent allo-HSCT from 2008 through 2014, and survived without relapse of hematologic diseases at least 300 days after transplant, were included in this study. In 10 of 22 patients, partial results were previously reported [11]. Median age was 38.5 years with 8 “old recipients” 50 years or older. We compared these old recipients with young recipients (<50 years) to estimate the contribution of age-related thymic involution to the reconstitution of T cells in allo-HSCT. One patient, a 59-year-old female with acute lymphoblastic leukemia, had received total thymectomy to treat invasive thymoma 5 months prior to allo-HSCT.
Patients received tacrolimus and methotrexate as prophylaxis for GVHD, except three who received cyclosporine and methotrexate. All patients received standard supportive treatments including use of laminar airflow units meeting allo-HSCT standards during myelosuppression, prophylaxis of viral infection with acyclovir, administration of granulocyte colony-stimulating factor, and blood transfusion, for which thresholds of 8 g/dL hemoglobin and 20 × 109/L platelets were generally used for packed irradiated red cells and irradiated apheresis platelet concentrates, respectively. Incidences of cGVHD [31] were first diagnosed by standard criteria. Among patients with any cGVHD, we deemed clinically significant cGVHD as that requiring systemic steroid therapy with no less than 30 mg/day of prednisolone, as described elsewhere [14].
This study was approved by Ethics Review Board of Fukushima Medical University, which is guided by local policy, national law, and the Declaration of Helsinki. All investigations were performed after properly documented informed consent. Information and records of patients were anonymized and de-identified prior to analysis.
Samples
Peripheral blood samples were collected for flow cytometry of Treg cells and Tcon cells at 1 month and/or 1 year after allo-HSCT as described previously [11]. In later evaluation at 1 year after allo-HSCT, we also evaluated naïve and effector cell fractions of each cell type as recently described by Sugiyama et al. [12]. Samples obtained from six age-matched healthy volunteers were used as controls.
Cell separation, flow cytometry, and chimerism analysis
Peripheral blood mononuclear cells were isolated using Ficoll-Hypaque (Lymphoprep; Axis-shield, Oslo, Norway) and suspended in phosphate-buffered saline containing 0.5% bovine serum albumin (Wako, Osaka, Japan). Anti-CD4 (OKT4; BioLegend, San Diego, CA, USA), anti-CD25 (B1.49.9; Beckman Coulter, Brea, CA, USA), anti-CD45RA (HI100; ALPCO Diagnostics, Salem, NH, USA) and/or anti-FOXP3 (PCH101; eBioscience, San Diego, CA, USA) were used to label PB MNCs after treatment with fixation Permeabilization Buffer (eBioscience). Non-specific control antibodies of the same subclasses were used as negative controls. These PB MNCs were counted by flow cytometry using a FACSCanto II (BD Biosciences), and the data were analyzed with Flow Jo software (FLOW JO LLC, Ashland, OR, USA). For chimerism analysis of CD3+ cells from the thymectomized patient, cells were sorted using EazySep T cell Enrichment Kit (StemCell Technologies, Vancouver, BC, Canada) according to the manufacturer’s protocol. The purity of CD3+ cells, evaluated by flow cytometry, was over 99% in each sample.
Chimerism analysis
Chimerism of PB MNCs, sorted CD3+ cells, and BM MNCs was determined by short tandem repeat polymerase chain reaction [32] for sex-matched transplants, or fluorescence in situ hybridization for sex chromosomes for sex-mismatched transplants.
Statistical analysis
To compare categorical variables, the Chi square test was used. The comparison of continuous variables between two groups was made using the unpaired Student’s t-test. Correlation coefficient was calculated to determine the correlation between two parameters. All data are shown as mean ± standard deviation unless otherwise specified. All P values are two-sided, with P < 0.05 considered significant.
Abbreviations
- Allo-HSCT:
-
allogeneic hematopoietic stem cell transplantation
- BM:
-
bone marrow
- BMT:
-
BM transplantation
- PB:
-
peripheral blood
- PBSCT:
-
PB stem cell transplantation
- GVHD:
-
graft-versus-host disease
- aGVHD:
-
acute GVHD
- cGVHD:
-
chronic GVHD
- Treg cells:
-
regulatory T cells
- FOXP3:
-
fork-head box P3
- Tcon cells:
-
conventional T cells
- iTreg cells:
-
induced Treg cells
- MNCs:
-
mononuclear cells
References
Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–26.
Beres AJ, Drobyski WR. The role of regulatory T cells in the biology of graft versus host disease. Front Immunol. 2013;4:1–9.
Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127:759–67.
Politikos I, Boussiotis VA. The role of the thymus in T-cell immune reconstitution after umbilical cord blood transplantation. Blood. 2014;124:3201–11.
Velardi E, Dudakov JA, van den Brink MR. Clinical strategies to enhance thymic recovery after allogeneic hematopoietic stem cell transplantation. Immunol Lett. 2013;155:31–5.
Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive Foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–35.
Zhang X, Chang Li X, Xiao X, Sun R, Tian Z, Wei H. CD4+CD62L+ central memory T cells can be converted to Foxp3+ T Cells. PLoS One. 2013;8:1–12.
Adeegbe DO, Nishikawa H. Natural and induced T regulatory cells in cancer. Front Immunol. 2013;4:1–14.
Karim M, Kingsley CI, Bushell AR, Sawitzki BS, Wood KJ. Alloantigen-induced CD25+CD4+ regulatory T cells can develop in vivo from CD25−CD4+ precursors in a thymus-independent process. J Immunol. 2004;172:923–8.
Heining C, Spyridonidis A, Bernhardt E, Schulte-Mönting J, Behringer D, Grüllich C, et al. Lymphocyte reconstitution following allogeneic hematopoietic stem cell transplantation: a retrospective study including 148 patients. Bone Marrow Transpl. 2007;39:613–22.
Ngoma AM, Ikeda K, Hashimoto Y, Mochizuki K, Takahashi H, Sano H, et al. Impaired regulatory T cell reconstitution in patients with acute graft-versus-host disease and cytomegalovirus infection after allogeneic bone marrow transplantation. Int J Hematol. 2012;95:86–94.
Sugiyama D, Nishikawa H, Maeda Y, Nishioka M, Tanemura A, Katayama I, et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci USA. 2013;110:17945–50.
Velardi E, Tsai JJ, Holland AM, Singer NV, West ML, Smith OM, et al. Sex steroid blockade enhances thymopoiesis by modulating notch signaling. Blood. 2013;122:2341–9.
Matsuoka K, Kim HT, McDonough S, Bascug G, Warshauer B, Koreth J, et al. Altered regulatory T cell homeostasis in patients with CD4+ lymphopenia following allogeneic hematopoietic stem cell transplantation. J Clin Invest. 2010;120:1479–93.
Zorn E, Kim HT, Lee SJ, Floyd BH, Litsa D, Arumugarajah S, et al. Reduced frequency of FOXP3+CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood. 2005;106:2903–11.
Imanguli MM, Cowen EW, Rose J, Dhamala S, Swaim W, Lafond S, et al. Comparative analysis of FoxP3+ regulatory T cells in the target tissues and blood in chronic graft versus host disease. Leukemia. 2014;28:2016–27.
Dong S, Maiella S, Xhaard A, Pang Y, Wenandy L, Larghero J, et al. Multi-parameter single-cell profiling of human CD4+FOXP3+ regulatory T cell populations in homeostatic conditions and during graft-versus-host disease. Blood. 2013;122:1802–12.
Halnon NJ, Cooper P, Chen DYH, Boechat MI, Uittenbogaart CH. Immune dysregulation after cardiothoracic surgery and incidental thymectomy: Maintenance of regulatory T cells despite impaired thymopoiesis. Clin Dev Immunol. 2011;2011:915864.
Nishizuka Y, Sakakura T. Thymus and reproduction: sex-linked dysgenesia of the gonad after neonatal thymectomy in mice. Science. 1969;166:753–5.
Samy ET, Wheeler KM, Roper RJ, Teuscher C, Tung KSK. Autoimmune disease in day 3 thymectomized mice is actively controlled by endogenous disease-specific regulatory T cells. J Immunol. 2008;180:4366–70.
Douek DC, Vescio RA, Betts MR, Brenchley JM, Hill BJ, Zhang L, et al. Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstitution. Lancet. 2000;355:1875–81.
Weinberg K, Blazar BR, Wagner JE, Agura E, Hill BJ, Smogorzewska M, et al. Factors affecting thymic function after allogeneic hematopoietic stem cell transplantation. Blood. 2001;97:1458–66.
Wils E-J, van der Holt B, Broers AEC, Posthumus-van Sluijs SJ, Gratama J-W, Braakman E, et al. Insufficient recovery of thymopoiesis predicts for opportunistic infections in allogeneic hematopoietic stem cell transplant recipients. Haematologica. 2011;96:1846–54.
Clave E, Busson M, Douay C, De Latour RP, Berrou J, Carmagnat M, et al. Acute graft-versus-host disease transiently impairs thymic output in young patients after allogeneic hematopoietic stem cell transplantation. Blood. 2009;113:6477–84.
Marmont AM, Bacigalupo A, Gualandi F, Bregante S, van Lint MT, Geroldi S. Systemic lupus erythematosus complicated with thymoma and pure red cell aplasia (PCRA). CR of both complications following thymectomy and allogeneic haematopoietic SCT (HSCT), but persistence of antinuclear antibodies (ANA). Bone Marrow Transpl. 2014;49:982–3.
Arber C, BitMansour A, Sparer TE, Higgins JP, Mocarski ES, Weissman IL, et al. Common lymphoid progenitors rapidly engraft and protect against lethal murine cytomegalovirus infection after hematopoietic stem cell transplantation. Blood. 2003;102:421–8.
Nguyen VH, Shashidhar S, Chang DS, Ho L, Kambham N, Bachmann M, et al. The impact of regulatory T cells on T-cell immunity following hematopoietic cell transplantation. Blood. 2008;111:945–53.
Koreth J, Matsuoka K, Kim HT, McDonough SM, Bindra B, Alyea EP III, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011;365:2055–66.
Veerapathran A, Pidala J, Beato F, Yu X-Z, Anasetti C. Ex vivo expansion of human Tregs specific for alloantigens presented directly or indirectly. Blood. 2011;118:5671–80.
Dudakov JA, Hanash AM, Jenq RR, Young LF, Ghosh A, Singer NV, et al. Interleukin-22 drives endogenous thymic regeneration in mice. Science. 2012;336:91–5.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transpl. 2005;11:945–56.
Nollet F, Billiet J, Selleslag D, Criel A. Standardisation of multiplex fluorescent short tandem repeat analysis for chimerism testing. Bone Marrow Transpl. 2001;28:511–8.
Author’s contributions
HT, SS, AMN, YM, and KU performed experiments, analyzed the data, and wrote the manuscript. KI organized the research, performed experiments, interpreted the data, and wrote the manuscript. MF, ASN, H.O. saw patients and wrote the manuscript. KO, KEN, HO, and YT organized the research and wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors are grateful to MTs Kinuyo Kawabata, Masami Kikuchi, Satoshi Ono, Keiji Minakawa, and Takako Ono for their skillful assays.
Compliance with ethical guidelines
Competing interests The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Takahashi, H., Ikeda, K., Ogawa, K. et al. CD4+ T cells in aged or thymectomized recipients of allogeneic stem cell transplantations. Biol Res 48, 41 (2015). https://doi.org/10.1186/s40659-015-0033-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40659-015-0033-8