Abstract
Background
Actinium-225 is an alpha-particle emitter under investigation for use in radiopharmaceutical therapy. To address limited supply, accelerator-produced 225Ac has been recently made available. Accelerator-produced 225Ac via 232Th irradiation (denoted 225/7Ac) contains a low percentage (0.1–0.3%) of 227Ac (21.77-year half-life) activity at end of bombardment. Using pharmacokinetic modeling, we have examined the dosimetric impact of 227Ac on the use of accelerator-produced 225Ac for radiopharmaceutical therapy. We examine the contribution of 227Ac and its daughters to tissue absorbed doses. The dosimetric analysis was performed for antibody-conjugated 225/7Ac administered intravenously to treat patients with hematological cancers. Published pharmacokinetic models are used to obtain the distribution of 225/7Ac-labeled antibody and also the distribution of either free or antibody-conjugated 227Th.
Results
Based on our modeling, the tissue specific absorbed dose from 227Ac would be negligible in the context of therapy, less than 0.02 mGy/MBq for the top 6 highest absorbed tissues and less than 0.007 mGy/MBq for all other tissues. Compared to that from 225Ac, the absorbed dose from 227Ac makes up a very small component (less than 0.04%) of the total absorbed dose delivered to the 6 highest dose tissues: red marrow, spleen, endosteal cells, liver, lungs and kidneys when accelerator produced 225/7Ac-conjugated anti-CD33 antibody is used to treat leukemia patients. For all tissues, the dominant contributor to the absorbed dose arising from the 227Ac is 227Th, the first daughter of 227Ac which has the potential to deliver absorbed dose both while it is antibody-bound and while it is free. CONCLUSIONS: These results suggest that the absorbed dose arising from 227Ac to normal organs would be negligible for an 225/7Ac-labeled antibody that targets hematological cancer.
Similar content being viewed by others
Introduction
Alpha-particle emitter radiopharmaceutical therapy (αRPT) is a promising new approach to cancer therapy. It has been found impervious to conventional resistance mechanism that make traditional therapy ineffective [1]. Encouraging results have been observed in early clinical studies utilizing 225Ac to deliver alpha-particles for both hematologic and solid tumor treatment, including programs targeting CD33 in acute myeloid leukemia and PSMA in castrate-resistant prostate cancer [2, 3]. However, current limitation of available 225Ac supply, due to the fixed output from 229Th generator, has been a concern that has impacted preclinical and clinical use of 225Ac-based αRPT [4]. Accordingly, a number of alternative production methods have been examined as potential sources of large and sustainable quantities of 225Ac [5,6,7]. Accelerator-produced 225Ac via 232Th irradiation (hereafter denoted as 225/7Ac) contains 0.1 to 0.3% 227Ac (21.77-year half-life) activity at end of bombardment [8]. To account for the time elapsed for processing, transport and injectate preparation, we consider a scenario where the injected, 225/7Ac radiolabeled conjugate contains 0.7% 227Ac.
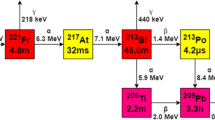
Actinium-227 decays by beta-particle emission primarily (99%) to thorium-227 (227Th; 18.68-d half-life) which in turn decays to radium-223 (223Ra, 11.43-d half-life) and a series of other alpha- and beta-emitting daughters to stable lead-207 (207Pb) (Fig. 1).
Actinium-227 decay scheme [28]
Using pharmacokinetic modeling, this work examines the contribution of 227Ac and its daughters to tissue absorbed doses when 225/7Ac-labeled antibody is administered intravenously to treat patients with hematological cancers (e.g., acute myeloid leukemia (AML) and/or myelodysplastic syndrome (MDS)).
Methods
Overview
Published pharmacokinetic models are used to obtain the distribution of 225/7Ac-labeled antibody and also the distribution of either free or antibody-conjugated 227Th. Since 227Th is obtained from the beta decay branch (99% yield) of 227Ac rather than a more energetically disruptive alpha-emitter decay, as has been observed with the 212Pb/212Bi delivery for α-emitter radiopharmaceutical therapy, [9,10,11,12], it is likely that a significant fraction of the 227Th generated remains antibody-conjugated. A pharmacokinetic model representing the distribution of radiolabeled antibody in patients with hematologically distributed cancer is adapted from reference [13] to obtain the pharmacokinetics for 225/7Ac and 227Th-labeled antibody. A model representing the pharmacokinetics of free 227Th is used to model the distribution of unconjugated 227Th [14]. Under both circumstances, 223Ra generated by 227Th decay is simulated using a pharmacokinetic model that is relevant to free 223Ra [15]. The 1% of 227Ac that decays to francium-223 (223Fr, T ½ = 22 min) is considered to have a negligible impact on tissue absorbed dose relative to that from 227Th which is already expected to be very low because of the low initial amount of 227Ac in 225/7Ac. Calculations were performed assuming 1 kg (1012 antigen-positive cells) in an adult female. The individual model simulations (i.e., Ab model, and the free 227Th and 223Ra models) are not coupled to each other. Rather, the biodistribution of free 227Th or 223Ra generated in the course of the simulation is assumed distributed throughout the body as it is created according to the kinetics described by the corresponding model (see Eqs. 17 and 18 of the “Appendix”).
Biokinetic modeling
Table 1 summarizes the various models that were used to simulate the pharmacokinetics (PK) of each radionuclide. All compartmental models were solved using the simulation analysis and modeling software package (SAAM II, The Epsilon Group, Charlottesville, VA). Detailed model equations are listed in the “Appendix”.
Antibody pharmacokinetic modeling
The radiolabeled antibody model is depicted in Fig. 2. This model is used to derive the kinetics of antibody-bound radionuclides. Radiolabeled antibody (Ab) is administered in the vascular space (compartment 1). It binds to antigen sites via saturable (non-linear) binding represented by the rate parameter k(2,1), which is a function of the affinity constant and the number of free antigen sites available (see equations in the “Appendix”). Antigen-bound antibody (AbAg) in compartment 2 may dissociate to return to the free radiolabeled antibody state by a rate constant, k(1,2) that is equal to the dissociation rate of antigen-bound antibody; AbAg may also internalize via k(3,2) into an intracellular compartment (compartment 3) where it is no longer available for dissociation but is cleared via catabolism at a rate represented by k(0,3). This lumped parameter model neglects aspects related to spatial gradients and transport across vasculature and is, therefore, specific to a radiopharmaceutical therapy of rapidly accessible antigen-positive cells within a vascular space that includes the plasma volume and the extracellular fluid (ECF) volume of the liver, spleen and red marrow (represented by the dotted box). The essential components of this model have been previously validated using patient data [13].
Pharmacokinetic model for radiolabeled antibody. The dotted line corresponds to the distribution volume of IV-administered antibody; ECF = extracellular fluid volume. Compartments 1 and 2 represent the free, (Ab) and antigen-bound antibody (AbAg) states, respectively. Compartment 3 represents internalized AbAg. The figure is adapted from reference [13]
Thorium biokinetic modeling
Time–activity curves for the fraction of 227Th that is not antibody-bound following the decay of 227Ac are given by the biokinetic model shown in Fig. 3. This model was developed and has been validated by Committee 2 of the International Commission on Radiological Protection (ICRP) [14, 16].
ICRP biokinetic model for thorium. Details and parameter values are provided in reference [14]
Radium biokinetic modeling
The biokinetic model for free radium is depicted in Fig. 4. It is based on an ICRP model describing the behavior of alkaline earth elements (“Appendix” of reference [17]), as implemented by Lassmann et al. to calculate normal tissue dosimetry for 223RaCl2 [15].
Biokinetic model for radium as represented in reference [15]. The solid arrows are relevant to the biokinetics of 223Ra
Time-integrated activity coefficients (TIACs)
The time-integrated activity (TIA), for each source region, \(r_{i}\), (\(\tilde{A}\left( {r_{i} } \right)\)) was obtained by integrating model-derived pharmacokinetic data. The TIAC is given by dividing TIA by the administered activity of 225Ac or by expressing the pharmacokinetics as a fraction of the administered activity. Equations 4–7 in the “Appendix” were integrated numerically with the substitutions indicated in equations: 8–10; 11–13, and 14–16, to get TIAC for 225Ac, 227Ac, and the antibody-bound fraction of 227Th, respectively. The TIAC for free 227Th and 223Ra was obtained by numerically integrating Eqs. 17 and 18. Numerical integration was performed using the trapezoidal method.
TIAC apportionment
Model-derived TIAC was apportioned to tissue parenchyma as specified by the pharmacokinetic models. TIAC calculated for blood (central compartment) was apportioned to all tissue according to their blood volume [18]. The daughters of 225Ac up to 213Bi, respectively, were assumed to decay at the site of 225Ac decay. Likewise the daughters of 213Bi were assumed to decay at the site of 213Bi decay. The TIAC, in each case was adjusted by the net yield of each daughter relative to the corresponding parent.
The same approach was taken for the daughters of 223Ra.
Absorbed dose calculations
Absorbed dose calculations were performed using the MIRD Committee S-value based method as described in pamphlet 21 [19]. The International Commission on Radiological Protection (ICRP) recently released absorbed fractions for a new series of phantoms that include far more tissues than were previously available [20]. The new absorbed fractions handle electron emissions far better than prior absorbed fractions which assumed that all or none of the energy associated with electron emissions was absorbed in tissues; absorption of alpha-particle energy is also appropriately considered [18]. A detailed comparison of the results obtained using OLINDA [21] and the new set of ICRP data has been published [22]. The calculations were performed using, newly developed software package, 3D-RD-S (Radiopharmaceutical Imaging and Dosimetry, LLC (Rapid), Baltimore MD), designed to account for the complexity of alpha-particle emitter dosimetry, in particular the differential fate of alpha-particle emitting daughters [23]. Absorbed doses from alpha-particles would ordinarily be multiplied by an RBE value of 5 [24, 25]. We have chosen not to use this factor and rather report the absorbed dose for each emission type directly. This approach provides all the information needed to apply an RBE value to the alpha-component of the absorbed dose.
Radionuclide decay scheme data
Decay schemes and half-lives for 225Ac and 227Ac and their daughters were obtained from ICRP publication 107 [26].
PK model parameter values
Table 2 lists the parameter values for the antibody PK model, and the free 227Th model and the 223Ra model parameters are available in the publications related to the models that are cited above.
Results
Ab–Ag pharmacokinetic model
The time–activity data obtained from Ab PK model simulations are plotted in Fig. 5A.
The PK for 227Ac-bound Ab is identical to that shown in Fig. 5, except that all data are scaled by 0.07% (= \(f_{Ac227}\), Table 2).
Since 99% of 227Ac decays by beta-particle emission, which is less energetically disruptive than alpha-particle decay, the assumption is made that 70% (= \(f_{Ab}\)) of the daughter radionuclide, 227Th, remains antibody-bound and obeys PK that is identical to that shown in Fig. 5A, except that all data are scaled by \(f_{Ac227} \cdot f_{Ab}\). The remaining 30% is assumed to obey the pharmacokinetics of free 227Th (Fig. 5B); this value was chosen as it is consistent with the retention of 212Bi following decay of 212Pb, also a beta-decay transition [27]. Since 227Th decays by alpha-particle emission to 223Ra, all of the 223Ra generated, regardless of whether the 227Th was Ab-bound or free is assumed to follow free radium kinetics (Fig. 5C).
As indicated in the text, Fig. 5A is scaled relative to an arbitrary amount of administered 225/7Ac that is antibody-bound. In other words 1 MBq of 225/7Ac-Ab administered should be multiplied by the fraction of injected activity (FIA) values indicated on the y-axis to obtain the corresponding amount of 227Ac or 227Th activity. The PK data plotted on Fig. 5B, C should be multiplied by \(f_{Ac227} \cdot (1 - f_{Ab} )\) and \(f_{Ac227}\), respectively, to convert the results to per MBq of 22/75Ac administered.
The resulting time–activity curves for each “species” were numerically integrated to obtain the TIAC for each of the indicated tissues (Table 3).
Absorbed doses
Table 4 lists absorbed doses for 225Ac and 227Ac; the absorbed dose from each particle type is provided separately. Table 5 lists the absorbed dose to selected tissues from 225Ac, 227Ac and their respective daughters. (Contributions from the 1% decay of 227Ac to 223Fr and daughters with a yield of less than 10–4% are not included.)
The absorbed dose from 225Ac and its daughters, along with the 227Ac to 225Ac absorbed dose ratio for the top 6 tissues by total absorbed dose, is depicted in Fig. 6.
Discussion
The alpha emitter 225Ac is a promising radionuclide for the generation of potent radiopharmaceutical agents for hematologic and solid tumor malignancies. However, commercial-scale supply concerns regarding purified 225Ac have limited more widespread clinical research and development of 225Ac-based αRPT. As a result, a number of alternative production methods have been examined as potential sources of large and scalable quantities of 225Ac, including accelerator-produced 225Ac via 232Th irradiation. However, accelerator-produced 225Ac contains 227Ac as an impurity in the purified material. We undertook this work to investigate the dosimetric impact of the 227Ac present in accelerator-produced 225Ac used for αRPT therapy in hematologic malignancies. Our modeling results determined that the tissue absorbed dose from 227Ac would be negligible in the context of therapy, less than 0.02 mGy/MBq for the top 6 highest absorbed dose tissues and less than 0.007 mGy/MBq for all other tissues. Compared to that from 225Ac, the absorbed dose from 227Ac would make up a very small component (< 0.04%) of the total absorbed dose delivered to the 6 highest dose tissues: red marrow, spleen, endosteal cells, liver, lungs and kidneys when accelerator produced 225/7Ac-conjugated anti-CD33 antibody would be used to treat leukemia patients. For all tissues evaluated, the dominant contributor to the absorbed dose arising from the 227Ac is 227Th, the first daughter of 227Ac, which has the potential to deliver absorbed dose both while it is antibody-bound and while it is free. These results suggest that the absorbed dose arising from 227Ac to normal organs would be negligible for an 225/7Ac-labeled antibody that targets hematological cancer.
In addition to the models used in these simulations and their related parameters, the following series of assumptions were used to arrive at these conclusion: (1) At time of administration there is 0.7% 227Ac in the 225/7Ac-conjugated anti-CD33 antibody. (2) 70% of the 227Th resulting from 227Ac decay remains antibody-bound and follows the same kinetics as the actinium-conjugated antibody. (3) 227Th decay releases free 223Ra. Under the simulation conditions described above, the spleen, red marrow, endosteal cells and liver would receive the highest absorbed doses from 227Ac and its daughters. The simulation also assumes high purity in the radiolabeled material so that loss of the labeled Ab also removes the Ac-227 conjugated to the Ab. It should be noted that different simulation models, parameter values and assumptions will give different results. In particular, these results may not apply to non-antibody carriers. The simulations and absorbed dose calculations were performed assuming 1 kg of antigen-positive cells in an adult female. Within the context of antibody-targeting of hematologic malignancies, alternative assumptions regarding percent 227Ac in the injectate and the fraction of 227Th that remains-antibody-bound may be implemented by scaling the listed absorbed dose values by the ratio of the new values with those used in this paper (e.g., by considering the scaling applied in Eqs. 8–16. For example, lower Ab retention of 227Th following decay of 227Ac may be obtained by scaling 227Th and daughter absorbed doses by the new retention fraction divided by 0.7 (= \(f_{Ab}\)). Such scaling can also account for injectate purity.
Conclusions
Using a pharmacokinetic model relevant to treating patients with leukemia and models describing the PK of free thorium and radium, the dose contribution of a 0.7% 227Ac in accelerator-produced 225Ac would be negligible in the context of αRPT therapy, less than 0.02 mGy/MBq for the top 6 highest absorbed tissues and less than 0.007 mGy/MBq for all other tissues.
The conclusion above is specific to the parameter values and assumptions outlined and may not apply to lower molecular weight agents or other cancer targets.
Availability of data and materials
The paper describes a series of simulations, all information required to repeat the simulations is included in the manuscript.
References
Sgouros G. α-particle–emitter radiopharmaceutical therapy: resistance is futile. Cancer Res. 2019;79:5479–81.
Jurcic JG. Targeted alpha-particle therapy for hematologic malignancies. Semin Nucl Med. 2020;50:152–61.
Lawal IO, Bruchertseifer F, Vorster M, Morgenstern A, Sathekge MM. Prostate-specific membrane antigen-targeted endoradiotherapy in metastatic prostate cancer. Curr Opin Urol. 2020;30:98–105.
REPORT ON JOINT IAEA-JRC WORKSHOP “SUPPLY OF ACTINIUM-225, 2018; Vienna, Austria.
John K. US DOE tri-lab production effort to provide accelerator-produced 225Ac for radiotherapy: 2019 update. J Nucl Med. 2019;60:1612–1612.
Hoehr C, Bénard F, Buckley K, et al. Medical isotope production at TRIUMF—from imaging to treatment. Phys Procedia. 2017;90:200–8.
Walsh KM. Producing Radioisotopes for Medical Imaging and Disease Treatment. In: Collide BNLRHi, ed. 2017. https://www.bnl.gov/rhic/news2/news.asp?a=12043&t=today. Accessed 14 May 2020.
Zewei J, Ekaterina R, Darrell RF, Ekaterina D. In vivo evaluation of free and chelated accelerator-produced Actinium-225—radiation dosimetry and toxicity results. Curr Radiopharm. 2018;11:215–22.
Stallons TAR, Saidi A, Tworowska I, Delpassand ES, Torgue JJ. Preclinical investigation of Pb-212-DOTAMTATE for peptide receptor radionuclide therapy in a neuroendocrine tumor model. Mol Cancer Ther. 2019;18:1012–21.
Delpassand E, Tworowska I, Shanoon F, et al. First clinical experience using targeted alpha-emitter therapy with Pb-212-DOTAMTATE (AlphaMedix (TM)) in patients th SSTR(+) neuroendocrine tumors. J Nucl Med. 2019;60:559.
Brechbiel MW. Bifunctional chelates for metal nuclides. Q J Nucl Med Mol Imaging. 2008;52:166–73.
Ruble G, Wu C, Squire RA, Ganswo OA, Strand M. The use of 212Pb-labeled monoclonal antibody in the treatment of murine erythroleukemia. Int J Radiat Oncol Biol Phys. 1996;34:609–16.
Sgouros G, Graham MC, Divgi CR, Larson SM, Scheinberg DA. Modeling and dosimetry of monoclonal antibody M195 (anti-CD33) in acute myelogenous leukemia. J Nucl Med. 1993;34:422–30.
Thorium. Annals of the ICRP. 1995;25:39–55.
Lassmann M, Nosske D. Dosimetry of 223Ra-chloride: dose to normal organs and tissues. Eur J Nucl Med Mol Imaging. 2013;40:207–12.
Leggett RW. Reliability of the ICRP’s dose coefficients for members of the public. 1. Sources of uncertainty in the biokinetic models. Radiat Prot Dosim. 2001;95:199–213.
ICRP. Publication 67, Age-dependent Doses to Members of the Public from Intake of Radionuclides: Part 2; Ingestion Dose Coefficients,: ICRP; 1993. 67.
Bolch WE, Jokisch D, Zankl M, et al. ICRP Publication 133: The ICRP computational framework for internal dose assessment for reference adults: specific absorbed fractions. Ann ICRP. 2016;45:5–73.
Bolch WE, Eckerman KF, Sgouros G, Thomas SR. MIRD pamphlet No. 21: a generalized schema for radiopharmaceutical dosimetry—standardization of nomenclature. J Nucl Med. 2009;50:477–84.
Menzel HG, Clement C, DeLuca P. ICRP Publication 110. Realistic reference phantoms: an ICRP/ICRU joint effort. A report of adult reference computational phantoms. Ann ICRP. 2009;39:1–164.
Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med. 2005;46:1023–7.
Josefsson A, Hobbs RF, Ranka S, et al. Comparative dosimetry for (68)Ga-DOTATATE: impact of using updated ICRP phantoms, S values, and tissue-weighting factors. J Nucl Med. 2018;59:1281–8.
Hamacher KA, Sgouros G. A schema for estimating absorbed dose to organs following the administration of radionuclides with multiple unstable daughters: a matrix approach. Med Phys. 1999;26:2526–8.
Feinendegen LE, McClure JJ. Meeting report—alpha-emitters for medical therapy—workshop of the United States department of energy—Denver, Colorado, May 30–31, 1996. Radiat Res. 1997;148:195–201.
Sgouros G, Allen BJ, Back T, et al. MIRD monograph: radiobiology and dosimetry for radiopahrmaceutical therapy with alpha-particle emitters. Sgouros G (editor). Reston VA: SNMMI; 2015.
Eckerman K, Endo A. ICRP Publication 107. Nuclear decay data for dosimetric calculations. Ann ICRP. 2008;38:7–96.
Westrom S, Generalov R, Bonsdorff TB, Larsen RH. Preparation of Pb-212-labeled monoclonal antibody using a novel Ra-224-based generator solution. Nucl Med Biol. 2017;51:1–9.
https://radioisotopes.pnnl.gov/isotopes/thorium-227.stm. Accessed 4 May 2018.
Acknowledgements
Not applicaple.
Funding
Work performed under contract for Actinium Pharmaceuticals, Inc. Additional support from NIH NCI grants R01CA116477 and R01CA187037.
Author information
Authors and Affiliations
Contributions
GS contributed to design and methodology, calculations, and manuscript writing/review. BH and EF performed calculations and manuscript writing/review. NR and DL performed manuscript writing/review. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicaple.
Consent for publication
Not applicaple.
Competing interests
Dr. Sgouros is a founder of, and holds equity in, Rapid. He serves as a member of Rapid's Board of Directors. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. Drs. He and Frey are employees of Rapid. Drs. Ray and Ludwig are employees of Actinium Pharmaceuticals, Inc. No other potential conflicts of interest relevant to this article exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Work performed under contract for Actinium Pharmaceuticals, Inc.
Appendix: Model equations
Appendix: Model equations
The model is described by the following differential equations:
with \(k\left( {1,2} \right)\) = \(k_{ - }\), the AbAg dissociation rate; \(k\left( {2,1} \right) = \frac{{k_{ + } }}{{V_{d} }} \cdot \left( {Ag_{0} - AbAg} \right)\), the time-dependent Ab association rate to free antigen (Ag) sites; \(k\left( {0,1} \right) = \frac{{{\text{ln}}\left( 2 \right)}}{{T_{c} }}\), the clearance rate of Ab; \(k\left( {3,2} \right) = \frac{{{\text{ln}}\left( 2 \right)}}{{T_{i} }}\), the internalization rate of AbAg; \(k\left( {0,3} \right) = \frac{{{\text{ln}}\left( 2 \right)}}{{T_{ci} }}\), loss/catabolism rate of AbAgi; \(k_{ + }\), the Ab, Ag association rate; \(V_{d}\), initial distribution volume of antibody; \(Ag_{0}\), total number of antigen sites; \(T_{c}\), Ab clearance half-time; \(T_{i}\), AbAg internalization half-time; \(T_{ci}\), AbAg loss or catabolism half-time of internalized AbAg.
Using this model, the amount of radiolabeled antibody in plasma (\(Q_{P} \left( t \right)\)), liver (\(Q_{L} \left( t \right)\)), spleen (\(Q_{S} \left( t \right)\)) and red marrow (\(Q_{RM} \left( t \right)\)) as a function of time, t, may be obtained using the equations below:
with \(f_{L1} ,f_{S1}\), fraction of Ab in the vascular or extracellular fluid space of the liver (L), or spleen (S); \(f_{L2} ,f_{S2}\), fraction of AbAg in the liver (L), or spleen (S); \(V_{RMECF} ,V_{d}\), red marrow ECF volume and total Ab distribution volumes.
Further details regarding this model, including the derivation of Eqs. 1–3, are in reference [13].
The time–activity curves for 225Ac, in plasma, liver, spleen and red marrow are given by substituting for \(Ab\left( t \right)\), \(AbAg\left( t \right)\), and \(AbAg_{int} \left( t \right)\) in Eqs. 4–7 as follows:
with \(f_{Ac227}\), fraction of total radioactivity arising from 227Ac; \(\lambda_{Ac225}\), transformation rate (= \(\frac{{{\text{ln}}\left( 2 \right)}}{{T_{Ac225} }}\)) of 225Ac; \(T_{Ac225}\), physical half-life of 225Ac.
Similar substitutions apply for 227Ac and 227Th, in plasma, liver, spleen and red marrow are given by substituting for \(Ab\left( t \right)\), \(AbAg\left( t \right)\), and \(AbAg_{int} \left( t \right)\) in Eqs. 4–7 as follows:
The corresponding equations for 227Th activity in plasma, liver, spleen and red marrow that remains antibody-bound are:
with \(f_{Ab}\), fraction of 227Th generated by the decay of 227Ac that remains Ab-bound; \(\lambda_{Th227}\), transformation rate of 227Th (= \(\frac{{{\text{ln}}\left( 2 \right)}}{{T_{Th227} }}\)); \(T_{Th227}\), physical half-life of 227Th.
The activity of free 227Th (per MBq 225Ac-Ab administered) as a function of time in tissue, \(i\), (\(a_{i} \left( t \right)_{Th227}\)) is obtained as follows:
with \(q_{i} \left( t \right)_{thorium}\), thorium content in tissue, \(i\), at time, \(t\) assuming one (arbitrary) unit of thorium is injected at \(t = 0\).
The activity of free 223Ra (per MBq 225/7Ac-Ab administered) as a function of time in tissue, \(i\), (\(a_{i} \left( t \right)_{Ra223}\)) is obtained assuming all daughters of radium remain at the site of parent decay:
with \(\lambda_{Ra223}\), transformation rate of 223Ra (= \(\frac{{{\text{ln}}\left( 2 \right)}}{{T_{Ra223} }}\)); \(T_{Ra223}\), physical half-life of 223Ra; \(q_{i} \left( t \right)_{radium}\), radium content in tissue, \(i\), at time, \(t\) assuming one (arbitrary) unit of radium is injected at \(t = 0\).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sgouros, G., He, B., Ray, N. et al. Dosimetric impact of Ac-227 in accelerator-produced Ac-225 for alpha-emitter radiopharmaceutical therapy of patients with hematological malignancies: a pharmacokinetic modeling analysis. EJNMMI Phys 8, 60 (2021). https://doi.org/10.1186/s40658-021-00410-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40658-021-00410-6