Abstract
Background
Ursodeoxycholic acid (UDCA) is an important clinical drug in the treatment of liver disease. In previous work, ursodeoxycholic acid was prepared by traditional organic synthesis. The preparation of ursodeoxycholic acid through an electrochemical method with higher stereoselectivity and environmental friendliness is described herein.
Results
Dimethyl sulfoxide (DMSO), dimethylformamide (DMF), and N-methyl-2-pyrrolidone (NMP) were used as stereoselectivity additives during electroreduction. With 107.5 mM DMSO in methanol containing potassium bromide and a continuous current of 20 mA, 936 Coulombs was passed into the electrolysis system, achieving 88.5 % conversion of 7-ketone lithocholic acid (7K-LCA), while the yield of UDCA reached 72.8 %. Cyclic voltammetry (CV) was used to explore the electrochemical behavior of the reaction, and the electrolysis results were consistent with the cyclic voltammograms.
Conclusions
Ursodeoxycholic acid can be prepared by electroreduction with high stereoselectivity. The method developed here offers a potential application for large-scale production of ursodeoxycholic acid and an interesting reference to asymmetric electrochemical reduction of the keto group.
Similar content being viewed by others
Background
Ursodeoxycholic acid (3α, 7β-2-hydroxy-5β-bile acid, UDCA) was first found by Shoda [1] in the bile of a black bear. It is used as a clinical drug in the treatment of gallstones, cholecystitis, PBC, and PSC and has broad market prospects and important scientific research value [2–9]. Kanazawa et al. [10] first synthesized ursodeoxycholic acid in 1954 by reducing 7-ketolithocholic acid (3α-hydroxy-7-oxo-5β-cholanic acid, 7K-LCA) in n-propyl alcohol by using metallic sodium with a 14 % yield. At present, the industrial production of ursodeoxycholic acid is based on the synthesis process outlined by Kanazawa. Bharucha and Slemon [11] demonstrated that 7K-LCA could be reduced to UDCA in an electrolyte containing short-chain alcohol, preferably with weakly acidic dipolar additives such as hexamethylphosphoric triamide (HMPA) with ruthenized titanium or mercury electrodes, and a 91 % yield of UDCA could be obtained. Magni et al. [12] stated that a 97 % yield of UDCA could be obtained by catalytic hydrogenation of 3α-hydroxy-7-oxo-5β-cholanate using Raney nickel as the catalyst and beta-branched alcohols as the solvent in the presence of a base at 40 °C and atmospheric pressure. 3α-Hydroxy-7-oxo-5β-cholanate dissolved in alcohols could also be reduced to UDCA by hydrogen at a pressure of 0.5 MPa and a temperature of 80 °C catalyzed by Raney nickel, giving a 92.5 % yield of UDCA [13]. In addition, some biological methods are developed in recent years and showed high potential for industrial application [14, 15]. Herein, 3α-hydroxy-7-oxo-5β-cholanate was formed by adding hydrate to 7K-LCA. Currently, there have been very few reports on the preparation of ursodeoxycholic acid by an electrochemical method, despite the fact that it is a relatively simple and safe process that does not require harsh reaction conditions or complicated reaction steps.
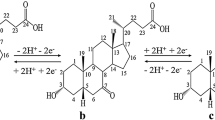
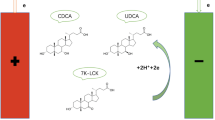
Electrochemical reduction of carbonyl compounds has progressed considerably during the past 20 years, not only in theory but also in application. A number of methods for creating a chiral environment have been reported using electrochemical stereoselective synthesis, such as the use of chiral solvents [16–18], chiral supporting electrolytes [17–20], intrinsic optical substrate conformation [19], chiral electrodes [21–23], and chiral catalytic systems [24, 25]. In the previous work [26], our group synthesized 7-ketolithocholic acid, which is an important intermediate in ursodeoxycholic acid synthesis, by indirect electrooxidation. Herein, we describe our attempts to prepare ursodeoxycholic acid through electroreduction. The electroreduction of ketone in the presence of a hydrogen source produces two main products: ursodeoxycholic acid and chenodeoxycholic acid (CDCA). During electrolysis of 7-ketolithocholic acid, the preferred predominantly chenodeoxycholic acid, with only small amounts of ursodeoxycholic acid, is formed. However, UDCA can be obtained stereoselectively in the presence of an aprotic polar solvent (Fig. 1).
The purpose of this paper was to electroreduce 7-ketolithocholic acid stereoselectively into ursodeoxycholic acid in a solution comprising a highly polar and aprotic solvent [dimethyl sulfoxide (DMSO), dimethylformamide (DMF), and N-methyl-2-pyrrolidone (NMP)]. With the correct amount of aprotic solvent, the C-7 carbonyl group of 7K-LCA can be selectively converted into a 7β-hydroxy group on the cathode surface. Experiments show that the aprotic solvent can significantly increase the conversion of 7-ketolithocholic acid and the yield of ursodeoxycholic acid, perhaps indicating the asymmetric reduction of the keto group.
Methods
General methods
UDCA (99 %) was purchased from Aladdin Chemistry Co. Ltd. 7K-LCA was prepared according to our previous work, via indirect electrooxidation of CDCA using a Br−/Br2 cycle. Methanol and potassium bromide were from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). Acetonitrile [high-performance liquid chromatography (HPLC) grade] was from Shanghai Xingke Biochemistry Co. Ltd. (Shanghai, China). All other reagents were of analytical grade, from the Shanghai Ling Feng Chemical Reagent Co. Ltd. (Shanghai, China). WY-30.5 DC power with a single regulator constant flow was from Shanghai Starfish Instrument Co. Ltd. (Shanghai, China). A H-divided electrolytic cell was manufactured by the Glass Instrument Factory of the East China University of Science and Technology (ECUST, Shanghai, China). Cathode material consisting of a high-purity lead plate electrode was obtained from the Engineering Training Center of ECUST. The size of the lead plate was 145 × 30 mm. The anode material consisted of a titanium-ruthenium mesh electrode from Henan Xinxiang Future Water Chemical Co. Ltd. (Xingxiang, Henan). The size of the titanium-ruthenium mesh electrode was 145 × 30 mm. A HF-101 fluoroplastic cation exchange membrane was purchased from the Shanghai Hua Kai Technology Company (Shanghai, China). A cyclic voltameter with a PARSTAT 2273 electrochemical workstation (Princeton Applied Research, Princeton, NJ, USA) was used to measure cyclic voltammograms.
Electroanalytical procedure
The electroanalytical experiments were carried out in an electrochemical workstation with a co-solvent (MeOH/H2O = 9.83/1, volume ratio) containing 0.08 M potassium bromide as the supporting electrolyte in a divided glass cell, with a lead plate as the working electrode, a titanium-ruthenium mesh as the counter electrode, and Ag|AgCl|KCl as the reference electrode. All experiments were performed at 65 °C under atmospheric pressure. In addition, oxygen was removed from the solution by continuous bubbling with nitrogen before the experiment.
Selective electroreduction of 7K-LCA
The galvanostatic electrolysis was carried out in a H-divided electrolytic cell with the HF-101 cation exchange membrane, and the reaction systems consisted of 0.04 M substrate 7-ketone lithocholic acid, 0.08 M supporting electrolyte, and 0.12 M aprotic solvent in a 131-ml co-solvent (MeOH/H2O = 9.83/1, volume ratio) bubbled with N2 to remove oxygen. A high-purity lead plate was used as the cathode and a titanium-ruthenium mesh as the anode. A continuous current of 20 mA was maintained during the reaction. After 936 Coulombs had passed through, the current was switched off. All experiments were carried out at 65 °C.
When the reaction was complete, the organic solvent from the catholyte was removed using a rotary evaporator to obtain a solid sample. It was then dissolved in NaOH solution, and the pH was adjusted to 2.0 using hydrochloric acid. The precipitate was collected by filtration and then dried in an oven. The final product was detected by HPLC.
Scale-up of electrolysis
During the scale-up of electrolysis, 6 kg of 7K-LCA was dissolved in 280 kg of methanol and 2.7 kg of KBr was dissolved in 24 kg of water; then, the two solutions were mixed and added to a hold-up vessel containing 6.5 kg of DMF, which was used as the catholyte. An equal volume of 5 % H2SO4 was used as the anolyte. A bipolar diaphragm electrolyzer comprised the reaction vessel separated by the HF-101 cation exchange membrane. A high-purity lead plate and titanium-ruthenium mesh were used as the cathode and anode, respectively. The effective overlap area of the electrodes was 70 × 35 cm. The electrolyte was pumped into the electrolyzer with a circulating pump before the reaction started. A continuous current of 20 A was used for electrolysis over 20 h at 55 °C. When the reaction was complete, the organic solvent from the catholyte was removed, and the final product was collected for detection.
Analytical methods
UDCA was analyzed by reversed phase HPLC, using a C-18 column with Welchrom-C18 5-μm resin in a 4.6 × 150 mm column (Welch Material Inc., Shanghai, China). The HPLC instrument (LC-20A) was from Shimadzu Corporation, Kyoto, Japan, and was used for quantitative analysis of the reaction products at 208 nm. The mobile phase was a mixture of acetonitrile and phosphate acid buffer (pH 3.0) with a volume ratio of 50:50 at a flow rate of 1.0 ml/min at 25 °C. Fifty percent methanol was used to clean the syringe in the SIL-20A autosampler (Shimadzu Corporation, Kyoto, Japan). The conversion of 7K-LCA was calculated as C = M t/M 0 × 100 %, where M t is the amount of 7K-LCA consumed and M 0 is the total amount of 7K-LCA used during the electrolysis. The yield of UDCA was calculated as Y = A r/A t × 100 %, where A r is the actual productivity of UDCA and A t is the theoretical productivity of UDCA. The electrolysis product was purified by silylation crystallization [27].
Results and discussion
Stereoselective electrochemical reduction is important both in research and applications. In this electrochemical reaction, ursodeoxycholic acid was synthesized by electroreduction of 7-ketolithocholic acid under certain conditions.
During electrolysis, the C-7 carbonyl group of 7K-LCA is converted into a 7β-hydroxy group on the cathode surface. Two side reactions may exist in this system. In the first, the C-7 carbonyl group of 7K-LCA is converted to a 7α-hydroxy group, and chenodeoxycholic acid is generated (Fig. 2). In the second, hydrogen is produced on the cathode surface. However, the added aprotic polar solvent can effectively suppress the generation of chenodeoxycholic acid.
Electroanalytical results
Cyclic voltammograms (CVs) of 7K-LCA using a lead electrode in methanol at different scan rates are shown in Fig. 3. These show that there is only one irreversible peak, located at −1.2 V, in the CVs from the positive to negative scan direction, at a scan rate of 10 mV/s (Fig. 3, curve a). This indicates that only the reduction process is taking place in the electrolysis system. From the results of the electrolysis reaction, this reduction peak may correspond to the reduction of the ketone group (C=O) of 7K-LCA because the main reaction is the conversion of the C-7 carbonyl group of 7K-LCA to a hydroxyl group. When the scan rate increased, the reduction peaks became more positive, with the peak current increasing concomitantly (Fig. 3, curves a–c).
To study the CVs of 7K-LCA in MeOH with 0.08 M KBr, a potential-controlled electrolysis experiment was carried out at −1.2 V, and a current-controlled electrolysis experiment was carried out at 60 mA, because the electrolysis was an irreversible electrochemical reaction.
To further study the effect of the aprotic solvent on the voltammetric behavior of 7-ketolithocholic acid, DMSO was added into the co-solvent (MeOH with 0.08 M KBr). As shown in curves b–d in Fig. 4, the reduction peak potential shifted to a more positive value with increasing amount of DMSO. This indicates strong interactions between DMSO and the 7-ketolithocholic acid, under which the peak potential shifted from −1.116 to −0.920 V. This is a suitable electroreduction condition for 7-ketolithocholic acid. Presumably, DMSO influenced the adsorption of 7K-LCA on the cathode interface and changed the structure of the electric double layer [28, 29].
Cyclic voltammograms recorded at the lead electrode at 65 °C at a scan rate of 10 mV s−1: (a) blank cyclic voltammetry in the solvent with 0.08 M KBr, (b) as (a) + 0.12 M DMSO, (c) as (a) + 0.36 M DMSO, and (d) as (a) + 0.6 M DMSO. The solvent was 130 ml of co-solvent (MeOH/H2O = 9.83/1, volume ratio)
Preparative electrolysis
Preparative electrolysis was carried out in a reaction system consisting of co-solvent (MeOH/H2O = 9.83/1), 0.08 M KBr, 0.04 M 7K-LCA, and 0.12 M DMSO in a divided cell equipped with a lead plate cathode and a titanium-ruthenium mesh anode at 65 °C. A continuous current of 20 mA was applied, and after 936 Coulombs had passed through, the current was switched off. The organic solvent from the catholyte was removed using a rotary evaporator to obtain a solid sample. It was then dissolved in NaOH and the pH was adjusted to 2.0 using hydrochloric acid. The precipitate was collected by filtration and dried in an oven.
The influence of DMSO on the yield of UDCA and conversion ratio of 7K-LCA
Table 1 presents the conversion of 7K-LCA and yield of UDCA with different amounts of DMSO. The electrolysis reaction without DMSO gave a 48 % 7K-LCA conversion and 18.8 % UDCA yield. Addition of 107.5 mM DMSO promoted the reduction of the C-7 carbonyl group of 7K-LCA, and the best results obtained give a 72.8 % UDCA yield and an 88.5 % 7K-LCA conversion. However, the yield of UDCA diminished with greater amounts of DMSO. It can be concluded from the experimental results that DMSO is beneficial to the reduction of the C-7 carbonyl group of 7K-LCA. Compared with the electrolysis in the absence of DMSO, the conversion of 7K-LCA rose from 48 to 88.5 %, and the yield of UDCA increased from 18.8 to 72.8 % which clearly means that the C-7 carbonyl group of 7K-LCA was selectively converted into the 7β-hydroxy group. However, higher amounts did not further promote the reaction, causing a higher resistance during electrolysis to be observed.
Influence of aprotic polar solvent type on the yield of UDCA and conversion ratio of 7K-LCA
As shown in Fig. 5a, the peak potential exhibited a positive shift (curves b–d in Fig. 5a) caused by the addition of DMF, while the increase in the 7-ketolithocholic acid reduction peak current can be ascribed to a higher resistance. In Fig. 5b, the peak potential of 7-ketolithocholic acid reduction was also shifted to a more positive position compared with the blank cyclic voltammetry in the solvent with 0.08 M KBr, but the peak current showed a minimal increase, in contrast to the increase shown in Fig. 5a.
Cyclic voltammograms recorded at the lead electrode at 65 °C at a scan rate of 10 mV s−1: a (a) blank cyclic voltammetry in the solvent with 0.08 M KBr, (b) as (a) + 0.11 M DMF, (c) as (a) + 0.33 M DMF, and (d) as (a) + 0.55 M DMF. b (a) Blank cyclic voltammetry in the solvent with 0.08 M KBr, (b) as (a) + 0.09 M NMP, (c) as (a) + 0.26 M NMP, and (d) as (a) + 0.44 M NMP. The solvent was 130 ml of co-solvent (MeOH/H2O = 9.83/1, volume ratio)
These results indicate that the addition of aprotic solvent was favorable to the electroreduction of 7-ketolithocholic acid, and the mechanism is similar to the situation when DMSO is added. However, different types of aprotic solvent have different influences on the reduction, and the addition of aprotic solvents changed the resistance, dielectric constant, and polarity of the electrolyte, which may lower the current efficiency.
Table 2 (No. 2–8) presents the results using different aprotic solvents (DMSO, DMF, NMP). With DMSO, the yield of UDCA reached 72.8 %, and the conversion of 7-ketolithocholic acid was 88.5 %. When DMSO was replaced by DMF, the conversion of 7K-LCA was augmented by an increase in the amount of DMF, but the yield of UDCA first increased and then decreased. Comprehensively considering the conversion of 7K-LCA and the yield of UDCA, 292.6 mM DMF was perhaps the best choice, with a 68.5 % 7K-LCA conversion ratio and a 65.4 % yield of UDCA. When adding NMP, at 79.0 mM NMP, the conversion ratio of 7K-LCA was 57 %, but the yield of UDCA was only 11.3 %. With an increased amount of NMP, 7K-LCA can be transformed, but the target product UDCA could not be produced. In addition, Table 2 (No. 1–8) reveals that the addition of aprotic solvents improves the product yield, consistent with the cyclic voltammograms shown in Fig. 5. However, the amount of aprotic solvent used has a different effect on the yield of UDCA.
Scale-up of electrolysis
In prior experiments, DMSO shows better results compared with DMF obviously. However, considering the solvent in separation by silylation crystallization and the price of raw materials, we chose DMF as the additive in the scale-up electrolysis.
During the scale-up of electrolysis, four experiments were conducted. Table 3 presents the main information of the four experiments. After considering the condition of scale-up, we changed the temperature and water content. As shown in Fig. 6, in the first experiment of scale-up, the 7K-LCA conversion reached 64.2 % and the UDCA yield was 44.5 %, but the other experiments gave lower UDCA yield. UDCA yield of the last experiment was 18.7 %, less than half of the first experiment. This result was lower than the electrolysis with 292.6 mM DMF in the laboratory significantly. There are two possible reasons for this result, evaporation of the solvent and the use of impure reused solvent. In our previous work, the water content of the electrolyte has great influence on the electrolysis result [30]. We intend to optimize the electrolyte in future research.
Characterization of the product
The product was determined by HPLC after purification and drying. As shown in Fig. 7, the retention times of UDCA and 7K-LCA were 6.0 and 8.2 min, respectively. Many impurity peaks can be seen between 2.0 and 6.0 min, and some of the corresponding materials were likely contained in the raw material and others were produced by the electroreduction process. The yield of UDCA was calculated to reach 72.8 %.
Conclusions
In summary, the electrochemical stereoselective reduction of 7-ketolithocholic acid into UDCA was conducted using aprotic polar solvents. A higher conversion (88.5 %) of 7-ketolithocholic acid was observed with a 72.8 % yield of ursodeoxycholic acid upon addition of 107.5 mM DMSO during the electrolysis process. In the preliminary scale-up, the conversion of 7K-LCA reached 64.2 % and the UDCA yield was 44.5 %. Compared with chemical and biological preparative processes, the electrochemical process offers the advantages of low cost, high stereoselectivity, and production safety.
References
Shoda M (1927) Über die ursodesoxycholsäure aus bärengallen und ihre physiologische wirkung. J Biochem 7(3):505–517
Talwalkar JA, Lindor KD (2003) Primary biliary cirrhosis. Lancet 362(9377):53–61
European Association for the Study of the Liver (2009) EASL clinical practice guidelines: management of cholestatic liver diseases. J Hepatol 51(2):237–267
Salvioli G, Igimi H, Carey MC (1983) Cholesterol gallstone dissolution in bile. Dissolution kinetics of crystalline cholesterol monohydrate by conjugated chenodeoxycholate-lecithin and conjugated ursodeoxycholate-lecithin mixtures: dissimilar phase equilibria and dissolution mechanisms. J Lipid Res 24:701–720
Shiraki K, Ito T, Sugimoto K et al (2005) Different effects of bile acids, ursodeoxycholic acid and deoxycholic acid, on cell growth and cell death in human colonic adenocarcinoma cells. Int J Mol Med 16(4):729–733
Liu F, Cheng Y, Wu J et al (2006) Ursodeoxycholic acid differentially affects three types of sphingomyelinase in human colon cancer Caco 2 cells. Cancer Lett 235(1):141–146
Galsky J, Bansky G, Holubova T et al (1999) Effect of ursodeoxycholic acid in acute viral hepatitis. J Clin Gastroenterol 28(3):249–253
Poupon RE, Lindor KD, Cauch DK et al (1997) Combined analysis of randomized controlled trials of ursodeoxycholic acid in primary biliary cirrhosis. Gastroenterol 113(3):884–890
Santos VN, Lanzoni VP, Szejnfeld J et al (2003) A randomized double-blind study of the short-time treatment of obese patients with nonalcoholic fatty liver disease with ursodeoxycholic acid. Braz J Med Biol Res 36(6):723–729
Kanazawa T, Shimazaki A, Sato T et al (1954) Syntheses of ursodeoxycholic acid and its conjugated bile acid. Proc Jpn Acad 30(5):391–392
Bharucha KR, Slemon CE (1985) Process for the electrochemical reduction of 7-ketolithocholic acid to ursodeoxycholic acid., US 4547271
Magni A, Piccolo O, Ascheri A (1987) Stereoselective reduction of the keto group at 7-position of a bile keto acid., EP 0230085
Hattori M, Mikami K, Sekine T (1993) Selective reduction of bile acid having keto group at 7-site., JP 5032692
Liu L, Braun M, Gebhardt G, Weuster-Botz D, Gross R, Schmid R (2013) One-step synthesis of 12-ketoursodeoxycholic acid from dehydrocholic acid using a multienzymatic system. Appl Microbiol Biotechnol 97:633–639
Zheng MM, Wang RF, Li CX, Xu JH (2015) Two-step enzymatic synthesis of ursodeoxycholic acid with a new 7β-hydroxysteroid dehydrogenase from Ruminococcus torques. Process Biochem 50:598–604
Seebach D, Oei HA (1975) Mechanism of electrochemical pinacolization. The first asymmetric synthesis in a chiral medium. Angew Chem internat Edit 14(9):634–636
Horner L, Degner D (1974) Studien zum vorgang der wasserstoffübertragung-33. Zur kenntnis des aufbaus der elektrochemischen doppelschicht. Die asymmetrische elektrochemische reduktion einiger alkyl-phenyl-ketone in gegenwart optisch aktiver leitsalze. Electrochim Acta 19(10):611–627
Horner L, Brich W (1978) Studien zum vorgang der wasserstoffübertragung, 49: zur frage der auslösung einer optischen induktion durch anwendung optisch aktiver ephedrinderivate und optisch aktiver kronenether als leitsalze bei der elektroreduktion von acetophenon. Chem Ber 111(2):574–578
Schuster C, Knollmueller M, Gaertner P (2006) Chiral linker. Part 4: diastereoselective addition of RZnX to α-keto esters using m-hydrobenzoin derived chiral auxiliaries in solution and on solid support and their application in the stereo-selective synthesis of frontalin. Tetrahedron: Asymmetry 17(16):2430–2441
Chen BL, Xiao Y, Xu XM, Yang HP, Wang H, Lu JX (2013) Alkaloid induced enantioselective electroreduction of acetophenone. Electrochim Acta 107:320–326
Yadav AK, Manju M, Chhinpa PR (2003) Enantioselective cathodic reduction of some prochiral ketones in the presence of (−)-N, N’-dimethylquininium tetrafluoroborate at mercury cathode. Tetrahedron: Asymmetry 14(8):1079–1081
Vago M, Williams FJ, Calvo EJ (2007) Enantioselective electrocatalytic hydrogenation of ethyl pyruvate on carbon supported Pd electrodes. Electrochem Commun 9:2725–2728
Vasudevan D, Kennady CJ (2008) Electroreduction of carbonyl compounds at a Ti/ceramic TiO2 cathode. J Appl Electrochem 38:403–408
Tascedda P, Dunach E (2000) Electrosynthesis of cyclic carbamates from aziridines and carbon dioxide. Chem Commun 6:449–450
Batanero B, Saez R, Barba F (2009) Electroreduction of quinones under aprotic conditions. Electrochim Acta 54:4872–4879
Zhao HB, Tian H, Jin YH, Cao XJ (2010) Synthesis of 7-ketolithocholic acid via indirect electrooxidation of chenodeoxycholic acid. J Appl Electrochem 40(7):1307–1316
Ma XL, Cao XJ (2014) Separation of ursodeoxycholic acid by silylation crystallization. Bioresources and Bioprocessing 1:5
Hecht M, Fawcett W (1995) Solvent effects in the electroreduction of [diamine-N, N'-polycarboxylato] chromate (III) complexes at a mercury electrode. J Electroanal Chem 396:473–483
Fawcett W (1997) The role of the metal and the solvent in simple heterogeneous electron transfer reactions. Electrochim Acta 42(5):833–839
Yuan XX, Ma XL, Cao XJ (2014) Preparation of ursodeoxycholic acid by direct electro-reduction of 7-ketolithocholic acid. Korean J Chem Eng 31(7):1276–1280
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
XH designed the study, collected, processed, and analyzed the data, and wrote the article. XC contributed to the study design and article corrections. Both authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Huang, X., Cao, X. Preparation of ursodeoxycholic acid from 7-ketone lithocholic acid by stereoselective electroreduction. Bioresour. Bioprocess. 2, 27 (2015). https://doi.org/10.1186/s40643-015-0058-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40643-015-0058-4