Abstract
Purpose
Animal models implicate candida colonization facilitating invasive bacterial infections. The clinical relevance of this microbial interaction remains undefined and difficult to study directly. Observations from studies of anti-septic, antibiotic, anti-fungal, and non-decontamination-based interventions to prevent ICU acquired infection collectively serve as a natural experiment.
Methods
Three candidate generalized structural equation models (GSEM), with Candida and Pseudomonas colonization as latent variables, were confronted with blood culture and respiratory tract isolate data derived from 464 groups from 279 studies including studies of combined antibiotic and antifungal exposures within selective digestive decontamination (SDD) interventions.
Results
Introducing an interaction term between Candida colonization and Pseudomonas colonization substantially improved GSEM model fit. Model derived coefficients for singular exposure to anti-septic agents (− 1.23; − 2.1 to − 0.32), amphotericin (− 1.78; − 2.79 to − 0.78) and topical antibiotic prophylaxis (TAP; + 1.02; + 0.11 to + 1.93) versus Candida colonization were similar in magnitude but contrary in direction. By contrast, the model-derived coefficients for singular exposure to TAP, as with anti-septic agents, versus Pseudomonas colonization were weaker or non-significant. Singular exposure to amphotericin would be predicted to more than halve candidemia and Pseudomonas bacteremia incidences versus literature benchmarks for absolute differences of approximately one percentage point or less.
Conclusion
GSEM modelling of published data supports the postulated interaction between Candida and Pseudomonas colonization towards promoting bacteremia among ICU patients. This would be difficult to detect without GSEM modelling. The model indicates that anti-fungal agents have greater impact in preventing Pseudomonas bacteremia than TAP, which has no impact.
Similar content being viewed by others
Take home message
Structural equation modelling of the ICU infection prevention data from 279 studies supports the postulated influence of prophylaxis using anti-fungal agents in preventing Pseudomonas bacteremia. The model implicates that anti-fungal agents have greater impact in preventing bacteremia versus antibiotics, which have no impact.
Introduction
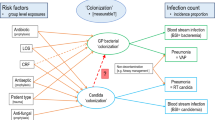
Animal models implicate candida colonization facilitating invasive bacterial infections [1, 2]. Which of over 600 catalogued immunological, biochemical, metabolic and mechanical processes might underlie this interaction between Pseudomonas aeruginosa and Candida albicans and, moreover, whether it has clinical relevance, remain challenging to investigate (Fig. 1) [3,4,5].
Theoretical model of clinical factors bearing on the interaction between Pseudomonas and candida colonization towards causing blood stream and other infections. ‘contextual’ refers to the contextual effect within each ICU setting. The blue boxes label the elements required to address the central research question here depicted by the vertical arrow labelled ‘?’. This research question would not be easily addressed within a single center study
Conceptually, clinical investigation of this postulated interaction would manipulate candida colonization among patients and measure the resulting Pseudomonas bacteremia incidence [6,7,8]. Such an investigation would be logistically complex. Additional challenges to such an approach are that the blood stream infection (BSI) endpoints are generally uncommon or rare, the key body site location of any postulated interaction, whether the oropharynx or elsewhere, remains unclear and measuring colonization, whether bacterial or candida, is problematic. Moreover, whether changes in the metabolic activity, hyphal growth or the mere viable count of Candida colonisation drives any interaction towards a propensity for invasive infection remains moot.
A novel approach uses the collective observations from numerous studies of assorted and variously formulated anti-septic, antibiotic, anti-fungal, and non-decontamination-based interventions in the prevention of ICU acquired infections such as ventilator associated pneumonia (VAP) [9,10,11,12,13,14,15,16,17]. Candidemia and bacteremia incidences, being occasional secondary endpoints within these studies, in association with varying Candida colonisation occurring as a bystander process to the study intervention, serve as a natural experiment.
Selective digestive decontamination (SDD), a widely studied antibiotic-based regimen, combines antibiotic and antifungal exposures. Moreover, the SDD concept invokes population effects which need to be considered in any analysis. Several SDD studies in the ICU setting avoided these anticipated contextual effects by using either non-concurrent or no control group patients [18,19,20,21,22].
The objectives here are threefold. First, to recapitulate the study level evidence for various ICU infection prevention interventions versus each of VAP and BSI with Candida or Pseudomonas. Second, to develop models of colonization and infection based on the postulated interaction between Candida and Pseudomonas colonization as impacted by various infection prevention interventions by confronting the models using group level infection data using GSEM modelling. Third, to estimate the relative impacts of anti-septic, antibiotic, and specific anti-fungal agents as singular or compound exposures on bacteremia and candidemia within the optimal GSEM model.
Materials and methods
Being an analysis of published work, ethics committee review of this study was not required.
Study selection and decant of groups
The literature search and study decant used here is as described previously [23,24,25] and is detailed in Fig. 2. The key inclusion criterion were as follows: patient groups requiring prolonged (> 24 h) ICU stay within either studies of ICU infection prevention interventions or observational studies without an intervention under study, and group level Candida, and Pseudomonas infection data was available. The studies were streamed into type of infection prevention intervention, being non-decontamination based, anti-septic based, antibiotic based or single anti-fungal (SAF) based methods. Studies without ICU infection prevention interventions were sourced to provide summary benchmark incidence data. Most of the studies had been cited in systematic reviews with additional studies being found by snowball sampling using the ‘Related articles’ function within Google Scholar [26].
Search method, screening criteria and resulting classification of eligible studies and subsequent decant of component groups. The six steps are as follows: (1) An electronic search for systematic reviews or meta-analysis (SR/MA) containing potentially eligible studies using search terms; “ventilator associated pneumonia”, “mechanical ventilation”, “intensive care unit”, each combined with either “meta-analysis” or “systematic review” up to November 2021; (2) The systematic reviews were then searched for studies of patient populations requiring prolonged (> 24 h) ICU admission (3) The studies were triaged from the systematic reviews into one of five categories; studies in which there was no intervention (observational studies), studies of various non-decontamination methods such as methods delivered either via the gastric route, the airway route or via the oral care route, studies of anti-septic methods, studies of antibiotic-based interventions, and studies of single drug antifungal (SAF) prophylaxis. (4) All studies were reviewed for potentially eligible studies and screened against inclusion and exclusion criteria. Any duplicate or ineligible studies were removed and (5) Studies identified outside of systematic reviews were included; (6) The component groups were decanted from each study being control (rectangles), intervention (ovals) and observation (diamond) groups. The total numbers do not tally as some systematic reviews provided studies in more than one category and some studies provided groups in more than one category and some studies have unequal numbers of control and interventions groups
The various study level and group level data were extracted for tabulation and to serve as indicator variables in the GSEM models.
Visual benchmarking
Scatter plots of VAP and blood stream infections in association with Candida and Pseudomonas infection were generated to facilitate a visual survey of the entire data versus benchmarks as derived from the observational groups.
Estimation of summary effect sizes
As study events were rare, summary effect sizes were derived using the Peto’s log odds ratio [27]. The Stata ‘meta’ command was used for deriving summary effect sizes, the associated measures of between-study heterogeneity and the caterpillar plots which display the results of individual studies.
GSEM models: measurement components
The incidences of VAP and blood stream infections in association with each of Candida and Pseudomonas were extracted. As Candida is generally not considered a cause of VAP, the count of Candida as a respiratory tract (RT Candida) isolate among patients with suspected VAP was recorded. These counts were each transformed to proportions using the number of patients with prolonged (> 24 h) ICU stay as the denominator. In the GSEM models, Candida and Pseudomonas colonization are each latent variables within a measurement model for which the infection count proportions serve as endogenous variables.
GSEM models: indicator variables
The following data constitute exogenous indicator variables of the GSEM models; origin from trauma ICU’s, being defined here as an ICU with > 50% of admissions being for trauma, whether more than 90% of patients of the group received more than 24 h of MV, and a mean (or median) length of ICU stay (ICU-LOS) for the group greater than 7 days. In the extraction of MV percentages, if this was not stated for any group, the percentage receiving MV was assumed to be less than 90%. In the extraction of ICU-LOS data from the studies, surrogate measures including mean (or median) length of mechanical ventilation were taken if the length of ICU-LOS was not available in order to generate a binary variable of ICU-LOS of greater or less than 7 days.
Also, the presence of any of the following group wide risk factors for candidemia (CRF) and invasive Candida infection were noted; liver transplantation or liver failure, use of parenteral nutrition, surgery for intestinal perforation, pancreatitis, and being colonized with Candida, however that was defined. Anti-septic exposure included use of agents such as chlorhexidine, povidone-iodine and iseganan regardless of whether the application was to the oropharynx, by tooth-brushing or by body-wash.
Antibiotic-based interventions were classified as follows. Topical antibiotic prophylaxis (TAP) is defined without regard to the specific antibiotic constituents and whether application was to the oropharynx and or gastrointestinal tract. Protocolized parenteral antibiotic prophylaxis (PPAP) is the prophylactic use of parenteral antibiotics as dictated by the study protocol whether to the intervention group alone or to both control and intervention groups (duplex studies). Exposure to anti-fungal prophylaxis was identified whether as a single agent (SAF) or in combination with antibiotic-based interventions as within SDD or selective oropharyngeal decontamination (SOD) regimens.
Structural equation modelling
In the GSEM models, the VAP and blood stream infection counts as proportion data, serve as the measurement components, the group level exposure parameters serve as the indicator variables and colonization with each of Candida and Pseudomonas, being represented as latent variables, link the indicator and measurement components.
Three candidate GSEM models were developed. The first two, with and without the inclusion of an interaction terms between the latent variables, being Candida colonization and Pseudomonas colonization and the third with the addition of concurrent control group membership within an antibiotic-based study as an indicator variable to identify postulated contextual effects.
Because the observations are clustered by study, study identifiers were used in the models to enable generation of robust variance covariance matrices of the coefficient estimate parameters. The GSEM model with the lowest Akaike's information criterion (AIC) score was selected as having parsimony and optimal fit from among the candidate models using the ‘GSEM’ command in Stata (Stata 17, College Station Texas, USA) [28]. The post model predictions were obtained using the Stata command ‘nlcom’ to obtain nonlinear combinations of estimators.
Results
Characteristics of the studies
Of the 279 studies identified by the search, most were sourced from 23 systematic reviews (Table 1; Fig. 2; Additional file 1: Tables S1–S5)), most were published between 1990 and 2010 and most had a mean ICU-LOS exceeding seven days. Twelve studies had more than one type of intervention group and 15 studies had either more than one or no control group. The majority of groups from studies of infection prevention interventions had less than 150 patients per group versus more than 150 patients in the observational studies.
The incidence of BSI (Fig. 3) and VAP (Additional file 1: Figs. S1, S2) with each of Candida and Pseudomonas ranged approximately 100-fold across the various observation, control and intervention groups of the 279 studies. In each case, the incidence was approximately 50% or more higher among concurrent control groups within studies where intervention groups received TAP versus a benchmark derived from observational groups. The candidemia incidence was generally higher among groups from SAF studies as patient selection for most of these studies was commonly based on presence of CRF.
a, b Scatter plots, on a logit scale, of the incidence proportions of Pseudomonas bacteremia (a) and candidemia (b) for groups from 289 studies as listed in Additional file 1: Tables S1 to S5. The mean proportion (and 95% CI) derived by random effect meta-analysis for each category of component (observational [Ob], control [_C] and intervention [_I]) group derived from observational [Ob], non-decontamination (non-D), antibiotic-based and single anti-fungal (SAF) studies, is displayed. In each plot, the benchmark proportion (solid vertical line) is the mean proportion derived from the observational groups. Those component groups that did (solid symbols) versus did not (open symbols) select patients with CRF’s are indicated. NCC non-concurrent control, CC concurrent control. Note that antibiotic groups received multiple exposures in association with compound regimens (e.g. SDD and SOD, which combine TAP, an antifungal together with or without PPAP)
Bacteremia and candidemia incidences were generally lower among studies of anti-septic interventions as there was a lower proportion with respect to each of the following versus other study categories: mean length of stay more than 7 days, trauma ICU’s, patient selection for CRF and use of MV for > 48 h.
Summary effect sizes
Among the summary prevention effects, the strongest was for antibiotic-based interventions versus Pseudomonas VAP (odds ratio 0.33; 95% CI; 0.26 to 0.42) (Table 1, Additional file 1: Fig S3). In the prevention of candidemia, the indicative summary effect size for the SAF and antibiotic-based interventions were similar. All other summary effect estimates for all other interventions versus the VAP, bacteremia, or candidemia end-points were either weaker or not significant (Table 1; Additional file 1: Table S6; Fig S3–S6). Of note, surprisingly, no significant prevention effect for Pseudomonas bacteremia was apparent for any intervention (Table 1; Additional file 1: Fig S4).
GSEM modelling
Three GSEM models of the relationship between various group level exposures on Candida and Pseudomonas colonization as latent variables were evaluated for fit and parsimony (Table 2; Additional file 1: Fig S7–S8; Fig. 4). The introduction of firstly Candida colonization as a cofactor towards Pseudomonas colonization (Model C to Model B), and then, addition to the model of membership of a concurrent control group within a study of an antibiotic-based interventions as an indicator variable (Model B to Model A), each improved the model fit towards the optimal model (Fig. 4).
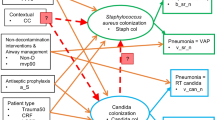
GSEM of the interaction model in relation to Pseudomonas and Candida infection data. Candida col and Pseudomonas col (ovals) are latent variables representing Candida and Pseudomonas colonization, respectively. The variables in rectangles are binary predictor variables representing the group level exposure to the following; patient selection for candidemia risk factors (CRF); trauma ICU setting (trauma50), mean or median length of ICU stay ≥ 7 days (los7), exposure to a topical anti-septic (a_S), exposure to TAP (tap), concurrency of a control group with an antibiotic-based intervention group (CC), exposure to a non-decontamination based prevention method (non-D), use of mechanical ventilation for more than 90% (mvp90) or exposure to PPAP (ppap). Note that the model factorizes exposures from compound regimens (e.g. SDD and SOD, which combine TAP, an antifungal together with or without PPAP) into singleton TAP, PPAP and anti-fungal exposures. The circles contain error terms. The three part boxes represent the binomial data for Candida and Pseudomonas VAP (v_can_n, v_ps_n) and candidemia (b_can_n) or bacteremia (b_ps_n) counts with the number of patients as the denominator which is logit transformed using the logit link function in the generalized model
In the optimal model (Model A), the coefficients for singular exposure to anti-septic agents (− 1.23; − 2.1 to − 0.32), amphotericin (− 1.78; − 2.79 to − 0.78) and topical antibiotic prophylaxis (TAP; + 1.02; + 0.11 to + 1.93) versus Candida colonization were similar in magnitude but contrary in direction.
In all models, group wide exposure to CRF, anti-septics and singular exposures to each of TAP and the antifungals, although less so for nystatin, displayed strong and significant associations with the Candida colonization latent variable, and these were generally consistent across the three models. By contrast, the same exposures versus Pseudomonas colonization were generally weaker, less consistent between models and variably significant.
Of note, the size of the association between Pseudomonas colonization and, on the one hand, membership of a concurrent control group within a study of antibiotic-based interventions, and on the other, with exposure to TAP, were similar in magnitude but contrary in direction and significance. By contrast, the size of these two factors on Candida colonization were similar in direction but differed in magnitude and significance.
Post GSEM modelling predictions
Post model predictions of Pseudomonas bacteremia and Candidemia incidences were estimated for a putative group of non-trauma ICU patients with group mean LOS greater than seven days and without patient selection for CRF. Predictions were made for various combination and singleton group wide exposures to anti-septic agents, TAP, PPAP, nystatin and amphotericin versus a benchmark derived for an equivalent putative non-concurrent control group (Fig. 5). In every case, singleton exposure to either amphotericin or to anti-septics outperformed singleton exposure to TAP towards lower predicted bacteremia incidences. Exposure to TAP combined with amphotericin, but not nystatin, was associated with significantly lower predicted bacteremia incidences versus benchmark.
a, b Model predictions derived from model A (Fig. 4) for the incidence proportions of Pseudomonas bacteremia (a), and candidemia (b) for a putative group of patients in a non-trauma ICU with mean LOS > 7 days without selection for CRF. The projections are for control (top) or intervention (bottom panel) groups receiving prophylaxis with various singleton or combination interventions. In each plot, the benchmark proportion (solid vertical line) is the mean prediction derived for an equivalent NCC group without exposures. NCC non-concurrent control, CC concurrent control, non-D non-decontamination, a_s anti-septic, TAP topical antibiotic prophylaxis, amb amphotericin, ny nystatin, ppap protocolized parenteral antibiotic prophylaxis
Discussion
There were three objectives here. First, the study level evidence for various ICU infection prevention interventions toward preventing VAP and BSI derived here broadly recapitulates prior summary estimates among systematic reviews in the literature (Additional file 1: Table S6) [9,10,11,12,13,14,15,16,17]. The effect sizes are indicative as each category includes broadly selected studies, with some having compound interventions, such as TAP combined with an anti-fungal, together with or without PPAP given to either or both of control and intervention groups in antibiotic-based studies. The selection of studies in the summaries here is congruous with that in the literature summaries. Of note, the concurrent control groups within antibiotic-based intervention studies have unusually high incidences for several end-points that are unexplained in previous meta-regression models, taking into account several other group level variables not included here, such as year of publication, and likely represents a strong contextual effect [29, 30].
The second objective is the development of GSEM models. The optimal model includes both the postulated interaction between Candida and Pseudomonas colonization and the contextual influences of TAP on concurrent control groups. In confronting the optimal GSEM model with published group level infection data, the anti-fungal agents, such as azoles and amphotericin, and the anti-septic agents, each showed strong prevention effects versus Candida colonization. By contrast, TAP as a singular exposure versus Pseudomonas colonization and versus Candida colonization demonstrated effects that were weaker and variable in direction and significance.
Thirdly, in post GSEM model predictions, the estimated effects of singular exposure to topical amphotericin and anti-septic agents would each more than halve the incidence of candidemia and Pseudomonas bacteremia, although for absolute differences being approximately one percentage point or less. These differences would be challenging to detect. For example, a cluster-randomized trial demonstrating halving in Pseudomonas bacteremia incidence from 1% in the control group to 0.5% in the intervention group would need to enrol over 2000 ICU’s each providing 500 patients per arm to provide 80% power.
By contrast, singular TAP exposure, with or without PPAP in combination, was either neutral or promoted Pseudomonas bacteremia and candidemia incidence. Of note, the most common PPAP among SDD studies is cefotaxime, which is not generally active against Pseudomonas. That antibiotic exposure in the ICU environment paradoxically promotes Pseudomonas acquisition is a finding with precedent [31, 32]. Moreover, bacterial colonization rebounds following cessation of TAP with effects on the whole-of-ICU population [33]. Whether rebound contributes to colonization pressure, and the contextual effects of TAP exposure, needs to be further examined [34].
The observations here are paradoxical. On the one hand, antibiotic-based interventions (being in combination with anti-fungal agents as used within studies of SDD) appear to show strong prevention effects against VAP that is most apparent for Pseudomonas VAP together with the appearance of a halving in candidemia among studies of antibiotic-based interventions.
On the other hand, despite these two strong prevention effects of antibiotic-based interventions, there is insignificant prevention of Pseudomonas bacteremia. Moreover, the incidences of candidemia and Pseudomonas bacteremia are generally higher among the concurrent control groups of antibiotic-based studies and reflect the contextual effects.
The interaction underlying these paradoxical observations, potentially explains how ICU-acquired gram-negative bacteremia might occur seemingly without preceding colonization [18, 35, 36]. They also could account for the bacteremia prevention effects observed in large studies of antibiotic-based interventions of SDD regimens containing topical polymyxin and tobramycin combined with amphotericin as the anti-fungal [20, 21], whereas the largest SDD study, where the same TAP regimen was combined with nystatin [22], failed to demonstrate any prevention effects against any bacteremia, or candidemia, end-points.
The SEM technique is an emerging method among critical care and infection pathogenesis research that enables group level modelling of multiple simultaneously observed variables [37, 38]. This technique enables the testing of causal concepts that might underlie relationships between observed variables mediated through latent variables. GSEM allows generalized linear response functions in addition to the linear response functions allowed by non-generalized SEM. A strength of GSEM modelling is the ability to incorporate observations from clusters with missing observations under the assumption of missing at random. This enables the inclusion of groups from studies either lacking control groups or providing data for only some end-points.
Limitations
There are multiple limitations and cautions in the interpretation of the modelling here. The GSEM is a group level modelling of two latent variables, Candida and Pseudomonas within a postulated model of interaction. These latent variables and the coefficients derived in the GSEM are indicative only. They have no counterpart at the level of any one patient or study and cannot be directly measured.
The second limitation is that there was no ability nor purpose to adjust for the underlying patient level risk. There was considerable heterogeneity in the interventions, populations, and study designs among the studies, which were conducted up to three and a half decades ago, meeting intentional broad inclusion criteria. This stands in contrast to the technique of network meta-analysis (NMA) applied to data obtained from studies selected according to tight inclusion criteria towards satisfying transitivity assumptions. With transitivity, a NMA enables comparisons of multiple interventions as allocated to comparable patient groups within randomized controlled trials towards estimating patient level effects on a defined end point from study level data. By contrast, the GSEM technique enables causal modelling of group-level associations towards deriving group level inferences among patient groups experiencing compound exposures within studies assembled with less stringent inclusion criteria. This allows the inclusion of concurrent control groups from antibiotic-based studies where contextual effects invalidate the transitivity assumption.
The third limitation is that the GSEM model is deliberately simplistic. There are only limited numbers of key group level factors, the exposures are entered as binary variables, and with interaction between the latent variables being the only interactions tested. In reality, the relationships between expoures and outcomes will likely be graded and complex with potentially compound expoure interactions.
The impact of anti-fungal prophylaxis on Pseudomonas colonization and infection is the only bacterial species examined here. However, other bacterial species, such as Acinetobacter warrant examination given the observed paradoxical incidences that occur in association within studies of topical antibiotic prophylaxis [39, 40].
Finally, the various regimens of antibiotic-based, anti-septic and anti-fungal interventions used within the various studies have been considered as similar within each category. This is a deliberate simplification. For example, some SAF interventions were administered parenterally rather than topically. In addition, the intensity and duration of application, and the body site targeted by the various interventions, varied among the studies and have not been modelled. On the other hand, a strength of this analysis is that the various compound interventions, as for example within SDD regimens comprising TAP, PPAP, and anti-fungal components, are factorized towards estimating their separate singleton associations on the latent variables within the GSEM model.
Conclusion
GSEM modelling of Pseudomonas and candida colonization, each as latent variables versus group level exposures, demonstrates complex and paradoxical relationships that would not be apparent in any single study examined in isolation nor within a summary effect of the collective studies as derived by conventional meta-analytic modelling. The model provides support to the postulated interaction between candida and Pseudomonas colonization in facilitating invasive Pseudomonas infections. Anti-fungal interventions have potentially stronger prevention impact on Pseudomonas bacteremia, mediated via candida colonization, than does singleton TAP or PPAP exposures, which either have no impact or which promote Pseudomonas bacteremia.
Availability of data and materials
All data generated or analysed during this study are included in this published article and the online Additional material (ESM).
Abbreviations
- BSI:
-
Blood stream infection
- CRF:
-
Candida risk factors
- ICU:
-
Intensive care unit
- MV:
-
Mechanical ventilation
- SAF:
-
Single anti-fungal
- VAP:
-
Ventilator associated pneumonia
- PPAP:
-
Protocolized parenteral antibiotic prophylaxis
- RT Candida:
-
Respiratory tract Candida
- SDD:
-
Selective digestive decontamination
- TAP:
-
Topical antibiotic prophylaxis
- GSEM:
-
Generalized structural equation models
References
Bergeron AC, Seman BG, Hammond JH, Archambault LS, Hogan DA, Wheeler RT (2017) Candida albicans and Pseudomonas aeruginosa interact to enhance virulence of mucosal infection in transparent zebrafish. Infect Immun 85(11):e00475-e517
Grainha T, Jorge P, Alves D, Lopes SP, Pereira MO (2020) Unravelling Pseudomonas aeruginosa and Candida albicans communication in coinfection scenarios: insights through network analysis. Front Cell Infect Microbial. https://doi.org/10.3389/fcimb.2020.550505
Albert M, Williamson D, Muscedere J, Lauzier F, Rotstein C, Kanji S et al (2014) Candida in the respiratory tract secretions of critically ill patients and the impact of antifungal treatment: a randomized placebo controlled pilot trial (CANTREAT study). Intensive Care Med 40:1313–1322
Lindau S, Nadermann M, Ackermann H, Bingold TM, Stephan C, Kempf VA et al (2015) Antifungal therapy in patients with pulmonary Candida spp. colonization may have no beneficial effects. J Intensive Care 3(1):31
Timsit JF, Schwebel C, Styfalova L, Cornet M, Poirier P, Forrestier C et al (2019) Impact of bronchial colonization with Candida spp. on the risk of bacterial ventilator-associated pneumonia in the ICU: the FUNGIBACT prospective cohort study. Intensive Care Med 45(6):834–843
Azoulay E, Timsit JF, Tafflet M, de Lassence A, Darmon M, Zahar JR et al (2006) Candida colonization of the respiratory tract and subsequent pseudomonas ventilator-associated pneumonia. Chest 129(1):110–117
Nseir S, Jozefowicz E, Cavestri B, Sendid B, Di Pompeo C, Dewavrin F et al (2007) Impact of antifungal treatment on Candida–Pseudomonas interaction: a preliminary retrospective case–control study. Intensive Care Med 33(1):137–142
Huang D, Qi M, Hu Y, Yu M, Liang Z (2019) The impact of Candida spp. airway colonization on clinical outcomes in patients with ventilator-associated pneumonia: a systematic review and meta-analysis. Am J Infect Control. https://doi.org/10.1016/j.ajic.2019.11.002
Minozzi S, Pifferi S, Brazzi L, Pecoraro V, Montrucchio G, D’Amico R (2021) Topical antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving mechanical ventilation. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD000022.pub4
Pileggi C, Bianco A, Flotta D, Nobile CG, Pavia M (2011) Prevention of ventilator-associated pneumonia, mortality and all intensive care unit acquired infections by topically applied antimicrobial or antiseptic agents: a meta-analysis of randomized controlled trials in intensive care units. Crit Care 15:R155
Silvestri L, Van Saene HK, Casarin A, Berlot G, Gullo A (2008) Impact of selective decontamination of the digestive tract on carriage and infection due to Gram-negative and Gram-positive bacteria: a systematic review of randomised controlled trials. Anaesth Intensive Care 36(3):324–338
Hurley JC (1995) Prophylaxis with enteral antibiotics in ventilated patients: Selective decontamination or selective cross-infection? Antimicrob Agents Chemother 39:941–947
Silvestri L, Van Saene HK, Milanese M, Gregori D, Gullo A (2007) Selective decontamination of the digestive tract reduces bacterial bloodstream infection and mortality in critically ill patients. Systematic review of randomized, controlled trials. J Hosp Infect 65(3):187–203
Silvestri L, Weir WI, Gregori D, Taylor N, Zandstra DF, van Saene JJ, van Saene HK (2017) Impact of oral chlorhexidine on bloodstream infection in critically ill patients: systematic review and meta-analysis of randomized controlled trials. J Cardiothorac Vasc Anesth 31(6):2236–2244
Labeau SO, Van de Vyver K, Brusselaers N, Vogelaers D, Blot SI (2011) Prevention of ventilator-associated pneumonia with oral antiseptics: a systematic review and meta-analysis. Lancet Infect Dis 11:845–854
Klompas M, Speck K, Howell MD, Greene LR, Berenholtz SM (2014) Reappraisal of routine oral care with chlorhexidine gluconate for patients receiving mechanical ventilation: systematic review and meta-analysis. JAMA Intern Med 174(5):751–761
Silvestri L, Van Saene HK, Milanese M, Gregori D (2005) Impact of selective decontamination of the digestive tract on fungal carriage and infection: systematic review of randomized controlled trials. Intensive Care Med 31:898–910
Hurley JC (2021) Selective digestive decontamination, a seemingly effective regimen with individual benefit or a flawed concept with population harm? Crit Care 25(1):1
Stoutenbeek CP, Van Saene HK, Miranda DR et al (1984) The effect of selective decontamination of the digestive tract on colonisation and infection rate in multiple trauma patients. Intensive Care Med 10:185–192
de Smet AMGA, Kluytmans JAJW, Cooper BS, Mascini EM, Benus RFJ, van der Werf TS, van der Hoeven JG, Pickkers P, Bogaers-Hofman D, van der Meer NJ, Bernards AT, Kuijper EJ, Joore JC, Leverstein-van Hall MA, Bindels AJ, Jansz AR, Wesselink RM, de Jongh BM, Dennesen PJ, van Asselt GJ, te Velde LF, Frenay IH, Kaasjager K, Bosch FH, van Iterson M, Thijsen SF, Kluge GH, Pauw W, de Vries JW, Kaan JA, Arends JP, Aarts LP, Sturm PD, Harinck HI, Voss A, Uijtendaal EV, Blok HE, Thieme Groen ES, Pouw ME, Kalkman CJ, Bonten MJ (2009) Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med 360:20–31
Oostdijk EAN, Kesecioglu J, Schultz MJ et al (2017) Notice of retraction and replacement: Oostdijk et al (2014) Effects of decontamination of the oropharynx and intestinal tract on antibiotic resistance in ICUs: a randomized clinical trial. JAMA 312(14):1429–1437
Wittekamp BH, Plantinga NL, Cooper BS et al (2018) Decontamination strategies and bloodstream infections with antibiotic-resistant microorganisms in ventilated patients: a randomized clinical trial. JAMA 320:2087–2098
Hurley JC (2015) ICU-acquired candidemia within selective digestive decontamination studies: a meta-analysis. Intensive Care Med 41(11):1877–1885
Hurley JC (2020) Structural equation modelling the ‘control of gut overgrowth’ in the prevention of ICU acquired Gram-negative infection. Crit Care 24:189
Hurley JC (2016) Impact of selective digestive decontamination on respiratory tract Candida among patients with suspected ventilator-associated pneumonia. A meta-analysis. Eur J Clin Microbiol Infect Dis 35(7):1121–1135
Goodman LA (1961) Snowball sampling. Ann Math Stat. https://doi.org/10.1214/aoms/1177705148
Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, Mantel N, McPherson K, Peto J, Smith PG (1977) Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. Analysis and examples. Br J Cancer 35:1–39. https://doi.org/10.1038/bjc.1977.1
Stata corporation (2021) Stata structural equation modelling reference manual, in Stata 17 documentation. College Station, TX, USA. https://www.stata.com/bookstore/structural-equation-modeling-reference-manual/. Accessed 6 July 2021.
Hurley JC (2018) Incidences of Pseudomonas aeruginosa-associated ventilator-associated pneumonia within studies of respiratory tract applications of polymyxin: testing the Stoutenbeek concurrency postulates. Antimicrob Agents Chemother 62(8):e00291-e318
Hurley JC (2018) Unusually high incidences of Pseudomonas bacteremias within topical polymyxin based decolonization studies of mechanically ventilated patients: benchmarking the literature. Open Forum Infect Dis 5(11):ofy256
Venier AG, Leroyer C, Slekovec C, Talon D, Bertrand X, Parer S, Alfandari S, Guerin JM, Megarbane B, Lawrence C, Clair B (2014) Risk factors for Pseudomonas aeruginosa acquisition in intensive care units: a prospective multicentre study. J Hosp Infect 88(2):103–108
Hoang S, Georget A, Asselineau J, Venier AG, Leroyer C, Rogues AM, Thiébaut R (2018) Risk factors for colonization and infection by Pseudomonas aeruginosa in patients hospitalized in intensive care units in France. PLoS ONE 13(3):e0193300
Tetteroo GWM, Wagenvoort JHT, Bruining HA (1994) Bacteriology of selective decontamination: efficacy and rebound colonization. J Antimicrob Chemother 34:139–148
Boyer A, Doussau A, Thiébault R, Venier AG, Tran V, Boulestreau H, Bébéar C, Vargas F, Hilbert G, Gruson D, Rogues AM (2011) Pseudomonas aeruginosa acquisition on an intensive care unit: relationship between antibiotic selective pressure and patients’ environment. Crit Care 15(1):1
Frencken JF, Wittekamp BH, Plantinga NL, Spitoni C, van de Groep K, Cremer OL, Bonten MJ (2017) Associations between enteral colonization with gram-negative bacteria and intensive care unit-acquired infections and colonization of the respiratory tract. Clin Infect Dis 66(4):497–503
Oostdijk EA, de Smet AM, Kesecioglu J, Bonten MJ (2011) The role of intestinal colonization with gram-negative bacteria as a source for intensive care unit-acquired bacteremia. Crit Care Med 39(5):961–966
Carver S, Beatty JA, Troyer RM, Harris RL, Stutzman-Rodriguez K, Barrs VR et al (2015) Closing the gap on causal processes of infection risk from cross-sectional data: structural equation models to understand infection and co-infection. Parasites Vectors 8(1):658
Bojan M, Duarte MC, Ermak N, Lopez-Lopez V, Mogenet A, Froissart M (2016) Structural equation modelling exploration of the key pathophysiological processes involved in cardiac surgery-related acute kidney injury in infants. Crit Care 20(1):1
Hurley JC (2020) Candida–Acinetobacter–Pseudomonas interaction modelled within 286 ICU infection prevention studies. J Fungi. 6(4):252
Hurley JC (2018) Paradoxical Acinetobacter associated ventilator associated pneumonia incidences within prevention studies using respiratory tract applications of topical polymyxin: benchmarking the evidence base. J Hosp Infect 100:105–113
Acknowledgements
Not applicable.
Funding
This work was supported by the Australian Government Department of Health and Ageing through the Rural Clinical Training and Support (RCTS) program.
Author information
Authors and Affiliations
Contributions
As sole author, JH produced the design of the study, collated the data, performed the statistical analysis and wrote the manuscript. JH read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable for a modelling analysis.
Consent for publication
Not applicable.
Competing interests
The author declares that he has no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Observational studies (Benchmark groups). Table S2. Groups of non-decontamination studies. Table S3. Groups of anti-septic studies. Table S4. Groups of antibiotic-based (= TAP ± PPAP ± antifungal) studies. Table S5. Groups from single drug anti-fungal (SAF) studies. Table S6. Review of effect sizes in the literature. Fig S1. a & b. Pseudomonas; VAP and bacteremia count data. Fig S2. a & b. Candidemia and RT candida count data. Fig S3. Effect size VAP Pseudomonas. Fig S4. Effect size Pseudomonas bacteremia. Fig S5. Effect size RT candida. Fig S6. Effect size Candidemia. Figs. S7, S8. GSEM model C & GSEM model B.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hurley, J.C. Structural equation modelling the relationship between anti-fungal prophylaxis and Pseudomonas bacteremia in ICU patients. ICMx 10, 2 (2022). https://doi.org/10.1186/s40635-022-00429-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40635-022-00429-8