Abstract
Purpose
This technical note describes a reconstructive technique of the distal tibial articular surface using autologous iliac crest bone graft.
Methods
Following curettage and high-speed burring of giant cell tumor of bone (GCTB) of the distal tibial articular surface, the resulting cavity was filled, and the articular surface was reconstructed using autologous tricortical iliac crest bone graft. The graft was fixed to the tibia with a plate.
Results
The smooth congruent articulating surface of the distal tibia was restored. Full ankle range of motion was achieved. No recurrence was detected in the follow-up imaging.
Conclusions
The currently reported technique using autologous tricortical iliac crest bone graft is a viable option for reconstructing the articular surface of the distal tibia.
Similar content being viewed by others
Introduction
Giant cell tumor of bone (GCTB) is a benign locally aggressive tumor representing 5% of all primary bone tumors [22]. This tumor rarely metastasizes, but it has a high tendency for local recurrence [10, 20].
GCTB usually occurs in patients aged 20–40 and commonly involves the epi-metaphysis of long bones and may compromise the articular surface integrity [7, 9].
The distal femur, proximal tibia, distal radius, and sacrum are the most common sites of GCTB [3]. Foot and ankle involvement with GCTB is uncommon, accounting for less than 4% of all GCTBs [15].
Treatment of GCTB should aim for local control without sacrificing joint function [9, 21]. The functional preserving surgery for GCTB is extended curettage with high-speed burring and chemical adjuvants such as liquid nitrogen, alcohol, or phenol and filling the resulting cavity with polymethylmethacrylate (PMMA) bone cement, bone substitutes, or bone graft [9, 18, 20].
In advanced cases where joint salvage is not feasible, en-bloc resection and endoprosthetic reconstruction may be an option but may result in increased morbidity and unfavorable functional outcomes in GCTB patients, who are frequently young and active [11, 19].
The incidence of GCTB in the distal tibia is rare [13, 15]. Management of tumors of distal tibia after curettage or resection varies depending on plenty of influencing factors, and the reported options include allograft reconstruction, ankle fusion or endoprosthetic reconstruction [1, 16, 17].
This article reports a reconstructive technique for the ankle joint after curettage and high-speed burring of GCTB using an autologous iliac crest bone graft to reconstruct the articulating part of the distal tibia.
Operative technique
The technique presented in this article was approved by our Institutional Review Board (IRB) (IRB number: ORTH14-2) for reconstructing the ankle joint with an autologous tricortical iliac crest bone graft after curettage of GCTB of the distal tibia destructing the articular surface.
1-Preoperative evaluation
Proper history taking is crucial, including the duration of symptoms. General and local ankle examinations should be performed, including examination of the swelling if present, the site of tenderness and ankle range of motion. Imaging studies in the form of ankle X-rays, computed tomography (CT), and magnetic resonance imaging (MRI) are done for provisional diagnosis and proper assessment of tumor characteristics, Fig. 1. The size and extent of the osteolytic lesion should be precisely measured in the CT and MRI with the evaluation of cortical breach and extra-osseous tissue extension. Tumors should be classified using the Campanacci radiographic grading [5]. CT-guided biopsy should be obtained to confirm the diagnosis of GCTB.
A 22-year-old female presented with limping, pain aggravated on walking, and restricted movement of the left ankle for 7 months. The patient had no history of trauma and no constitutional symptoms. Examination revealed tenderness over the anterior aspect of the distal tibia, with painful and restricted range of motion of the left ankle. A Preoperative anteroposterior and lateral X-rays showing a well-defined expansile osteolytic lesion in the epi-metaphyseal region of the left distal tibia suggestive of GCTB. The tumor was classified as Campanacci grade III. B Sagittal, coronal and axial CT scans showing an osteolytic lesion occupying the anterior two-thirds of the epi-metaphyseal region of the left distal tibia with erosion of the articular surface. C Sagittal and coronal MRI images showing an eccentric expansile osteolytic lesion measuring 4 (craniocaudal) × 3.5 (transverse) × 2.5 (anteroposterior) cm breaching the articular surface with only the posterior one-third of the tibial plafond preserved with no soft tissue or intra-articular extension
2-Set‑up
The patient is placed supine under spinal anesthesia. Prophylactic 3rd generation cephalosporin antibiotic should be administrated, and an above-knee tourniquet should be used.
3-Surgical approach
Sterile precautions and careful soft tissue handling are crucial. A standard anterior ankle approach is performed. A midline anterior longitudinal incision is made over the ankle. Subcutaneous tissue is dissected, and the extensor retinaculum is incised in line with the skin incision. The extensor hallucis longus tendon and the neurovascular bundle are retracted medially, and the extensor digitorum longus tendon is retracted laterally. The joint capsule is then opened. Bone is exposed, and a cortical window is made if the cortical bone is intact.
4-Curettage and high-speed burring
Curettage is done by different-sized bone curettes. A high-speed burr is then used to extend the curettage beyond the tumor margin. A thorough wash with H2O2 and saline solutions is done. After curettage, the resulting cavity should be measured in order to obtain a matched-sized tricortical iliac crest bone graft.
5-Tricortical iliac crest bone graft harvesting
Ipsilateral tricortical iliac crest bone graft is harvested in the usual manner. The graft size should be based on the size of the defect, and it must be large enough to fully reconstruct the bone defect. After appropriate size calculation, the bone graft is cut using an oscillating saw.
6‑Reconstruction of the distal tibial defect
The tricortical iliac crest bone graft is then prepared and fashioned using a bone nibbler to adapt to the cavity and the articular surface. The graft is placed and impacted in the cavity in a reversed manner so that the iliac crest cartilaginous cap reconstructs the articular surface. A space of 2 mm is left between the graft and the articular cartilage of the talus. A suitable-sized anterior plate, such as a distal radial plate, is then used to fix the graft to the tibia, Fig. 2. C-arm photos are obtained. A drain is placed, and the wound is closed. The specimen should be sent to histopathology for further examination.
Intraoperative photographs A) Curettage of the lesion using different-sized curettes. B The cavity after curettage and high-speed burring with exposed articular cartilage of the talus. C The harvested tricortical iliac crest autograft. D Testing the shape and size of the graft in relation to the defect. E Placing the graft in the defect after trimming and fashioning. F Fixation of the graft to the tibia using a distal radius plate
7-Postoperative follow-up and rehabilitation
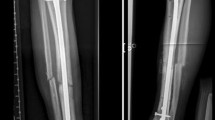
The operated leg should be placed in a below-knee splint. Immediate postoperative ankle X-rays are obtained, Fig. 3. The drain is removed after 24 h. The splint and the stitches should be removed in 2–3 weeks. Thereafter, the patient is instructed to perform ankle flexion–extension range of motion exercises, with no weight bearing for the first 6 weeks postoperatively. Partial weight bearing as tolerated is allowed in the second 6 weeks postoperatively. Full weight bearing should commence 3 months postoperatively.
Regular follow-up visits should include clinical examination and ankle X-rays and CT scans to evaluate the union of the graft and the reconstruction of the articular surface and to detect any tumor recurrence, Figs. 4 and 5. It is recommended to have X-rays every 6 months for the first 2 years, and then annually till 5 years.
Six months follow-up radiological images showing complete union of the graft and reconstruction of the distal tibial articular surface. No donor site morbidity symptoms were reported. Clinical photos showing pain-free full range of motion of the ankle joint. A Anteroposterior and lateral plain X-rays. B Sagittal CT cuts. C Coronal CT cuts. D Axial CT cuts. E Ankle dorsiflexion. F Ankle plantar flexion
Discussion
GCTB should be considered a differential diagnosis in all lytic bone lesions. While GCTBs usually occur in the distal femur, the proximal tibia, the distal radius, and the sacrum, they can also occur in rare locations, such as the distal tibia [3, 15].
Management of GCTB of the distal tibia is challenging, especially for tumors encroaching on the articular surface [15]. Moreover, having inadequate soft tissue coverage in the ankle may increase the risk of poor wound healing, especially with multiple surgeries; therefore, it is essential to avoid recurrence [15].
Several management options are reported in the literature. In their retrospective study of 31 patients with GCTB of the distal tibia, AlSulaimani et al. [2] recommended extended curettage for adequate control of the tumor. Despite having a 29% rate of local recurrence, recurrences were manageable with repeated curettage [2].
Paul et al. [15] reported 8 (42.1%) cases with GCTB of the distal tibia out of 19 patients with GCTBs of the foot and ankle over 9 years, treated with excision and bone graft (n = 3), extended curettage and bone graft (n = 2), and excision and mega prosthesis (n = 2), extended curettage and bone cement (n = 1). Wound infection (n = 2) and chronic osteomyelitis (n = 1) were reported as complications in patients who had previous surgeries. No local recurrence was detected after the index surgery in a mean follow-up of 36.2 months [15]. Previous studies reported no recurrence following intralesional curettage with filling the cavity with bone cement in patients with GCTB of the distal tibia [4, 13, 14].
Cribb et al. [6] reported a technique of curettage, high-speed burring, and ankle stabilization using Ilizarov fixator and reported excellent functional results with no tumor recurrence. Saglik et al. [16] described distal tibial resection and ankle arthrodesis using fibular autograft and reported pain-free daily activities. Economopoulos et al. [8] described ankle arthrodesis using a custom-made porous tantalum spacer and reported pain-free walking and no recurrence. Wiratnaya [23] reported a technique of wide-margin resection followed by tibialisation of fibula and ankle arthrodesis as an alternative option, with good functional outcomes and with no complications over 2 years of follow-up.
However, the drawback of ankle arthrodesis is the elimination of the ankle range of motion which is critical in those active young patients in their work years [17]. Moreover, the endoprosthetic replacement of the ankle joint has unpredictable long-term outcomes and implant survival [1].
Osteoarticular allograft reconstruction is another reported option, but it has drawbacks, including infection, graft lysis, and osteoarthritis [17, 24].
In the case of articular surface destruction, an autologous tricortical iliac crest bone graft can be used with the technical considerations reported in this technical note. This technique is an excellent option to fill the cavity and achieve anatomical restoration of the articular surface while preserving the ankle range of motion. Furthermore, curettage and high-speed burring and filling the resulting cavity with autografts give excellent results and reduce the chances of recurrence. The drawbacks of using autografts include donor site morbidity such as infection, residual scar, pain, or sensory changes [12]. Additionally, there is difficulty in detecting recurrence in X-rays with grafts in place. Further clinical studies are needed to validate the long-term efficacy and functional outcomes of the current technical note.
Conclusion
In the setting of GCTB destroying the articular surface of the distal tibia, the current technical description of autologous tricortical iliac crest bone graft is sufficient to fill the resulting cavity after curettage and high-speed burring and could restore the smooth congruent articulating surface of the distal tibia, with preservation of the range of motion.
Availability of data and materials
The dataset used in this study is available from the corresponding author on request.
References
Ajit Singh V, Nasirudin N, Bernatt M (2013) Endoprosthetic reconstruction for giant cell tumors of the distal tibia: a short term review. Asia Pac J Clin Oncol 9(2):182–189. https://doi.org/10.1111/j.1743-7563.2012.01553.x
AlSulaimani SA, Turcotte RE, Canadian Orthopaedic Oncology Society c (2013) Iterative curettage is associated with local control in giant cell tumors involving the distal tibia. Clin Orthop Relat Res 471(8):2668–2674. https://doi.org/10.1007/s11999-013-2965-z
AlYami AH, Nazer A, Bashawieh HH, Dabroom AA, SaemAldahar M, AlYami AA, AlMaeen BN (2022) Outcomes in bone giant cell tumors treated with surgical resection with and without Denosumab injection: a single-institution retrospective study. Cureus. 14(7):e26869. https://doi.org/10.7759/cureus.26869
Bami M, Nayak AR, Kulkarni S, Kulkarni A, Gupta R (2013) Giant cell tumor of lower end of tibia. Case Rep Orthop. 2013:429615. https://doi.org/10.1155/2013/429615
Campanacci M, Baldini N, Boriani S, Sudanese A (1987) Giant-cell tumor of bone. J Bone Joint Surg Am 69(1):106–114
Cribb GL, Cool P, Hill SO, Mangham DC (2009) Distal tibial giant cell tumour treated with curettage and stabilisation with an Ilizarov frame. Foot Ankle Surg 15(1):28–32. https://doi.org/10.1016/j.fas.2008.04.003
Ebeid WA, Badr IT, Mesregah MK, Hasan BZ (2021) Risk factors and oncological outcomes of pulmonary metastasis in patients with giant cell tumor of bone. J Clin Orthop Trauma 20:101499. https://doi.org/10.1016/j.jcot.2021.101499
Economopoulos K, Barker L, Beauchamp C, Claridge R (2010) Case report: reconstruction of the distal tibia with porous tantalum spacer after resection for giant cell tumor. Clin Orthop Relat Res 468(6):1697–1701. https://doi.org/10.1007/s11999-009-1097-y
Gundavda MK, Agarwal MG (2021) Extended curettage for giant cell tumors of bone: a surgeon’s view. Essent Surg Tech 11(3):e20.00040. https://doi.org/10.2106/JBJS.ST.20.00040
Hasan O, Ali M, Mustafa M, Ali A, Umer M (2019) Treatment and recurrence of giant cell tumors of bone - a retrospective cohort from a developing country. Ann Med Surg (Lond) 48:29–34. https://doi.org/10.1016/j.amsu.2019.10.010
He H, Zeng H, Luo W, Liu Y, Zhang C, Liu Q (2019) Surgical treatment options for giant cell tumors of bone around the knee joint: extended curettage or segmental resection? Front Oncol 9:946. https://doi.org/10.3389/fonc.2019.00946
Hill NM, Horne JG, Devane PA (1999) Donor site morbidity in the iliac crest bone graft. Aust N Z J Surg 69(10):726–728. https://doi.org/10.1046/j.1440-1622.1999.01674.x
Mohapatra AR, Choudhury P, Patel PS, Malhotra RS, Patil AB (2018) An unusual case of giant cell tumor of the distal tibia. J Orthop Case Rep 8(4):29–31. https://doi.org/10.13107/jocr.2250-0685.1144
Osman W, Jerbi M, Ben Abdelkrim S, Maaref K, Ben Maitigue M, Ben Ayeche ML (2015) Giant cell tumor of the lower end of tibia. Curettage and cement reconstruction. Foot Ankle Surg 21(1):e16-20. https://doi.org/10.1016/j.fas.2014.09.002
Paul AJ, Shreemal BB, Titus VTK (2021) Foot and ankle giant cell tumors are not so aggressive after all: a retrospective study. J Foot Ankle Surg 60(1):176–181. https://doi.org/10.1053/j.jfas.2020.07.004
Saglik Y, Yildiz Y, Atalar H, Gunay C (2008) The use of fibular autograft and ankle arthrodesis for aggressive giant cell tumor in the distal tibia: a case report. Foot Ankle Int 29(4):438–441. https://doi.org/10.3113/FAI.2008.0438
Sambri A, Dalla Rosa M, Scorianz M, Guido D, Donati DM, Campanacci DA, De Paolis M (2020) Different reconstructive techniques for tumours of the distal tibia. Bone Joint J 102-B(11):1567–1573. https://doi.org/10.1302/0301-620X.102B11.BJJ-2020-0127.R1
Tripathy P, Bansal MC, Upadhyay R (2022) A Surgical approach to giant cell tumor of lower end of tibia with curettage and reconstruction by bone grafting harvested from left iliac crest and k-wire fixation: a case report. Eur J Med Health Sci 4(1):1–3
van der Heijden L, Dijkstra S, van de Sande M, Gelderblom H (2020) Current concepts in the treatment of giant cell tumour of bone. Curr Opin Oncol 32(4):332–338. https://doi.org/10.1097/CCO.0000000000000645
van der Heijden L, Lipplaa A, van Langevelde K, Bovee J, van de Sande MAJ, Gelderblom H (2022) Updated concepts in treatment of giant cell tumor of bone. Curr Opin Oncol 34(4):371–378. https://doi.org/10.1097/CCO.0000000000000852
van der Heijden L, Dijkstra PD, van de Sande MA, Kroep JR, Nout RA, van Rijswijk CS, Bovee JV, Hogendoorn PC, Gelderblom H (2014) The clinical approach toward giant cell tumor of bone. Oncologist 19(5):550–561. https://doi.org/10.1634/theoncologist.2013-0432
Vari S, Riva F, Onesti CE, Cosimati A, Renna D, Biagini R, Baldi J, Zoccali C, Anelli V, Annovazzi A, Covello R, Ascione A, Casini B, Ferraresi V (2022) Malignant transformation of giant cell tumour of bone: a review of literature and the experience of a referral centre. Int J Mol Sc. 23(18):10721
Wiratnaya IGE (2019) Wide margin excision followed by tibialisation of fibula and ankle arthrodesis as novel surgical technique in giant cell tumor patient. J Clin Orthop Trauma 10(5):1004–1007. https://doi.org/10.1016/j.jcot.2018.05.011
Xu L, Zhou J, Wang Z, Xiong J, Qiu Y, Wang S (2018) Reconstruction of bone defect with allograft and retrograde intramedullary nail for distal tibia osteosarcoma. Foot Ankle Surg 24(2):149–153. https://doi.org/10.1016/j.fas.2017.01.006
Acknowledgements
Not applicable.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No financial support was received for this study.
Author information
Authors and Affiliations
Contributions
B.Z.H. designed the study, performed the surgery, and revised the manuscript. M.K.M. revised the literature and wrote the preliminary manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by Menoufia University Institutional Review Board (IRB). Informed consent to participate in the study was taken from the patient.
Consent for publication
Consent to publish individual data was obtained.
Competing interests
All authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hasan, B.Z., Mesregah, M.K. A technical note on autologous iliac crest bone grafting for restoration of the distal tibial articular surface. J EXP ORTOP 10, 50 (2023). https://doi.org/10.1186/s40634-023-00612-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40634-023-00612-0