Abstract
Purpose
The knee stiffness acquired following an Anterior Cruciate Ligament (ACL) injury might affect clinical knee tests, i.e., the pivot-shift maneuver. In contrast, the motor effects of spinal anesthesia could favor the identification of rotatory knee deficiencies prior to ACL reconstruction. Hence, we hypothesized that the intra-operative pivot-shift maneuver under spinal anesthesia generates more acceleration in the lateral tibial plateau of patients with an injured ACL than without.
Methods
Seventy patients with unilateral and acute ACL rupture (62 men and 8 women, IKDC of 55.1 ± 13.8 pts) were assessed using the pivot-shift maneuver before and after receiving spinal anesthesia. A triaxial accelerometer was attached to the skin between Gerdys’ tubercle and the anterior tuberosity to measure the subluxation and reduction phases. Mixed ANOVA and multiple comparisons were performed considering the anesthesia and leg as factors (alpha = 5%).
Results
We found a higher acceleration in the injured leg measured under anesthesia compared to without anesthesia (5.12 ± 1.56 m.s− 2 vs. 2.73 ± 1.19 m.s− 2, p < 0.001), and compared to the non-injured leg (5.12 ± 1.56 m.s− 2 vs. 3.45 ± 1.35 m.s− 2, p < 0.001). There was a presence of significant interaction between leg and anesthesia conditions (p < 0.001).
Conclusions
The pivot-shift maneuver performed under anesthesia identifies better rotatory instability than without anesthesia because testing the pivot-shift without anesthesia underestimates the rotatory subluxation of the knee by an increased knee stiffness. Thus, testing under anesthesia provides a unique opportunity to determine the rotational instability prior to ACL reconstruction.
Similar content being viewed by others
Introduction
The anterior cruciate ligament (ACL) rupture has an incidence of 68.6 per 100,000 people-years [31], and most cases (~ 80%) involve rotatory instability [3, 25]. Clinically, the rotatory instability is tested through the pivot-shift maneuver [30], and it has high specificity for ACL insufficiency [12] and correlates well with self-reports of knee function [20]. However, the altered activity of gamma motor neurons may increase knee stiffness after an ACL rupture [17, 25]. Hence, the knee rotation in patients with an ACL injury may be restricted.
When the ACL is insufficient during the pivot-shift maneuver, the lateral tibial plateau displaces more anteriorly and medially (subluxation) followed by the stretching of the iliotibial band [3]. Because of that, a sudden higher posterior and lateral shift of the lateral tibial plateau (reduction) is caused [11, 16]. Unfortunately, this joint movement relates to residual knee instability, mainly triggered during cutting, twisting, or pivoting motions [34]. In consequence, the scintigraphy activity in the subchondral bone and the risk of early knee osteoarthrosis increase [34].
On the other hand, meniscal injuries (bucket handle meniscus tear), collateral ligamentous laxity, or the quality of pivot-shift execution among clinicians can increase a false-negative diagnosis (type II error) of rotatory knee instability [17, 25, 26]. This false-negative diagnosis can affect clinical decisions. For example, the pertinence of an extraarticular anterolateral tenodesis could be affected if there is a false-negative rotatory instability [6, 22]. Thus, the improvement of pivot-shift execution has an important clinical significance.
Regarding the last improvements of the test, the pivot-shift maneuver has added accelerometry measures to quantify the rotatory knee laxity [1, 22]. However, applying the pivot-shift accelerometry for a better rotatory knee laxity detection after an ACL injury remains unclear. As anesthesia can selectively reduce the motor response of skeletal muscles, here we hypothesized that the intra-operative pivot-shift maneuver under spinal anesthesia generates more acceleration in the lateral tibial plateau of patients with an injured ACL than without. Therefore, the goal of our study was to determine the acceleration of the lateral tibial plateau during the pivot-shift maneuver in patients with an acute ACL injury under and without spinal anesthesia.
Methods
Patients
Seventy patients (62 men and 8 women) diagnosed with an acute ACL rupture were recruited between 2019 and 2020. Table 1 presents the demographic variables of the sample.
The inclusion criteria were: i) unilateral and acute ACL rupture (low edema and flexion range of motion of at least 90° within the first 3 weeks after injury) [9]; ii) positive Lachman and draw tests, iii) sports non-contact ACL-rupture [2]; iv) direct signs of ACL rupture in the magnetic resonance imaging [4]; v) arthroscopy confirmation of the ACL-rupture [25, 36]; vi) age between 20 and 50 years old; and vii) diagnosis and surgery conducted at the MEDS clinic (Santiago, Chile). The exclusion criteria were: i) ACL re-rupture; ii) presence of associated injuries (bone, ligament, or meniscus [10, 15]; iii) the presence of a previous orthopedic condition (patellofemoral dysfunction, anterior knee pain, leg length differences, knee varus or valgus, or osteoarthritis); iv) not being eligible for anesthesia [13]; v) rheumatological and metabolic conditions; vi) neurological conditions; vii) active infection; and viii) cognitive impairment. All cases satisfying the admissibility criteria provided written consent to participate. The study was approved by the institutional ethics board from MEDS clinic (Santiago, Chile) and conducted according to the Declaration of Helsinki.
ACL rupture diagnosis
A senior orthopedic medical doctor Roberto Yañez confirmed the ACL rupture through a combination of clinical history, physical exam, magnetic resonance images, and arthroscopy. An acute unilateral rupture of the ACL was fully diagnosed when patients indicate a sport non-contact ACL rupture mechanism (whether in flexion, abduction, internal rotation or any combinations) [18], positive signs for acute inflammation, a positive Lachman and anterior draw test [12], and the non-integrity of ACL determined by magnetic resonance imaging [4].
Experiment
The pivot-shift testing [24] was performed before and after the anesthesia delivery, with the patient lying supine position on an operating table. A disinfected wireless accelerometry was attached over the lateral tibial plateau to acquire the accelerometry data when the pivot-shift maneuver was executed. The same senior orthopedic surgeon performed the pivot-shift maneuver in all patients. The orthopedic surgeon had more than 30 years of experience with regular use of the test and a minimum of 100 ACL reconstructions per year in a regular year.
Prior to ACL reconstruction, spinal anesthesia was applied to patients receiving bupivacaine (10 to 12 mg) and intravenous sedation together with fentanyl 20 μg, while receiving oxygen via a facemask to ensure 98% oxygen saturation. Non-invasive monitors were used for electrocardiography, blood pressure, and pulse oximeter monitoring [13]. The femoral nerve block for bone-to-bone patellar allograft and a saphenous nerve block for semitendinosus-gracilis allograft was performed guided by ultrasonography [13]. The effect of the anesthesia on the quadriceps-hamstring strength was checked by the surgeon performing manual strength testing. A second pivot-shift maneuver was executed when the quadriceps strength reached an M-score equal to zero (non-visible or palpable muscle contraction). Finally, the accelerometer was detached, and the cleaning and sterilization procedures were performed to proceed with the ACL reconstruction.
Pivot-shift testing and accelerometry measurement
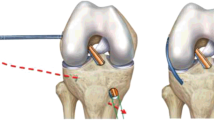
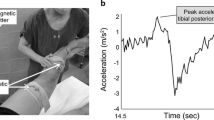
The pivot-shift test was repeated five times under conditions of anesthesia and non-anesthesia on both injured and non-injured legs. The movement was tracked by a triaxial accelerometer (Trigno™ accelerometry system, Delsys Inc., USA) with 3 degrees of freedom, range of ±3 g, sampling resolution of 20 ± 5 Hz, > 40 dB*dec − 1 and 450 ± 50 Hz > 80 dB*dec− 1, basal noise (RMS) of 0.016 g for a range of ±1.5 g and 0.032 g for a range of ±6 g, a bandwidth of DC − 50 ± 5 Hz with 20 dB*dec− 1, offset error of ±0.21 g for the X-axis and − 0.42 g for the Z-axis, and an accelerometer resolution depth of 10 bits [8]. The accelerometer was placed in the middle point between the patient’s Gerdy’s tubercle and the tibial tuberosity, with the patient in a supine position [1]. The accelerometer was aligned to the longitudinal axis of the tibia bone [1]; see Fig. 1.
The variable of interest was the difference between the maximal and minimum value of the resultant acceleration on the lateral aspect of the tibia during each pivot-shift maneuver (Fig. 2). We chose the delta acceleration of the resultant acceleration to obtain a physical measure that can transduce equally the flexion, valgus, and medial rotation induced by the clinical test. Furthermore, the mean of five repetitions was used to attenuate the possible outlier accelerations obtaining an averaged pattern. Hence, the resultant acceleration was obtained from \(\boldsymbol{a}=\sqrt[]{{{\boldsymbol{a}}_{\boldsymbol{x}}}^{\mathbf{2}}+{{\boldsymbol{a}}_{\boldsymbol{y}}}^{\mathbf{2}}+{{\boldsymbol{a}}_{\boldsymbol{z}}}^{\mathbf{2}}}\) measured in m*s− 2. Thus, the acceleration pattern of each pivot-shift maneuver was clearly observed during the data acquisition. For methodological details, see Figs. 1 and 2.
Statistical analysis
Data were reported as the mean and standard deviation. The Kolmogorov-Smirnov test confirmed the normality of data distribution. The data homoscedasticity was confirmed using Levene’s test, and sphericity was confirmed using Mauchly’s test. The main effects for anesthesia and injury, and possible interactions, were determined using a mixed-ANOVA of two factors 2 × 2 (anesthesia factor with two levels: without and under anesthesia; and injury factor with two levels: injured and non-injured leg). A two-tailed paired t-test was applied to identify paired differences for repeated measures and a two-tailed independent t-test for independent data. The partial-eta squared was interpreted as small (> 0.01–0.06), moderate (> 0.06–0.14), and large (> 0.14) [27]. The Cohen’s d effect was interpreted as small (> 0.2–0.5), moderate (> 0.5–0.8), and large (> 0.8) [27]. The statistical significance was set at 0.05 for all analyses performed using the statistical software SPSS 26 (IBM Corp., USA). The post-hoc power analysis was described and estimated using the G*Power 3.1.9.2 software (Kiel & Dusseldorf University, Germany).
Results
A main effect was found for anesthesia (F(1,69) = 16.3, p < 0.001, and ŋ2 = 0.106; moderate effect size) and for injury factors (F(1,69) = 82.4, p < 0.001, and ŋ2 = 0.374; large effect size). There was an interaction between anesthesia and injury factors (F(1,69) = 36.8, p < 0.001, and ŋ2 = 0.211; large effect size). See Fig. 3 for details.
Acceleration was higher when the pivot-shift maneuver was performed under anesthesia compared to without anesthesia in the injured leg (5.12 ± 1.56 m.s− 2 vs. 2.73 ± 1.19 m.s− 2, Δ = 2.39 m·s− 2, p < 0.001, t(1,69) = − 10.32, d = 1.72; large effect size). In the injured leg the acceleration was also higher compared to the non-injured leg under anesthesia (5.12 ± 1.56 m.s− 2 vs. 3.45 ± 1.35 m.s− 2, Δ = 1.67 m.s− 2, p < 0.001, t(1,69) = 6.76; d = 1.66, large effect size), see Fig. 3 and Table 2 for details. The post-hoc power analysis obtained was 0.98 for an n = 70.
Discussion
In this study, we demonstrated that the pivot-shift maneuver conducted without spinal anesthesia generates lower acceleration of the lateral tibial plateau during the reduction phase than under anesthesia in ACL injury. This suggests that false-negative outcomes may be induced during the pivot-shift maneuver for ACL injury conducted without anesthesia. Accordingly, we recommend assessing the pivot-shift maneuver under anesthesia. We highlight that the use of accelerometry performed by the same clinician adds sensitivity to identify rotatory instability and diminishes subjective appreciations. Knowing residual rotatory instability tested under anesthesia without the increased co-activation of the hamstrings and quadriceps is crucial to choosing a better surgical and clinical management.
Several factors can increase the activity of the hamstrings in patients with ACL injury resulting in an attenuated pivot-shift sign, as we observed in this study. It is known that the pivot-shift maneuver can induce knee joint instability, but this is typically controlled in healthy knees without disproportionated increasing knee muscle stiffness [3]. However, the maneuver may induce hamstrings activation to restrict the knee rotation and displacement [32]. The anterior displacement and rotatory instability movement caused by the pivot-shift maneuver excites muscle spindles by stretching stimulus, eliciting hamstring activation in an involuntary attempt to restore joint stability [32].
Also, there is a neural link between ligaments and spindles, where mechanical changes in stress over the ligaments triggers muscle spindle activity [32]. Hence, it is highly probable that both the ruptured and non-ruptured ACL may induce the activity of hamstrings during testing to counteract the anterior tibial displacement and medial knee rotation [5]. As a consequence, our results agree with the muscle-ligament-reflex phenomena described by Solomonow and Krogsgaard [19, 23, 32]. This increased neuromuscular response of knee muscles is supported by the reduced acceleration found in the injured ACL patients without anesthesia and by the increased tibial acceleration during the subluxation phase of the pivot-shift maneuver when the anesthesia is delivered, blocking the nerve innervation of hamstrings. Thus, the motor effects of the anesthesia are clinically useful to determine the residual rotatory instability in ruptured ACL.
Unfortunately, a hiding pivot-shift sign results in early osteoarthrosis [34], and an instability sensation can often remain in patients [21]. The fact that rotational instability (positive pivot shift sign) may be an important precursor of knee osteoarthrosis [7], supports the efforts that minimize the false-negative rotatory knee laxity. A recent cadaveric study with sectioned ACL has found that rotational instability is not adequately resisted by intact anterolateral ligaments or the iliotibial band [28]. When both structures are sectioned, 7 out of 10 knees developed high rotational instability [28]. In the long-term, this unrestricted pivoting movement is associated with knee osteoarthrosis [14]. Unfortunately, patients can hide symptoms [35], showing persistent joint movement alterations after the ACL reconstruction [33]. These changes and consequences reaffirm the importance of implementing better alternatives to measure rotatory knee instability.
Regarding the false-positive diagnosis of rotatory instability, muscle activation and stiffness are important factors in assessing injured ACL patients. Indeed, exploring the positive pivot-shift sign should encourage clinicians and researchers. Thus, performing the pivot-shift maneuver with patients under anesthesia is a unique opportunity to determine the rotational knee deficits in ACL ruptured patients. We could mention the reproducibility of the pivot-shift maneuver as a relevant limitation, such as it has been described previously by the literature [26]. Also, we believe that accelerometers are a good measurement tool but not the best for assessing dynamic knee laxity [29].
Conclusions
The pivot-shift maneuver executed without anesthesia elicits lower acceleration of the tibial plateau during the reduction phase than under anesthesia due to increased knee stiffness. Hence, performing the pivot-shift maneuver executed under anesthesia permitted a more sensitive identification of rotational knee laxity.
Availability of data and materials
Data will be available after publication in an external repository. The external link is: https://www.researchgate.net/publication/354628903_Supplementary_data_Intraoperative_pivotshift_accelerometry_combined_with_anesthesia_improves_the_measure_of_rotatory_knee_instability_in_anterior_cruciate_ligament_injury.
References
Abusleme S, Strömbäck L, Caracciolo G, Zamorano H, Cheyre J, Vergara F, Yañez R (2021) Lateral extra-articular tenodesis: a technique with an iliotibial band strand without implants. Arthrosc Tech 10:e85–e89
Alentorn-Geli E, Myer GD, Silvers HJ, Samitier G, Romero D, Lázaro-Haro C, Cugat R (2009) Prevention of non-contact anterior cruciate ligament injuries in soccer players. Part 1: mechanisms of injury and underlying risk factors. Knee Surg Sports Traumatol Arthrosc 17:705–729
Amis AA (2017) Anterolateral knee biomechanics. Knee Surg Sports Traumatol Arthrosc 25:1015–1023
Bining J, Andrews G, Forster BB (2009) The ABCs of the anterior cruciate ligament: a primer for magnetic resonance imaging assessment of the normal, injured and surgically repaired anterior cruciate ligament. Br J Sports Med 43:856–862
Blackburn T, Pietrosimone B, Goodwin JS, Johnston C, Spang JT (2019) Co-activation during gait following anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon) 67:153–159
Borgstrom PH, Markolf KL, Wang Y, Xu X, Yang PR, Joshi NB, Yeranosian MG, Petrigliano FA, Hame SL, Kaiser WJ, McAllister DR (2015) Use of inertial sensors to predict pivot-shift grade and diagnose an ACL injury during preoperative testing. Am J Sports Med 43:857–864
Butler RJ, Minick KI, Ferber R, Underwood F (2009) Gait mechanics after ACL reconstruction: implications for the early onset of knee osteoarthritis. Br J Sports Med 43:366–370
De la Fuente C, Weinstein A, Guzman-Venegas R, Arenas J, Cartes J, Soto M, Carpes FP (2019) Use of accelerometers for automatic regional chest movement recognition during tidal breathing in healthy subjects. J Electromyogr Kinesiol 47:105–112
Evans S, Shaginaw J, Bartolozzi A (2014) Acl reconstruction - it’s all about timing. Int J Sports Phys Ther 9:268–273
Frank RM, Hamamoto JT, Bernardoni E, Cvetanovich G, Bach BR, Verma NN, Bush-Joseph CA (2017) ACL reconstruction basics: quadruple (4-Strand) hamstring autograft harvest. Arthrosc Tech 6:e1309–e1313
Horvath A, Meredith SJ, Nishida K, Hoshino Y, Musahl V (2020) Objectifying the pivot shift test. Sports Med Arthrosc Rev 28:36–40
Huang W, Zhang Y, Yao Z, Ma L (2016) Clinical examination of anterior cruciate ligament rupture: a systematic review and meta-analysis. Acta Orthop Traumatol Turc 50:22–31
Iamaroon A, Tamrongchote S, Sirivanasandha B, Halilamien P, Lertwanich P, Surachetpong S, Rungwattanakit P (2016) Femoral nerve block versus intra-articular infiltration: a preliminary study of analgesic effects and quadriceps strength in patients undergoing arthroscopic anterior cruciate ligament reconstruction. J Med Assoc Thail 99:578–583
Ikuta F, Yoneta K, Miyaji T, Kidera K, Yonekura A, Osaki M, Gamada K (2020) Knee kinematics of severe medial knee osteoarthritis showed tibial posterior translation and external rotation: a cross-sectional study. Aging Clin Exp Res 32:1767–1775
Katakura M, Horie M, Watanabe T, Katagiri H, Otabe K, Ohara T, Nakamura K, Katagiri K, Ueki H, Zaffagnini S, Sekiya I, Muneta T, Koga H (2019) Effect of meniscus repair on pivot-shift during anterior cruciate ligament reconstruction: objective evaluation using triaxial accelerometer. Knee 26:124–131
Kim S-J, Bae J-H, Lim H (2014) Comparison of Achilles and tibialis anterior tendon allografts after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 22:135–141
Kim SJ, Kim HK (1995) Reliability of the anterior drawer test, the pivot shift test, and the Lachman test. Clin Orthop Relat Res 317:237–242
Kopkow C, Lange T, Hoyer A, Lützner J, Schmitt J (2018) Physical tests for diagnosing anterior cruciate ligament rupture. Cochrane Database Syst Rev 2018(12):CD011925
Krogsgaard MR, Dyhre-Poulsen P, Fischer-Rasmussen T (2002) Cruciate ligament reflexes. J Electromyogr Kinesiol 12:177–182
Kujala UM, Nelimarkka O, Koskinen SK (1992) Relationship between the pivot shift and the configuration of the lateral tibial plateau. Arch Orthop Trauma Surg 111:228–229
Kuroda R, Hoshino Y (2016) Electromagnetic tracking of the pivot-shift. Curr Rev Musculoskelet Med 9:164–169
Matsushita T, Oka S, Nagamune K, Matsumoto T, Nishizawa Y, Hoshino Y, Kubo S, Kurosaka M, Kuroda R (2013) Differences in knee kinematics between awake and anesthetized patients during the Lachman and pivot-shift tests for anterior cruciate ligament deficiency. Orthop J Sports Med 1:2325967113487855
Melnyk M, Faist M, Gothner M, Claes L, Friemert B (2007) Changes in stretch reflex excitability are related to “giving way” symptoms in patients with anterior cruciate ligament rupture. J Neurophysiol 97:474–480
Musahl V, Hoshino Y, Ahlden M, Araujo P, Irrgang JJ, Zaffagnini S, Karlsson J, Fu FH (2012) The pivot shift: a global user guide. Knee Surg Sports Traumatol Arthrosc 20:724–731
Musahl V, Karlsson J, Kuroda R, Zaffagnini S (2016) Rotatory knee instability: an evidence based approach. Springer
Naendrup J-H, Patel NK, Zlotnicki JP, Murphy CI, Debski RE, Musahl V (2019) Education and repetition improve success rate and quantitative measures of the pivot shift test. Knee Surg Sports Traumatol Arthrosc 27:3418–3425
Nakagawa S, Cuthill IC (2007) Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc 82:591–605
Noyes FR, Huser LE, Levy MS (2017) Rotational knee instability in ACL-deficient knees: role of the anterolateral ligament and iliotibial band as defined by tibiofemoral compartment translations and rotations. J Bone Joint Surg Am 99:305–314
Pappas E, Zampeli F, Xergia SA, Georgoulis AD (2013) Lessons learned from the last 20 years of ACL-related in vivo-biomechanics research of the knee joint. Knee Surg Sports Traumatol Arthrosc 21:755–766
Schilaty ND, Nagelli C, Hewett TE (2016) Use of objective neurocognitive measures to assess the psychological states that influence return to sport following injury. Sports Med 46:299–303
Snoeker B, Turkiewicz A, Magnusson K, Frobell R, Yu D, Peat G, Englund M (2020) Risk of knee osteoarthritis after different types of knee injuries in young adults: a population-based cohort study. Br J Sports Med 54:725–730
Solomonow M, Krogsgaard M (2001) Sensorimotor control of knee stability. A review. Scand J Med Sci Sports 11:64–80
Tengman E, Grip H, Stensdotter A, Häger CK (2015) Anterior cruciate ligament injury about 20 years post-treatment: a kinematic analysis of one-leg hop. Scand J Med Sci Sports 25:818–827
Vaudreuil NJ, Rothrauff BB, de Sa D, Musahl V (2019) The pivot shift: current experimental methodology and clinical utility for anterior cruciate ligament rupture and associated injury. Curr Rev Musculoskelet Med 12:41–49
Yang C, Tashiro Y, Lynch A, Fu F, Anderst W (2018) Kinematics and arthrokinematics in the chronic ACL-deficient knee are altered even in the absence of instability symptoms. Knee Surg Sports Traumatol Arthrosc 26:1406–1413
Yaqoob J, Alam MS, Khalid N (2015) Diagnostic accuracy of magnetic resonance imaging in assessment of meniscal and ACL tear: correlation with arthroscopy. Pak J Med Sci 31:263–268
Code availability
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or non-profit sectors.
Author information
Authors and Affiliations
Contributions
The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The IRB approved the investigation.
Each patient gave their written consent before starting the study.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Caracciolo, G., Yáñez, R., Silvestre, R. et al. Intraoperative pivot-shift accelerometry combined with anesthesia improves the measure of rotatory knee instability in anterior cruciate ligament injury. J EXP ORTOP 8, 80 (2021). https://doi.org/10.1186/s40634-021-00396-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40634-021-00396-1