Abstract
Background
We aimed to identify the association of pH value in blood gas assessment with neurological outcome among out-of-hospital cardiac arrest (OHCA) patients treated by extracorporeal cardiopulmonary resuscitation (ECPR).
Methods
We retrospectively analyzed the database of a multicenter prospective observational study on OHCA patients in Osaka prefecture, Japan (CRITICAL study), from July 1, 2012 to December 31, 2016. We included adult OHCA patients treated by ECPR. Patients with OHCA from external causes such as trauma were excluded. We conducted logistic regression analysis to identify the odds ratio (OR) and 95% confidence interval (CI) of the pH value for 1 month favorable neurological outcome adjusted for potential confounders including sex, age, witnessed by bystander, CPR by bystander, pre-hospital initial cardiac rhythm, and cardiac rhythm on hospital arrival.
Results
Among the 9822 patients in the database, 260 patients were finally included in the analysis. The three groups were Tertile 1: pH ≥ 7.030, Tertile 2: pH 6.875–7.029, and Tertile 3: pH < 6.875. The adjusted OR of Tertiles 2 and 3 compared with Tertile 1 for 1 month favorable neurological outcome were 0.26 (95% CI 0.10–0.63) and 0.24 (95% CI 0.09–0.61), respectively.
Conclusions
This multi-institutional observational study showed that low pH value (< 7.03) before the implementation of ECPR was associated with 1 month unfavorable neurological outcome among OHCA patients treated with ECPR. It may be helpful to consider the candidate for ECPR.
Similar content being viewed by others
Background
Extracorporeal cardiopulmonary resuscitation (ECPR) is a mechanical hemodynamic support for out-of-hospital cardiac arrest (OHCA) patients using veno-arterial extracorporeal membrane oxygenation (V-A ECMO). Although this advanced resuscitation is expected to improve outcomes among patients with refractory cardiac arrest, it is invasive and expensive and requires considerable human resources [1,2,3]. Therefore, it is important to judge whether this is appropriate for a patient immediately after hospital arrival, based on the available information associated with neurological outcomes [4, 5].
Blood gas assessment (BGA) is performed easily and commonly to identify the treatable causes and predict prognosis in resuscitation for OHCA [6,7,8,9,10]. Among the factors assessed in BGA, the pH value in particular is influenced by metabolic and respiratory acidosis and is representative of hemodynamic and respiratory conditions [11]. Some observational studies show that the pH value after the return of spontaneous circulation (ROSC) is associated with neurological outcomes among OHCA patients [6,7,8]. These results may be helpful in considering the indication of intensive care admission or targeted temperature management after ROSC; however, the decision to start ECPR needs to be made before ROSC. Thus, these results may not be generalizable to ECPR candidates. One other observational study indicated that the pH value during resuscitation was related to neurological outcome; however, this study did not include patients with ECPR [6]. Currently, little is known about the association between pH value before the implementation of ECPR and neurological outcome among OHCA patients treated by ECPR. Our study aimed to determine the association between the pH value before implementation of ECPR and neurological outcome, among OHCA patients treated with ECPR.
Methods
We have reported the methodology of this study according to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement [12]. The Ethics Committee of Kyoto University and each participating institution approved this study protocol (R1045), and written informed consent was waived.
Study design and settings
We performed retrospective analysis of the database of the Comprehensive Registry of Intensive Care for OHCA Survival (CRITICAL) study. This is a multicenter prospective observational study to collect pre-hospital and in-hospital data among OHCA patients in Osaka prefecture, Japan. The pre-hospital data was obtained from the All-Japan Utstein Registry of the Fire and Disaster Management Agency (FDMA) [13,14,15,16]. In-hospital data were obtained from 13 tertiary critical care medical centers (CCMCs) and 1 non-CCMC community hospital with an emergency department, all located in Osaka prefecture in Japan. Osaka prefecture is an urban area of 1905 km2, and it had a residential population of about 8.8 million in 2015 [17]. In Osaka prefecture, a total of 7500 OHCA cases occur every year [18], and approximately 1 in 4 OHCA patients (approximately 2000 cases or more) have been registered every year from 2012 to 2016. This registry is still ongoing, with an undefined study period. In-hospital data were recorded by the physicians in charge of the patients and were registered by the physicians or medical administrators using a predefined online form. Finally, the working group checked and confirmed the quality of data. If the data were incomplete, they were returned to each institution and completed [16]. A detailed description of the All-Japan Utstein Registry of FDMA and the CRITICAL study has been published previously [16].
Study patients
From the CRITICAL database, we included all adult (aged ≥ 18 years) patients with OHCA due to internal medical causes, who were treated with ECPR, between July 1, 2012 and December 31, 2016. We defined ECPR as the initiation of cardiopulmonary bypass using V-A ECMO with the emergency cannulation of a large vein and artery for OHCA patients on hospital arrival during the resuscitation [19]. We excluded the following patients: those who did not receive any resuscitation or treatment in the hospital, with unavailable pre-hospital records, whose age was 17 years or less or unknown, who collapsed following cardiac arrest due to external causes such as trauma, drowning, or hanging, and those who did not undergo ECPR. We also excluded those without available BGA results before implementation of ECPR. In this cohort, the implementation of ECPR was decided by the physicians in charge of the patients or by each institution’s protocol.
Outcome
The primary outcome of our study was 1 month survival with favorable neurological outcome, defined as Cerebral Performance Category (CPC) 1 or 2. CPC is most commonly used to evaluate neurological status as follows: category 1, good cerebral performance; category 2, moderate cerebral disability; category 3, severe cerebral disability; category 4, coma or vegetative state; and category 5, death [15].
Data measurement and collection
From the CRITICAL database, we obtained the following clinical information: sex, age (< 65, 65–74, ≥ 75), cause of cardiac arrest (cardiac, others), witnessed by bystander (yes, no), CPR performed by bystander (yes, no), pre-hospital initial cardiac rhythm (shockable, non-shockable), cardiac rhythm on hospital arrival (shockable, non-shockable, ROSC), pH in the BGA before the implementation of ECPR, resuscitation time course, and outcomes. Age categories were defined on the basis of a government reference [17]. The pH value in venous BGA can be used interchangeably with that in arterial BGA because they are well related to each other [20, 21]. Thus, we treated them as the same. The resuscitation time courses were defined as the time from emergency call (E-call) for ambulance to hospital arrival, BGA, and start of ECPR in the hospital. The included patients were divided into three groups of approximately equal size, based on the pH value in the BGA (Tertiles 1 to 3).
Potential bias
We excluded patients who lacked BGA data from the main analysis (complete case analysis). If data are missing completely at random, excluding patients with missing data does not lead to biased results; thus, it may be acceptable [22]. However, if the missing does not occur at random and depends on the outcome and exposure, then it would introduce selection bias [22]. Therefore, to demonstrate the robustness of our results and compensate for the risk of selection bias, we described the characteristics of patients with missing data and performed a sensitivity analysis presuming that the missing of data depended on exposure and outcome; this has been described in Additional File 1 (Details of the sensitivity analysis are also described in Additional File 1).
Statistical analysis
We described the patients’ characteristics in each patient group. To identify the associations of the pH with the primary outcome, we calculated crude odds ratios (OR) and adjusted OR with 95% confidence intervals (CI) of each patient group for the outcome, using a logistic regression model. We adjusted for the following potential confounders: sex (male, female), age (< 65, 65–74, and ≥ 75), witnessed by bystander, CPR by bystander, pre-hospital initial cardiac rhythm (shockable, non-shockable), and cardiac rhythm on hospital arrival (shockable, non-shockable, and ROSC). Moreover, for better understanding of the results, we also calculated the area under the curve of the receiver operating characteristic curve (AUC_ROC) to predict the neurological outcome, treating the pH values as continuous variables. We also described the characteristics of those who had favorable neurological outcomes in each group.
We did not estimate a sample size because our analysis involved secondary usage of already available data [12]. All statistical results were considered significant at a two-sided P value of < 0.05. All statistical analyses were performed using the JMP Pro® 14 software (SAS Institute Inc., Cary, NC, USA).
Results
Study participants
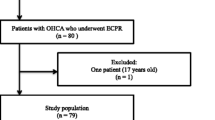
Among the 9822 patients in the CRITICAL database, 260 were finally included in the analysis (Fig. 1). The three groups into which the included patients were divided were Tertile 1: pH ≥ 7.03, Tertile 2: pH 6.875–7.029, and Tertile 3: pH < .875. The characteristics of the patients are shown in Table 1. In summary, the patients in Tertile 3 (pH < 6.875) were relatively young (age, median, [IQR] 55.5, [46–66] years), compared with those in Tertile 1 (pH ≥ 7.03) (67 [56.8–75.3] years) and Tertile 2 (pH 6.875–7.029) (63.5 [49.0–69.8] years). The other parameters were substantially similar among groups.
Primary outcome
The primary outcome (1 month survival with favorable neurological outcome) was 27.9% (24/86) in Tertile 1 (pH ≥ 7.03), 10.2% (9/88) in Tertile 2 (pH 6.875 to 7.029), and 9.3% (8/86) in Tertile 3 (pH < 6.875).
The crude OR with 95% CI for primary outcome of Tertiles 2 and 3, compared with Tertile 1 for reference, were 0.29 (95% CI 0.13–0.68) and 0.26 (95% CI 0.11–0.63), respectively (Fig. 2). Adjusted ORs with 95% CI for primary outcome of Tertiles 2 and 3 compared with Tertile 1 were 0.26 (95% CI 0.10–0.63) and 0.24 (95% CI 0.09–0.61), respectively (Fig. 2). According to these results, Tertile 2 (pH 6.875–7.029) and Tertile 3 (pH < 6.875) were associated with unfavorable neurological outcome, compared with Tertile 1 (pH ≥ 7.03). The crude and adjusted ORs of the other covariates are provided in the supplementary file. The discrimination ability of pH (AUC_ROC) was 0.675 [95% CI 0.573–0.763].
The characteristics of patients with favorable neurological outcome
The characteristics of patients with favorable neurological outcomes are shown in Table 2. Those in Tertiles 2 and 3 were more likely to be young, have OHCA witnessed by bystanders, and have ECPR implemented early after arrival.
Sensitivity analysis
Under this assumption, Tertile 2 (pH 6.875–7.029) and Tertile 3 (pH < 6.875) were also independently associated with neurological outcome (Additional file 1). This result demonstrates the robustness of this association, despite the exclusion of the patients with missing BGA.
Discussion
Key observations
This multi-institutional observational study including 14 emergency departments showed that the pH value before the implementation of ECPR was associated with 1 month neurological outcomes among OHCA patients treated with ECPR. It may be helpful to consider the candidate for ECPR.
Interpretation of the results
We suggest that our results may be explained as follows: severe acidemia, including metabolic and respiratory acidosis, is representative of the severe conditions of hypoperfusion of vital organs and insufficient discharge of carbon dioxide during resuscitation, and these conditions may lead to cerebral injury or multiple organ failure and unfavorable outcomes. Metabolic acidosis, particularly lactic acidosis, is caused by inadequate oxygen delivery, impaired tissue oxygenation, and anaerobic glycolysis [23]. In cardiac arrest patients, it may be affected by low cardiac output by chest compression during resuscitation [24]. Some observational studies have reported that metabolic acidosis after ROSC is correlated with the duration from arrest to ROSC and is associated with neurological outcome among OHCA patients [8, 25,26,27,28]. Respiratory acidosis, the other cause of severe acidemia, indicates inadequate discharge of carbon dioxide and is mostly caused by low venous return by chest compression and insufficient alveolar ventilation during resuscitation [24, 29]. Previous observational studies also reported that respiratory acidosis is associated with cerebral injury and unfavorable neurological outcomes among post-cardiac arrest patients or those with head trauma injury [30, 31]. Thus, it is possible that lower pH values may represent a longer duration of cardiac arrest, lower cerebral blood flow and venous return, and insufficient ventilation; these conditions are associated with an unfavorable neurological outcome.
Clinical implication
We conclude that pH measurement may be helpful to judge the indication of ECPR. If OHCA patients have a pH value higher than 7.03, they have a higher probability of favorable neurological outcome. The results of BGA are objective, reproductive, and available as soon as a blood sample is collected. Further, when ECPR is attempted, obtaining access to the femoral artery enables continual collection of blood samples. Therefore, pH measurement can be easily applied to real clinical settings.
It should be noted that in our results, some patients with severe acidemia did survive with favorable neurological outcomes. These patients were relatively young, with shockable rhythms and OHCA witnessed by bystanders. A previous case series also reported that some patients with severe acidemia could achieve good recovery from OHCA in some situations [32]. Furthermore, in our study, the discrimination ability of pH (AUC_ROC) was 0.675 [95% CI 0.573–0.763], which is not adequately high for predicting the neurological outcome definitely. According to these findings, physicians considering the indication of ECPR should not make quick decisions based only on the pH value.
Strengths and limitations
Compared with previous studies, the strength of our study was that we could identify the association between pH values and neurological outcomes by adjusting for potential confounders. Among ECPR patients, a previous systematic review reported that witnessed cardiac arrest, CPR performed by bystander, initial shockable rhythm, arrest to ECPR duration, and higher pH value during resuscitation may be potential predictors for survival [33]. In the meta-analysis including five observational studies in this review, there were statistical differences between survivors and non-survivors based on pH value (7.16 ± 0.04 vs 7.01 ± 0.06, mean difference 0.14 [95% CI 0.08–0.21]) during resuscitation [33]. However, this analysis did not consider the effect of confounding. Further, it did not identify an association with neurological outcome. Conversely, our analysis adjusted for several major confounders using a logistic regression model and showed the association with neurological outcome. Therefore, our results showed a more robust association than previous studies.
Our study also has several limitations. First, the timing of collecting blood samples and the collecting sites (arterial or venous) were not strictly defined; this may have caused measurement bias. Second, our sample size and the number of events were limited. For more precise estimation, a larger sample size would be better. Third, some potential unmeasured confounders may influence the results. Fourth, the indication of ECPR was decided by each physician or according to each institution’s protocol. Thus, there may be selection bias. Finally, this registry was derived from a critical care center in Osaka, Japan; it is unclear as to what extent the results can be generalized to other populations or other settings.
Conclusions
Our study showed that a lower pH value (< 7.03) was associated with unfavorable neurological outcomes among OHCA patients treated by ECPR. Our results may be helpful in deciding the indication of ECPR.
Availability of data and materials
Not applicable
Abbreviations
- BGA:
-
Blood gas assessment
- CCMC:
-
Critical care medical center
- CPC:
-
Cerebral Performance Category
- CRITICAL:
-
Comprehensive Registry of Intensive Care for OHCA Survival
- ECPR:
-
Extracorporeal cardiopulmonary resuscitation
- FDMA:
-
Fire and Disaster Management Agency
- OHCA:
-
Out-of-hospital cardiac arrest
- ROSC:
-
Return of spontaneous circulation
- STROBE:
-
STrengthening the Reporting of OBservational studies in Epidemiology
- V-A ECMO:
-
veno-arterial extracorporeal membrane
References
Sakamoto T, Morimura N, Nagao K, Asai Y, Yokota H, Nara S, et al. Extracorporeal cardiopulmonary resuscitation versus conventional cardiopulmonary resuscitation in adults with out-of-hospital cardiac arrest: a prospective observational study. Resuscitation. 2014;85:762–8.
Ortega-Deballon I, Hornby L, Shemie SD, Bhanji F, Guadagno E. Extracorporeal resuscitation for refractory out-of-hospital cardiac arrest in adults: a systematic review of international practices and outcomes. Resuscitation. 2016;101:12–20.
Yannopoulos D, Bartos JA, Raveendran G, Conterato M, Frascone RJ, Trembley A, et al. Coronary artery disease in patients with out-of-hospital refractory ventricular fibrillation cardiac arrest. J Am Coll Cardiol. 2017;70:1109–17.
Dennis M, Zmudzki F, Burns B, Scott S, Gattas D, Reynolds C, et al. Cost effectiveness and quality of life analysis of extracorporeal cardiopulmonary resuscitation (ECPR) for refractory cardiac arrest. Resuscitation. 2019;139:49–56.
Kawashima T, Uehara H, Miyagi N, Shimajiri M, Nakamura K, Chinen T, et al. Impact of first documented rhythm on cost-effectiveness of extracorporeal cardiopulmonary resuscitation. Resuscitation. 2019;140:74–80.
Shin J, Lim YS, Kim K, Lee HJ, Lee SJ, Jung E, et al. Initial blood pH during cardiopulmonary resuscitation in out-of-hospital cardiac arrest patients: a multicenter observational registry-based study. Crit Care. 2017;21:322.
Takaki S, Kamiya Y, Tahara Y, Tou M, Shimoyama A, Iwashita M. Blood pH is a useful indicator for initiation of therapeutic hypothermia in the early phase of resuscitation after comatose cardiac arrest: a retrospective study. J Emerg Med. 2013;45:57–64.
Momiyama Y, Yamada W, Miyata K, Miura K, Fukuda T, Fuse J, et al. Prognostic values of blood pH and lactate levels in patients resuscitated from out-of-hospital cardiac arrest. Acute Med Surg. 2017;4:25–30.
Hope Kilgannon J, Hunter BR, Puskarich MA, Shea L, Fuller BM, Jones C, et al. Partial pressure of arterial carbon dioxide after resuscitation from cardiac arrest and neurological outcome: A prospective multi-center protocol-directed cohort study. Resuscitation. 2019;135:212–20.
Truhlar A, Deakin CD, Soar J, Khalifa GE, Alfonzo A, Bierens JJ, et al. European Resuscitation Council Guidelines for Resuscitation 2015: section 4. Cardiac arrest in special circumstances. Resuscitation. 2015;95:148–201.
Cowley NJ, Owen A, Bion JF. Interpreting arterial blood gas results. BMJ. 2013;346:f16.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC. Vandenbroucke JP; STROBE Initiative. The STrengthening the Reporting of OBservational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–7.
Kitamura T, Iwami T, Kawamura T, Nagao K, Tanaka H. Hiraide A; Implementation working group for the All-Japan Utstein Registry of the Fire and Disaster Management Agency. Nationwide public-access defibrillation in Japan. N Engl J Med. 2010;362:994–1004.
Kitamura T, Kiyohara K, Sakai T, Matsuyama T, Hatakeyama T, Shimamoto T, et al. Public-access defibrillation and out-of-hospital cardiac arrest in Japan. N Engl J Med. 2016;375:1649–59.
Jacobs I, Nadkarni V, Bahr J, Berg RA, Billi JE, Bossaert L, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Councils of Southern Africa). Circulation. 2004;110:3385–97.
Yamada T, Kitamura T, Hayakawa K, Yoshiya K, Irisawa T, Abe Y, et al. Rationale, design, and profile of Comprehensive Registry of In-Hospital Intensive Care for OHCA Survival (CRITICAL) study in Osaka, Japan. J Intensive Care. 2016;4:10.
Statistics Bureau (Japan). https://www.stat.go.jp/english/. Accessed 17 Nov 2019.
Fire and Disaster Management Agency of the Ministry of Internal Affairs and Communications (Japan). http://www.fdma.go.jp/neuter/topics/fieldList9_3.html. Accessed 7 Jun 2018.
Brooks SC, Anderson ML, Bruder E, Daya MR, Gaffney A, Otto CW, et al. Part 6: Alternative techniques and ancillary devices for cardiopulmonary resuscitation: 2015 American heart association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132:S436–43.
Malatesha G, Singh NK, Bharija A, Rehani B, Goel A. Comparison of arterial and venous pH, bicarbonate, PCO2 and PO2 in initial emergency department assessment. Emerg Med J. 2007;24:569–71.
Kelly AM, McAlpine R, Kyle E. Venous pH can safely replace arterial pH in the initial evaluation of patients in the emergency department. Emerg Med J. 2001;18:340–2.
Hughes RA, Heron J, Sterne JAC, Tilling K. Accounting for missing data in statistical analyses: multiple imputation is not always the answer. Int J Epidemiol. 2019;48:1294–304.
Reddy AJ, Lam SW, Bauer SR, Guzman JA. Lactic acidosis: Clinical implications and management strategies. Cleve Clin J Med. 2015;82:615–24.
Magliocca A, Olivari D, De Giorgio D, Zani D, Manfredi M, Boccardo A, et al. LUCAS versus manual chest compression during ambulance transport: a hemodynamic study in a porcine model of cardiac arrest. J Am Heart Assoc. 2019;8:e011189.
Takasu A, Sakamoto T, Okada Y. Arterial base excess after CPR: the relationship to CPR duration and the characteristics related to outcome. Resuscitation. 2007;23:394–9.
Orban JC, Novain M, Cattet F, Plattier R, Nefzaoui M, Hyvernat H, et al. Association of serum lactate with outcome after out-of-hospital cardiac arrest treated with therapeutic hypothermia. PLoS One. 2017;12:e0173239.
Jamme M, Ben Hadj Salem O, Guillemet L, Dupland P, Bougouin W, Charpentier J, et al. Severe metabolic acidosis after out-of-hospital cardiac arrest: risk factors and association with outcome. Ann Intensive Care. 2018;8:62.
Carden DL, Martin GB, Nowak RM, Foreback CC, Tomlanovich MC. Lactic acidosis as a predictor of downtime during cardiopulmonary arrest in dogs. Am J Emerg Med. 1985;3:120–4.
Sandroni C, De Santis P, D'Arrigo S. Capnography during cardiac arrest. Resuscitation. 2018;132:73–7.
McKenzie N, Williams TA, Tohira H, Ho KM, Finn J. A systematic review and meta-analysis of the association between arterial carbon dioxide tension and outcomes after cardiac arrest. Resuscitation. 2017;111:116–26.
Tiruvoipati R, Pilcher D, Botha J, Buscher H, Simister R, Bailey M. Association of hypercapnia and hypercapnic acidosis with clinical outcomes in mechanically ventilated patients with cerebral injury. JAMA Neurol. 2018;75:818–26.
Ilicki J, Djarv T. Survival in extremely acidotic cardiac arrest patients depends on etiology of acidosis. Resuscitation. 2017;113:e25.
Wang J, Ma Q, Zhang H, Liu S, Zheng Y. Predictors of survival and neurologic outcome for adults with extracorporeal cardiopulmonary resuscitation: a systemic review and meta-analysis. Medicine (Baltimore). 2018;97:e13257.
Acknowledgements
We appreciate all members and institutions of the CRITICAL study for their contribution.
Funding
This study was supported by a scientific research grant from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (15H05006 and 19 K09393). Funding sources had no role in the study design, analysis and interpretation of the data, and writing of the paper.
Author information
Authors and Affiliations
Consortia
Contributions
YO: conceptualization, validation, verification, visualization, formal analysis, methodology, and writing original draft. Takeyuki K: conceptualization, methodology, validation, verification, formal analysis, and writing review and editing. Takuya I, KY, TY, KH, KN, TN, TI, YY, MK, HS, YH, TS, TM, HS, KS, FN, NN, TM, JS, and SM: resource and data curation. TS: supervision. KK: writing review and editing and supervision. Takashi K: methodology and writing review and editing. Tetsuhisa K: methodology resource, data curation, supervision, project administration, and funding acquisition. Taku I: methodology, writing review and editing, supervision, project administration, and funding acquisition. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Ethics Committee of Kyoto University and each participating institution approved this study protocol (R1045), and written informed consent was waived.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: S-Table 1.
The characteristics of patients with missing BGA. S-Table 2. Original cohort (N=260). S-Table 3. Expected outcome among the excluded patients in the assumption (N=123). S-Table 4. The crude and adjusted ORs of other covariates. S-Table 5. The results of sensitivity analysis. S-Figure. Flow chart in sensitivity analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Okada, Y., Kiguchi, T., Irisawa, T. et al. Association between low pH and unfavorable neurological outcome among out-of-hospital cardiac arrest patients treated by extracorporeal CPR: a prospective observational cohort study in Japan. j intensive care 8, 34 (2020). https://doi.org/10.1186/s40560-020-00451-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40560-020-00451-6