Abstract
Background
Morphological studies on fishes are important from various viewpoints. Studies carried out on the Caspian Sea fishes show that many species possess speciation and population formation microprocess running. Morphological characteristics of the native endangered Caspian lamprey, Caspiomyzon wagneri Kessler 1870, from migrating stocks of two major rivers in the southern Caspian Sea were analyzed to investigate the hypothesis population structure and morphologic sexual dimorphism.
Results
Univariate analysis of variance of 180 adult specimens showed significant differences between the means of the two studied groups for 15 standardized morphometric measurements out of 31 (P < 0.05). In morphometric trait linear discriminant function analysis, the overall assignments of individuals into their original groups in male and female specimens were 77.1 and 84.0 %, respectively. The discriminant analysis showed a morphological segregation of the studied populations based on the characters predorsal length, interdorsal, interorbital distance, tail length, and first dorsal fin length. The principal component analysis, scatter plot of individual component score between PC1 and PC2, showed the specimens grouped into two areas but with high and moderate overlap between two localities in males and females, respectively.
Conclusions
The present study indicated that there are at least two types of morphological forms of Caspian lamprey that had high morphometric differentiation in the rivers across the southern Caspian Sea, which can be considered in conservational policy of this valuable species.
Similar content being viewed by others
Background
Lampreys (Petromyzontiformes) are a significant ecological, cultural, and economically important fish groups in the world. There are about 43 lamprey species in 9 genera with only 1 recorded from Iran (Coad 2015). Caspian lamprey, Caspiomyzon wagneri, is a Eurasian anadromous non-parasitic species (Imanpoor and Abdollahi 2011). The Caspian lamprey is endemic to the Caspian Sea and related river system in its northern, western, and southern watersheds (Holčík 1986) and migrates to the Volga, Ural, Terek, and Kura rivers (Coad 2015; Holčík and Oláh 1992). The Caspian lamprey in the southern Caspian Sea basin migrates to such rivers as Shirud, Talar, Babolrud, Gorganrud, Tajan, Haraz, Sardabrud, Aras, Tonekabon, Polrud, Sefidrud, and Shafarud rivers and the Anzali Lagoon (Imanpoor and Abdollahi 2011; Kiabi et al. 1999; Nazari and Abdoli 2010). This species migrate upstream from the sea where they spend the feeding stage, and when migration starts, lampreys stop growing and begin to mature sexually (Larsen 1980). Adults die after spawning. During the spawning migration, the lamprey undergoes certain morphological changes, some of which have been linked to the sex of the fish (Coad 2015).
The Caspian lamprey is listed as vulnerable generally in Europe (Renaud 1997) and in Iran because it migrates into rivers which are polluted and dammed and because of its restricted and declining distribution (Coad 2015). Also, Kiabi et al. (1999) consider this species to be near threatened in the southern Caspian Sea basin according to the IUCN criteria. Because of the valuable ecological importance of Caspian lamprey, broad studies are performed, in terms of reproduction (Nazari and Abdoli 2010), age and growth parameters, morphology, endocrinology, and histology (Imanpoor and Abdollahi 2011).
The study of morphological characters, morphometric and meristic, with the objective of defining and characterizing populations, has a long tradition in ichthyology (Almeida et al. 2008). Morphological studies on fishes are important from various viewpoints including evolution, ecology, behavior, conservation, water resource management, and stock assessment (AnvariFar et al. 2011). Suitable and successful management of aquatic organism stock will be gained by the study of genetic stocks of endemic species and identification of populations (Coad 1980). The study of morphological characters with the aim of defining or characterizing fish stock units has for some time been a strong interest in ichthyology (Tudela 1999). Studies carried out on the Caspian Sea fishes show that many species possess speciation and population formation microprocess running, as the Black Sea species (Gholiev 1997). There are several reports on the southern Caspian Sea fishes (e.g., Abdolhay et al. 2010; AnvariFar et al. 2011, 2013) which indicate the existence of morphological variability in different parts of this basin. However, information on population variability and differentiation of Caspian lamprey in the southern Caspian Sea basin is still rather limited.
Holčík (1986) studied on the morphological characters of this species in the southern Caspian Sea basin. Also, Ginzburg (1936) described ammocoetes from Iran. Yamazaki and Goto (1996) compared the morphometric and meristic characters of the two groups of brook lamprey in Japan. Holcík (1999) stated that dramatic declines in migratory species such as lampreys, sturgeons, salmons, and clupeids were well known in Europe which require attention. Almeida et al. (2008) investigated the morphological variation and differentiation of sea lamprey ammocoetes in Portuguese river basins. Renaud (2011) gave details on the morphology of lampreys of the world. Hence, it is important to understand that this unit population had morphological differentiation. On the other hand, despite the biodiversity and ecological importance of Caspian lamprey, unfortunately there are limited studies available on population differentiation of the fish in the area. Considering the abovementioned facts, the main aims of this study were the following: (1) obtaining information about population differentiation of this species in two most important rivers at the reproductive migration time in the southern Caspian Sea basin, (2) identifying the best set of characters to establish the separation of the eventual groups, and (3) identifying morphometric sexual dimorphism and determining the characters that have sexual dimorphism. The results of this study can be employed in the conservation purpose of this species in the region.
Methods
Collection of samples
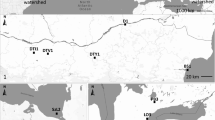
A total of 180 adult individuals of Caspian lamprey were collected from two sampling sites from September to October (2011) comprising 106 individuals from Babolrod (36° 42′ N, 52° 38′ E) and 74 individuals from Kheyrod (51° 30′ N, 36° 39′ E) rivers (Fig. 1). Samplings were done by hand netting reproductive migratory fish at nighttime.
Thirty traditional morphometric characters were measured using an electronic digital caliper to the nearest 0.1 mm (Fig. 2). Measurements follow Holcik et al. (1986) and Renaud (2011). All measurements were made by the same person. After measuring, fish were dissected to identify the sex by macroscopic examination of the gonads and external sexual characters (males with well-developed urogenital papillae and females crescent-shaped extra anal fin). Gender was used as the class variable in the analysis of variance (ANOVA) to test for the significant differences in the morphometric characters if any, between males and females of Caspian lamprey.
Morphometric characters measured on Caspian lamprey samples. Landmarks selected based on the studies of Holcik et al. (1986) and Renaud (2011). LC total length, LD predorsal length, LF predorsal distance, LS second postdorsal length, LG second postdorsal distance, LN head length, LO prenasal length, SC second predorsal fin, MP interbranchial opening distance, ZM postocular length, QA postdisc length, NC postbranchial length, FS interdorsal, LA preanal distance, VC tail length, MN branchial length, LM prebranchial distance, MC postbranchial distance, LE snout length, EM postorbital distance, LQ disc length, AV urogenital papilla length, DF first dorsal fin length, SG second dorsal fin length, R↓ first dorsal fin height, Y↓ second dorsal fin height, D↓ body width, O↓ head depth, EZ eye diameter, EE′ interorbital distance
Statistical analyses
Univariate ANOVA was performed for each morphometric character to evaluate the significant differences among the two locations (Zar 1984), and the morphometric characters which showed significant variation (P < 0.05) were only used so as to achieve the recommended ratio of the number of organisms (N) measured to the parameters (P) included in the analysis to be at least 3–3.5 (Kocovsky et al. 2009) for obtaining the stable outcome from the multivariate analysis. In the present study, linear discriminant function analyses (DFA), principal component analysis (PCA), and cluster analysis (CA) were employed to discriminate the two populations. Principal component analysis helps in morphometric data reduction, in decreasing the redundancy among the variables (Anvarifar et al. 2013; Mousavi-Sabet and Anvarifar 2013; Veasey et al. 2001), and in extracting a number of independent variables for population differentiation. Wilks’ lambda was used to compare the differences among all individuals of the two groups. The DFA was used to calculate the percentage of correctly classified (PCC) fish. A cross-validation using PCC was done to estimate the expected actual error rates of the classification functions. As a complement to discriminant analysis, morphometric distances among the individuals of the two groups were inferred to cluster analysis (Veasey et al. 2001) by adopting the Euclidean distance as a measure of dissimilarity and the UPGMA (unweighted pair group method with arithmetical average) as the clustering algorithm.
Size-dependent variation was corrected by adapting an allometric method as suggested by Elliott et al. (1995):
where M is the original measurement, M adj is the size-adjusted measurement, L 0 is the standard length of the fish, L s is the overall mean of the standard length for all fish from all samples in each analysis, and b is estimated for each character from the observed data as the slope of the regression of log M on log L 0 using all fish from both groups. The results derived from the allometric method were confirmed by testing the significance of the correlation between transformed variables and standard length (Turan 1999).
Statistical analyses for morphometric data were performed using the SPSS version 16 software package, Numerical Taxonomy and Multivariate Analysis System (NTSYS-pc), and Excel (Microsoft office, 2010).
Results
Descriptive data for the sex ratio, range (minimum-maximum), and mean and standard deviation (SD) of length and weight in the case of sampled specimens are shown in Table 1. The correlation between transformed morphometric variables and standard length was non-significant (P > 0.05) that confirmed size or allometric signature on the basic morphological data was accounted. Morphological differences (P < 0.05) were observed between two populations of Caspian lamprey in Babolrod and Kheyrod in the southern Caspian Sea basin for 15 out of 30 morphometric characters (Table 2), and these significant variables were used further for multivariate analysis (PCA, DFA, and CA). The ANOVA revealed effective morphologic characters on sexual dimorphism (P < 0.05) in 14 out of the 30 studied measurements which include LC, LD, LS, SC, QA, LA, LE, LQ, AV, R↓, Y↓, D↓, O↓, and EE′. Therefore, the analyses of morphometric characters were conducted with the sexes separated.
A common problem with many fish morphology studies that use multivariate analysis is potentially inadequate sample size. For decades, authors of theoretical works on PCA and DFA recommended that the ratio of the number of organisms measured (N) relative to the parameters included (P) in the analysis is at least 3–3.5 (Johnson 1981; Kocovsky et al. 2009). Small N values may fail to adequately capture covariance or morphological variation, which may lead to false conclusions regarding differences among groups (Kocovsky et al. 2009). In this study, for multivariate analysis, we used only morphometric characters that were significant at a high level (P < 0.05), and under these circumstances, the N:P ratio was 12 (180/15) for these traits including LD, LF, SC, ZM, MC, FS, VC, MN, NC, LE, EM, DF, SG, O↓, and EE′.
To examine the suitability of the data for principal component analysis, Bartlett’s test of sphericity was performed, and it was significant (P < 0.01). In order to determine which morphometric measurement made most effectively differentiates between the populations, the contributions of variables to principal components (PC) were examined. Principal component analysis of 15 morphometric measurements extracted five factors with eigenvalues >1, explaining 68.98 % of the variance in male, and six factors with eigenvalues >1, explaining 78.86 % of the variance in female (Table 3). The first principal component (PC1) accounted for 26.78 and 26.65 % of the variation and the second principal component (PC2) for 12.96 and 14.52 % in males and females, respectively (Table 4). The most significant loadings on PC1 in males were ZM, MN, and EM and in females were the same. Also, the most significant loadings on PC2 in males were MC and NC and in females were the same. Visual examination of plots of PC1 and PC2 scores revealed that the male specimens were grouped into two areas but with high overlap between two stations. Also, in female visual examination of plots of PC1 and PC2 scores, specimens were grouped into two areas with moderate overlap between two stations (Fig. 3). In this analysis, the characteristics with an eigenvalue exceeding 1 were included and others discarded. It is worth mentioning out here that a factor loading greater than 0.30 is considered significant, 0.40 is considered more important, and 0.50 or greater is considered very significant (Nimalathasan 2009). According to Mousavi-Sabet and Anvarifar (2013), for parsimony, in this study, only those factors with loadings above 0.7 were considered significant.
Wilks’ lambda tests of discriminant analysis indicated significant differences in 15 morphometric characters of the two populations in both sexes. In this test, one function in male and female was highly significant (P < 0.01) (Table 5). The linear discriminant analysis in male gave an average PCC of 77.1 %. Medium classification success rates were obtained for Babolrod (73.1 %) and Kheyrod (84.2 %) that indicate a high correct classification of specimens into their original populations (Table 6). The discriminant analysis in female gave an average PCC of 84.0 % for morphometric characters. The proportion of individuals correctly classified into their original groups was 84.6 and 83.3 % in Babolrod and Kheyrod, respectively, indicating a high rate of correct classification of individuals into their original populations (Table 6). In both male and female, the cross-validation testing procedure was exactly the same as the PCC results. Figures 4 and 5 indicate the coordinates of two populations in the first two axes of DFA. In this analysis, there was a high degree of separation between Caspian lamprey specimens in the southern Caspian Sea basin. The measurements that were used in this analysis for males included LD, FS, and EE′ and for females were VC, DF, and EE′.
Clustering analysis based on Euclidean square distances between the groups of centroids using an UPGMA resulted two main clusters: Babolrod (male), Babolrod (female), and Kheyrod (female) in one group and Kheyrod (male) in the other group. Also, the results of this analysis demonstrated Babolrod (male and female) closed together and far from Kheyrod (male), although they are far apart geographically (Fig. 6).
Discussion
The aim of the present study was to investigate the hypothesis population differentiation and morphologic sexual dimorphism in Caspian lamprey populations using traditional method. The morphometric analysis was planned, whereas the previous studies revealed non-significant differences for meristic between the fish populations (Nazari et al. 2010). Our study results demonstrate that there is significant phenotypic variation between the two studied populations, also between the sexes. Discriminant function analysis could be a useful method to distinguish different stocks of the same species (Karakousis et al. 1991). In the present study, a high classification of individuals that were correctly classified into their respective groups by DFA was achieved (Fig. 4), and this segregation was partly confirmed by PCA. Although, there were some ranges of overlap somewhat in all of the characters examined between two groups. Yamazaki and Goto (1996) compared the morphometric and meristic characteristics between two groups of brook lamprey and reported some ranges of overlap in multivariate analysis. This survey indicated that the population differentiation which resulted from different multivariate analyses in females was higher than that in males. Abdolhay et al. (2010) have shown that the average coefficient of variation (CV %) of raw data, morphometric characters, and ratio in females were higher than those in males of Mahisefid populations. Almeida et al. (2008) stated that the discriminatory power of the meristic characters was comparatively weaker, being responsible for the reduced separation between groups of sea lamprey. The PCA and DFA showed a morphological segregation of the studied populations based on the characters postocular length, branchial length, postorbital distance, postbranchial distance, postbranchial length, predorsal length, interdorsal distance, interorbital distance, tail length, and first dorsal fin length. Almeida et al. (2008) reported that the DFA showed a morphological segregation of the sea lamprey ammocoete populations in Portuguese river basins based on the characters head, tail, and branchial length.
During the spawning migration, the lamprey undergoes certain morphological changes, some of which have been linked to the sex of the fish. Coad (2015) reported that females of Caspian lamprey reach larger sizes than males and have a smaller urogenital papilla. Also, the teeth become blunt, fin size increases, the dorsal fins become almost united at the base in males, and there is a change in color. The results of our survey have shown that half of the morphometric characters have differences between females and males which revealed sexual dimorphism. These characters include LC, LD, LS, SC, QA, LA, LE, LQ, AV, R↓, Y↓, D↓, O↓, and EE′.
These morphological differences may be solely related to body shape variation and not to size effect which was successfully accounted by allometric transformation. On the other hand, size-related traits play a predominant role in morphometric analysis, and the results may be erroneous if not adjusted for statistical analyses of data (Tzeng 2004). In the present study, the size effect had been removed successfully by allometric transformation, and the significant differences between the populations are due to the body shape variation when tested using ANOVA and multivariate analysis. The causes of morphological differences between populations are often quite difficult to explain (Poulet et al. 2004). It has been suggested that the morphological characteristics of fish are determined by genetic, environment, and the interaction between them (Pinheiro et al. 2005; Poulet et al. 2004; Swain and Foote 1999). The environmental factors prevailing during the early development stages, when the individual’s phenotype is more amenable to environmental influence, are of particular importance (Pinheiro et al. 2005). The influences of environmental parameters on morphometric characters are well discussed by several authors in the course of fish population segregation (e.g., Swain and Foote 1999). The habitat of this species in the southern Caspian Sea proper is unknown although some specimens have been caught in the Caspian at 600–700 m (Jolodar and Abdoli 2004; Coad 2015). It seems that isolation by distance and different environmental conditions such as variability of food items, growth pattern, and abiotic characteristics between two stations such as temperature, oxygen, turbidity, and water quality are the mechanisms responsible for population differentiation of Caspian lamprey in the southern Caspian Sea basin.
Conclusions
The present study showed that each sampling site represents an independent population, and there are at least two types of morphological forms of Caspian lamprey that had high morphometric differentiation in the southern Caspian basin. The results can be interesting for management and conservation programs of this valuable endangered species in this region. A detailed study involving the molecular genetics and environmental aspects may further confirm the present findings unambiguously. However, in order to have better conservational policy, further studies are recommended on determining other possible populations of this species in other regions of the Caspian Sea.
Abbreviations
- LC:
-
total length
- LD:
-
predorsal length
- LF:
-
predorsal distance
- LS:
-
second postdorsal length
- LG:
-
second postdorsal distance
- LN:
-
head length
- LO:
-
prenasal length
- SC:
-
second predorsal fin
- MP:
-
interbranchial opening distance
- ZM:
-
postocular length
- QA:
-
postdisc length
- NC:
-
postbranchial length
- FS:
-
interdorsal
- LA:
-
preanal distance
- VC:
-
tail length
- MN:
-
branchial length
- LM:
-
prebranchial distance
- MC:
-
postbranchial distance
- LE:
-
snout length
- EM:
-
postorbital distance
- LQ:
-
disc length
- AV:
-
urogenital papilla length
- DF:
-
first dorsal fin length
- SG:
-
second dorsal fin length
- R↓:
-
first dorsal fin height
- Y↓:
-
second dorsal fin height
- D↓:
-
body width
- O↓:
-
head depth
- EZ:
-
eye diameter
- EE′:
-
interorbital distance
References
Abdolhay HA, Daud SK, Pourkazemi M, Siraj SS, Rezvani S, Mostafa KAS, Hosseinzadeh Sahafi H (2010) Morphometric studies of Mahisefid (Rutilus frisii kutum, Kamensky, 1901) from selected rivers in the southern Caspian Sea. Iran J Fish Sci 9:1–18
Almeida PR, Tomaz G, Andrade NO, Quintella BR (2008) Morphological analysis of geographic variation of sea lamprey ammocoetes in Portuguese river basins. Fish and Diadromy in Europe (ecology, management, conservation). Dev Hydrobiol 200:47–59
AnvariFar H, Khyabani A, Farahmand H, Vatandoust S, AnvariFar H, Jahageerdar S (2011) Detection of morphometric differentiation between isolated up- and downstream populations of Siah Mahi (Capoeta capoeta gracilis) (Pisces: Cyprinidae) in the Tajan River (Iran). Hydrobiologia 673:41–52
AnvariFar H, Farahmand H, Silva DM, Bastos RP, AnvariFar H (2013) Fourteen years after the Shahid-Rajaei dam construction: an evaluation of the morphometric and genetic differentiation between isolated up- and downstream populations of Capoeta Capoeta gracilis (Siah Mahi, Pisces: Cyprinidae) in the Tajan River (Iran). Gen Mol Res 12:3465–3478
Coad BW (1980) Environmental change and its impact on the freshwater fishes of Iran. Biol Conserv 19:51–80
Coad BW (2015) Freshwater fishes of Iran., Available at http://www.briancoad.com (accessed on 16 June 2015)
Elliott NG, Haskard K, Koslow JA (1995) Morphometric analysis of orange roughly (Hoplostethus atianticus) off the continental slope of Southern Australia. J Fish Biol 46:202–220
Gholiev F (1997) Cypriniformes and Perciformes in Caspian Sea. In: (Translated from Russian to Farsi by Adeli, Y.) Iranian Fisheries Research and Training Organization., p 61 [in Farsi]
Ginzburg YAI (1936) Opisaniye lichinok kaspiiskoi minogi Caspiomyzon wagneri (Kessler) iz rechki Cheshmkelya (Iran) [Description of larvae of the Caspian lamprey (Caspiomyzon wagneri (Kessler)) from the Cheshmkelya stream (Iran)]. Izvestiya Azerbaidzhanskogo Filiala Akademii Nauk SSSR 1936(1):47–50
Holčík J (1986) The freshwater fishes of Europe. Balogh Scientific Books. Vol 1: part I., petromyzontidae., pp 117–140
Holcík J (1999) The impact of stream regulations upon the fish fauna and measures to prevent it. In: Stomboudi MT, Kottelat M, Barbieri R (eds) Workshop on Mediterranean stream fish ecology and conservation, Rhodes, Hellas, 1–3 November 1999., p 13
Holčík J, Oláh J (1992) Fish, fisheries and water quality in Anzali Lagoon and its watershed. In: Report prepared for the project-Anzali Lagoon productivity and fish stock investigations. FAO, Rome, FI:UNDP/IRA/88/001
Imanpoor MR, Abdollahi M (2011) Serum biochemical parameters of Caspian lamprey, Caspiomyzon wagneri during final spawning migration. World Appl Sci J 12(5):600–606
Johnson DH (1981) How to measure habitat: a statistical perspective. In: U.S. Forest Service General Technical Report RM, vol 87., pp 53–57
Jolodar MN, Abdoli A (2004) Fish species atlas of south Caspian Sea basin (Iranian waters). Iranian Fisheries Research Organization, Teheran, p 110, In Farsi and English
Karakousis Y, Triantaphyllidis C, Economidis PS (1991) Morphological variability among seven populations of brown trout, Salmon trutta L., in Greece. J Fish Biol 38:807–817
Kiabi BH, Abdoli A, Naderi M (1999) Status of the fish fauna in the South Caspian basin of Iran. Zool Middle East 18:57–65
Kocovsky PM, Adams JV, Bronte CR (2009) The effect of sample size on the stability of principal component analysis of truss-based fish morphometrics. T Am Fish Soc 138:487–496
Larsen LO (1980) Physiology of adult lampreys, with special regard to natural starvation, reproduction, and death after spawning. Can J Fish Aquat Sci 37:1762–1779
Mousavi-Sabet H, Anvarifar H (2013) Landmark-based morphometric variation between Cobitis keyvani and Cobitis faridpaki (Pisces: Cobitidae), with new habitat for C. faridpaki in the southern Caspian Sea basin. Folia Zool 62(3):167–175
Nazari H, Abdoli A (2010) Some reproductive characteristics of endangered Caspian lamprey (Caspiomyzon wagneri Kessler, 1870) in the Shirud River southern Caspian Sea, Iran. Environ Biol Fish 88:87–96
Nimalathasan B (2009) Determinants of key performance indicators (KPIs) of private sector banks in Sri Lanka: an application of exploratory factor analysis. In: The annals of the Stefan cel Mare University of Suceava, vol 9, Fascicle of the Faculty of Economics and Public Administration., pp 9–17
Pinheiro A, Teixeira CM, Rego AL, Marques JF, Cabral HN (2005) Genetic and morphological variation of Solea lascaris (Risso, 1810) along the Portuguese coast. Fish Res 73:67–78
Poulet N, Berrebi P, Crivelli AJ, Lek S, Argillier C (2004) Genetic and morphometric variations in the pikeperch (Sander lucioperca L.) of a fragmented delta. Archiv Fuer Hydrobiologie 159:531–554
Renaud CB (1997) Conservation status of northern hemisphere lampreys. J Appl Ichthyol 13:143–148
Renaud CB (2011) Lampreys of the world. In: An annotated and illustrated catalogue of lamprey species known to date, FAO Species Catalogue for Fishery Purposes. No. 5. FAO, Rome, p 109
Swain DP, Foote CJ (1999) Stocks and chameleons: the use of phenotypic variation in stock identification. Fish Res 43:113–128
Tudela S (1999) Morphological variability in a Mediterranean, genetically homogeneous population of the European anchovy, Engraulis encrasicolus. Fish Res 42:229–243
Turan C (1999) A note on the examination of morphometric differentiation among fish populations: the truss system. Turk J Zool 23:259–263
Tzeng TD (2004) Morphological variation between populations of spotted mackerel Scomber australasicus of Taiwan. Fish Res 68:45–55
Veasey EA, Schammass EA, Vencovsky R, Martins PS, Bandel G (2001) Germplasm characterization of Sesbania accessions based on multivariate analyses. Genet Resour Crop Ev 48:79–90
Yamazaki Y, Goto A (1996) Genetic differentiation of Lethenteron reissneri populations, with reference to the existence of discrete taxonomic entities. Ichthyol Res 43:283–299
Zar JH (1984) Biostatistical analysis. Prentice Hall, Englewood Cliffs, N.J.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SV provided the materials and drafted the manuscript. HM-S made comments on the manuscript and revised it. MR-M provided the materials and drafted the manuscript. HA carried out the experiments and drafted the manuscript. AH measured the specimens. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Vatandoust, S., Mousavi-Sabet, H., Razeghi-Mansour, M. et al. Morphometric variation of the endangered Caspian lamprey, Caspiomyzon wagneri (Pisces: Petromyzontidae), from migrating stocks of two rivers along the southern Caspian Sea. Zool. Stud. 54, 56 (2015). https://doi.org/10.1186/s40555-015-0133-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40555-015-0133-8