Abstract
Background
Solifenacin succinate is a competitive muscarinic acetylcholine receptor antagonist used in the treatment of overactive bladder with or without urge incontinence.
Methods
Liquid chromatography–tandem mass spectrometry method was used for quantification of Solifenacin (SF) in rat plasma. Solifenacin-d5 (SFD5) used as an internal standard. Chromatographic separation was performed Gemini-NX C18, 50 × 4.6 mm, 5 µm, 110 Å column. Mobile phase composed of 5 mM Ammonium formate, pH 3.0: methanol (20:80 v/v), with 0.4 mL/min flow-rate. Drug and IS were extracted by Liquid- liquid extraction method. SF and SFD5 were detected with proton adducts at m/z 363.2®193.2 and 368.2®198.2 in multiple reaction monitoring (MRM) positive mode respectively. The method was validated with the correlation coefficients of (r2) ≥ 0.9975 over a linear concentration range of 0.1-100.0 ng/mL.
Results
This method demonstrated intra and inter-day precision within 1.09 to 4.84 and 1.75 to 7.68 % and accuracy within 101.21 to 106.67 and 97.94 to 104.79 % for SF.
Conclusions
This method is successfully applied in the Pharmacokinetic study of rat plasma.
Similar content being viewed by others
Background
Solifenacin succinate is a competitive muscarinic acetylcholine receptor antagonist used in the treatment of overactive bladder with or without urge incontinence. Chemically, it is 1-azabicyclo[2.2.2]oct-8-yl(1S)-1-phenyl-3,4-dihydro-1H-isoquinoline-2-carboxylate.
The molecular formula of Solifenacin succinate is C23H26N2O2 with its molecular weight 362.46. Solifenacin is extensively metabolized in the liver. One pharmacologically active metabolite (4R-hydroxy Solifenacin) occurs at low concentrations and three pharmacologically inactive metabolites (N-glucuronide and the N-oxide and 4R-hydroxy-N-oxide of Solifenacin) in human plasma after oral dosing. After oral administration of vesicare to healthy volunteers, peak plasma levels (Cmax) of Solifenacin reached within 3 to 8 h after administration and at steady state ranged from 32.3 to 62.9 ng/mL for the 5 and 10 mg vesicare tablets, respectively. The terminal elimination half-life of Solifenacin (SF) is approximately 45 to 68 h. Solifenacin is approximately 98% (in vivo) bound to human plasma proteins, principally to alpha1-acid glycoprotein (Morales-Olivas and Estan [2010]; Doroshyenko and Fuhr [2009]; Hoffstetter and Leong [2009]; Kuipers et al. [2004]; Leone Roberti Maggiore et al. [2012]; Uchida et al. [2004]; Yamada et al. [2012]; Maruyama et al. [2008]; Kuipers et al. [2006]; Callegari et al. [2011]).
Literature survey reveals that quantification of Solifenacin in human plasma (Macek et al. [2010]; Mistri et al. [2008]), rat plasma (Yanagihara et al. [2007]), pharmaceutical compounds (Krishna et al. [2010]; Desai et al. [2012]; Rami Reddy et al. [2013]; Desai et al. [2011]), and industrial waste streams (Ann-Marie et al. [2011]) were reported. These methods were reported by using liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-MS/MS) (Macek et al. [2010]; Mistri et al. [2008]; Ann-Marie et al. [2011]), high-performance liquid chromatography (HPLC) (Yanagihara et al. [2007]; Krishna et al. [2010]; Desai et al. [2012]; Rami Reddy et al. [2013]), and HPTLC (Desai et al. [2011]). Among all, quantification of Solifenacin by LC-MS/MS in biological matrices (Macek et al. [2010]; Mistri et al. [2008]; Yanagihara et al. [2007]) was proved best results.
Macek et al. [2010] reported with the linearity range of 0.47 to 42 ng/mL and used PPT method for extraction of drug and internal standard (IS). They used 250 μL of plasma sample for extraction procedure and obtained good chromatography within 3 min run time. They used Solifenacin d5 as an internal standard. Mistri et al. ([2008]) reported with the linearity range of 0.6 to 60 ng/mL and used liquid-liquid extraction (LLE) method for extraction of drug and IS. They used 500 μL of plasma sample for extraction procedure and obtained good chromatography with in 3 min run time. They used propranolol as an internal standard. (Yanagihara et al. [2007]) reported with the linearity range of 2.0 to 2,000.00 ng/mL and used LLE method for extraction of drug and IS. They used 1,000 μL of plasma sample for extraction procedure and obtained good chromatography within 25 min run time. They used ((−)-[(1R or 1S),3_R]-quinuclidin-3_-yl 1-benzyl-1,2,3,4-tetrahydroisoquinoline-2-carboxylate monohydrochloride as an internal standard.
The reported methods have some drawbacks in terms of large amount of plasma sample usage, lower sensitivity (Macek et al. [2010]; Mistri et al. [2008]; Yanagihara et al. [2007]), long run analysis time, and internal standard usage (Yanagihara et al. [2007]). The main goal of the present study is to develop and validate the novel simple, higher sensitive, selective, rapid, rugged, and reproducible bioanalytical method for quantitative determination of SF in rat plasma by LC-MS/MS with a small amount of sample volume. The developed method would be applied in the pharmacokinetic study of rat plasma.
Methods
Chemicals and reagents
Solifenacin succinate, Solifenacin d5 hydrochloride obtained from Hetero Drugs, Hyderabad, India (Figure 1). HPLC grade methanol was purchased from Jt. Baker Mallinckrodt Baker, Inc., Phillipsburg, NJ, USA. Formic acid was purchased from S.D. Fine Chemicals, Mumbai, India. Diethyl ether was purchased from Merck Speciality Private Limited, Worli, Mumbai, India. Ultrapure water from Milli-Q system (Millipore, Bedford, MA, USA) was used through the study. All other chemicals in this study were of analytical grade. Rats were obtained from Bioneeds, Bangalore, Karnataka, India.
Instrumentation
HPLC system (1200 Series Agilent Technologies, Deutschland, Germany) connected with triple quadrupole mass spectrometer instrument (API 4000, Toronto, Canada). Data processing was performed with the Analyst 1.5.1 software package (SCIEX, Framingham, MA 01701, USA). Ionization was performed by electrospray positive mode with unit resolution.
Detection
Mass parameters were optimized to get the product ions of m/z, 193.2 and m/z, 198.2 from its respective precursor ions of SF [M + H]+ (m/z, 363.2) and SFD5 [M + H]+ (m/z, 368.2) with source temperature 500°C, ion spray voltage 5,500 V, heater gas, nebulizer gas 35 psi each, curtain gas 20 psi, CAD gas 5 psi (all gas channels with nitrogen), source flow rate 400 μL/min without split, entrance potential 10 V, declustering potential 55 V for analyte and 65 V for internal standard, collision energy 25 V for both analyte and internal standard, and collision cell exit potential 13 V for analyte and 12 V for internal standard.
Chromatographic conditions
Chromatography was performed using Gemini-NX C18, 50 × 4.6 mm, 5 μm, 110 Å analytical column at 30°C, with 5 mM ammonium formate, pH 3.0/methanol (20:80 v/v) as mobile phase at a flow rate of 0.4 mL/min. SFD5 was used as an internal standard in terms of chromatography and extractability. The drug and internal standard was eluted at 1.2 ± 0.2 min with 2 min total run time.
Preparation of standards and quality control samples
Standard stock solutions of SF (50.0 μg/mL) and SFD5 (50.0 μg/mL) was prepared in methanol. The internal standard spiking solution (50.0 ng/mL) was prepared in water from SFD5 standard stock solution (50.0 μg/mL). Standard stock solutions and internal standard spiking solutions were stored in refrigerator conditions (2° C to 8° C) until analysis. Standard stock solution of SF was added to screened drug-free rat plasma to obtain concentration levels of 0.1, 0.2, 1.0, 5.0, 10.0, 40.0, 60.0, 80.0, and 100.0 ng/mL for analytical standards and 0.1, 0.3, 50.0, 75.0 ng/mL for quality control (QC) standards and stored in a −30° C freezer until analysis. Respective aqueous standards were prepared in reconstitution solution (5 mM ammonium formate pH 3.0/methanol (20:80) and stored in refrigerator conditions 2° C to 8° C until analysis.
Sample preparation
Liquid-liquid extraction was used to isolate drug and IS from rat plasma. For this purpose, 50 μL of IS (40.0 ng/mL) and 100 μL of plasma sample (respective concentration) was added into labeled polypropylene tubes and vortexed briefly. Followed by 2.5 mL of extraction solvent (diethyl ether) was added and vortexed for 10 min. Then, the samples were centrifuged at 4,000 rpm for 10 min at 20°C temperature. Subsequently, the supernatant from each sample was transferred into respective polypropylene tubes. After that, all the samples were kept for evaporation under nitrogen at 40°C. The dried residue was reconstituted with 1,000 μL of reconstitution solution (5 mM ammonium formate pH 3.0/methanol (20:80)) and vortexed briefly. Finally, the extracted sample was transferred into auto sampler vials and injected into LC-MS/MS.
Selectivity and specificity
The selectivity of the method was determined by six different rat blank plasma samples, which were pretreated and analyzed to test the potential interferences of endogenous compounds co-eluting with analyte and IS. Chromatographic peaks of analyte and IS were identified based on their retention times and multiple reaction monitoring (MRM) responses. The peak area of SF at the respective retention time in blank samples should not be more than 20% of the mean peak area of limit of quantification (LOQ) of SF. Similarly, the peak area of SFD5 at the respective retention time in blank samples should not be more than 5% of the mean peak area of LOQ of SFD5.
Recovery
The extraction recovery of SF and SFD5 from rat plasma was determined by analyzing quality control samples. Recovery at three concentrations (1.5, 25.0, and 35.0 ng/mL) was determined by comparing peak areas obtained from the plasma sample and the standard solution spiked with the blank plasma residue. A recovery of more than 85% was considered adequate to obtain required recovery.
Limit of detection and limit of quantification
The limit of detection (LOD) is a parameter that provides the lowest concentration in a sample that can be detected from background noise but not quantitated. LOD was determined using the signal-to-noise ratio (S/N) of 3:1 by comparing test results from samples with known concentrations of analytes with blank samples.
The LOQ is defined as the lowest concentration of analyte that can be determined with acceptable precision and accuracy. The LOQ was found by analyzing a set of mobile phase and plasma standards with a known concentration of SF.
Matrix effect
To predict the variability of matrix effects in samples from individual subjects, matrix effect was quantified by determining the matrix factor, which was calculated as follows:
Six lots of blank biological matrices were extracted each in triplicates and postspiked with the aqueous standard at the low and high QC level and compared with aqueous standards of same concentration. The overall precision of the matrix factor is expressed as coefficient of variation (%CV) and %CV should be <15%.
Calibration curve, precision, and accuracy
The calibration curve was constructed using values ranging from 0.1 to 100.0 ng/mL of SF in rat plasma. Calibration curve was obtained by linear model with weighted 1/x2 regression analysis. The ratio of SF/SFD5 peak area was plotted against the ratio of SF concentration in ng/mL. Calibration curve standard samples and quality control samples were prepared in replicates (n = 6) for analysis. Precision and accuracy for the back-calculated concentrations of the calibration points, should be within ≤15 and ± 15% of their nominal values. However, for LLOQ, the precision and accuracy should be within ≤20 and ±20%.
Stability (freeze - thaw, auto sampler, bench top, long-term) of SF in plasma
Low-quality control and high-quality control samples (n = 6) were retrieved from a deep freezer after three freeze-thaw cycles according to the clinical protocol. Samples were stored at −30°C in three cycles of 24, 36 and 48 h. In addition, the long-term stability of SF in quality control samples was also evaluated by analysis after 45 days of storage at −30°C. Autosampler stability was studied following 61.5-h storage period in the autosampler tray with control concentrations. Bench top stability was studied for 25-h period with control concentrations. Stability samples were processed and extracted along with the freshly spiked calibration curve standards. The precision and accuracy for the stability samples must be ≤15 and ±15%, respectively, of their nominal concentrations.
Application to pharmacokinetic study
The developed LC-MS/MS method was successfully applied to a pharmacokinetic study by administration of SF to six male Sprague-Dawley rats by oral route using BD syringe attached with oral gavage needle (size 18) at the dose of 4 mg/200 g body weight. Required quantity of test item (4 mg SF) was accurately weighed and transferred to a mortar and triturated with 2% (w/w) Tween 80 using a pestle to make a smooth paste. Then, 0.5% (w/w) carboxymethyl cellulose (CMC) was gradually added and suspended to make a required concentration (1 mg/mL of SF). The final suspension was kept under continuous stirring till the dose administration. Approximately, 0.2 mL of blood samples from each anesthetized (isoflurane) rat at pre-determined time intervals was collected from the retro-orbital plexus using a capillary tube into pre-labeled eppendorf tubes containing 10% of K2EDTA anticoagulant (20 μL). The time intervals for the sample collection were 0 (predose), 0.5, 1, 2, 3, 4, 5, 6, 8, 16, 24, 36, 48, and 72 h (postdose). The total blood volume collected from each rat was approximately 1.9 to 2.2 mL which does not exceed the maximal recommended blood volume of 20% (2.0 mL for a 200 g body weight rat). Plasma was obtained by centrifuging blood samples at 3,000 rpm for 10 min. The obtained plasma samples were transferred into pre-labeled microcentrifuge tubes and stored at −30°C. All the samples were analyzed by the developed method. Pharmacokinetic parameters were calculated by non-compartmental analysis by using Win Nonlin® 5.1 software. Concentrations obtained from the above bio-analytical method were compiled (FDA [2002]; FDA [2003]).
Results and discussion
Method development and method validation
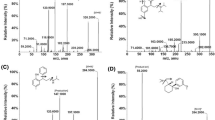
LC-MS/MS has been used as one of the most powerful analytical tools in clinical pharmacokinetics for its selectivity, sensitivity, and reproducibility. The goal of this work is to develop and validate a simple, sensitive, rapid, rugged, and reproducible assay method for the quantitative determination of SF from rat plasma samples. Chromatographic conditions, especially the composition and nature of the mobile phase, usage of different columns, and different extraction methods such as solid phase, precipitation, and liquid-liquid extraction methods were optimized through several trials to achieve the best resolution and increase the signal of SF and SFD5. The MS optimization was performed by direct infusion of solutions of both SF and SFD5into the ESI source of the mass spectrometer. The critical parameters in the ESI source that include the needle (ESI) voltage, capillary voltage, source temperature, and other parameters such as nebulizer gas, heater gas, and desolvation gasses were optimized to obtain a better spray shape, resulting in better ionization of the protonated ionic SF and SFD5 molecules. Product ion spectrum for SF and SFD5 yielded high-abundance fragment ions of m/z 193.2 and m/z 198.2, respectively (Figures 2 and 3). After mass spectrometer parameters optimized, chromatographic conditions such as mobile phase optimization, column optimization, and extraction method optimization was performed to obtain a fast and selective LC method.
A good separation and elution were achieved using 5 mM ammonium formate, pH 3.0/methanol (20:80 v/v) as the mobile phase at a flow rate of 0.4 mL/min and injection volume of 5 μL. Gemini-NX C18, 50 × 4.6 mm, 5 μm, 110 Å column, and liquid-liquid extraction method was optimized for the best chromatography (Figure 4).
The developed method was validated over a linear concentration range 0.1 to 100.0 ng/ml. The validation parameters that include selectivity and specificity, LOD and LOQ, matrix effect, precision and accuracy, recovery, stability (freeze - thaw, auto sampler, bench top, long-term) were evaluated under validation section (FDA [2001]).
Selectivity and specificity
The analysis of SF and SFD5 using MRM function was highly selective with no interfering compounds. Chromatograms obtained from plasma spiked with SF (0.1 ng/mL) and SFD5 (50.0 ng/mL) are shown in Figure 4.
Limit of detection and quantification
The limit of detection was used to determine the instrument detection levels for SF even at low concentrations. Five microliters of a 0.5-pg/mL solution was injected, and estimated LOD was 3.2 fg with S/N values ≥3 to 5. The limit of quantification for this method was proven as the lowest concentration of the calibration curve which was proven as 0.1 ng/ml.
Matrix effect
Six lots of blank biological matrices were extracted each in triplicates and postspiked with the aqueous standard at the mid-QC level and compared with neat standards of the same concentration in alternate injections. The overall precision of the matrix factor is 3.45 for Solifenacin. There was no ion suppression and ion enhancement effect observed due to IS and analyte at respective retention time.
Precision and accuracy
Calibration curves were plotted as the peak area ratio (SF/SFD5) versus (SF) concentration. Precision and accuracy of calibration curve standards and quality control standards are represented in Tables 1 and 2.
Stability (freeze - thaw, auto sampler, bench top, long-term)
Quantification of the SF in plasma subjected to three freeze-thaw cycles (−30°C to room temperature), autosampler, room temperature (benchtop), and long-term stability details was shown in Table 3.
Recovery
The recovery following the sample preparation using liquid-liquid extraction with diethyl ether was calculated by comparing the peak area of SF in plasma samples with the peak area of solvent samples and was estimated at control levels of SF. The recovery of SF was determined at three different concentrations; 1.5, 25.0, and 50.0 ng/mL were found as 82.05%, 80.94%, and 83.33%, respectively. The overall average recovery of SF and SFD5 were found to be 82.11% and 78.86%, respectively.
Pharmacokinetics and statistical analysis
The validated method has been successfully applied to quantify SF concentrations into a single dose (4 mg/200 g body weight of rat) in rats. The pharmacokinetic parameters evaluated were Cmax (maximum observed drug concentration during the study), AUC0 to 72 (area under the plasma concentration-time curve measured 24 h, using the trapezoidal rule), Tmax (time to observe maximum drug concentration), Kel (apparent first-order terminal rate constant calculated from a semi-log plot of the plasma concentration versus time curve, using the method of least square regression), and T1/2 (terminal half-life as determined by quotient 0.693/Kel). Pharmacokinetic details are shown in Table 4. The mean concentration versus time profile of SF in rat plasma is shown in Figure 5 FDA ([2002]), FDA ([2003]) Guidence.
Conclusions
The method described in this manuscript has been developed and validated over the concentration range of 0.1 to 100.0 ng/mL in rat plasma. The intrabatch precision was less than 4.84% and accuracy ranged from 101.21% to 106.67%. The interbatch precision was less than 7.68% and accuracy ranged from 97.94% to 104.79%. The selectivity, sensitivity, precision, and accuracy obtained with this method make it suitable for the purpose of the present study. The simplicity of the method, and using rapid liquid-liquid extraction and sample turnover rate of 2.0 min per sample, makes it an attractive procedure in high-throughput bioanalysis of Solifenacin. The validated method was successfully applied in the pharmacokinetic study of rats by oral administration of 4 mg/200 g in six healthy rats.
Abbreviations
- LC-MS/MS:
-
liquid chromatography-electrospray ionization-tandem mass spectrometry
- MRM:
-
multiple reaction monitoring
- SF:
-
Solifenacin
- SFD5:
-
Solifenacin d5
- LLOQ:
-
lower limit of quantification
- LOQ:
-
limit of quantification
- IS:
-
internal standard
- CAD:
-
collisionally activated dissociation
References
Ann-Marie D, Mark C, Michael O, Kieran N, John T, Anne M: A SPE-LC-MS/MS Method for the Detection of Low Concentrations of Pharmaceuticals in Industrial Waste Streams. Anal Lett 2011, 44(17):2808–2820.
Callegari E, Malhotra B, Bungay PJ, Webster R, Fenner KS, Kempshall S, LaPerle JL, Michel MC, Kay GG: A comprehensive non-clinical evaluation of the CNS penetration potential of antimuscarinic agents for the treatment of overactive bladder. Br J Clin Pharmacol 2011, 72(2):235–46.
Desai DJ, Patel G, Ruikar D, Jain RA, Rajput SJ: Development and validation of stability-indicating HPTLC method of solifenacin succinate. Asian J Phar Biol Res. 2011, 1(3):310–316.
Desai D, Patel G, Shukla N, Rajput S: Development and validation of stability-indicating HPLC method for solifenacin succinate: Isolation and identification of major base degradation product. Acta Chromatogr 2012, 24(3):399–418. [http://www.akademiai.com/content/48123rm7t8085531/] http://www.akademiai.com/content/48123rm7t8085531/
Doroshyenko O, Fuhr U: Clinical pharmacokinetics and pharmacodynamics of solifenacin. Clin Pharmacokinet 2009, 48(5):281–302.
FDA (2001) Guidance for industry: bioanalytical method validation, U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER)
FDA (2002) Guidance for industry: Food- effect bio availability and Fed Bio equivalence studies. U.S. Department of Health and Human services Food and Drug Administration Centre for Drug Evaluation and research (CDER)
FDA (2003) Guidance for industry Bio availability and Fed Bio equivalence Studies for Orally Administered Drug Products-General considerations U.S. Department of Health and Human services Food and Drug Administration Centre for Drug Evaluation and research (CDER)
Hoffstetter S, Leong FC: Solifenacin succinate for the treatment of overactive bladder. Expert Opin Drug Metab Toxicol 2009, 5(3):345–50.
Krishna SR, Rao BM, Rao NS: A validated rapid stability-indicating method for the determination of related substances in solifenacin succinate by ultra-fast liquid chromatography. J Chromatogr Sci 2010, 48(10):807–10.
Kuipers ME, Krauwinkel WJ, Mulder H, Visser N: Solifenacin demonstrates high absolute bioavailability in healthy men. Drugs R D 2004, 5(2):73–81.
Kuipers M, Smulders R, Krauwinkel W, Hoon T: Open-label study of the safety and pharmacokinetics of solifenacin in subjects with hepatic impairment. J Pharmacol Sci 2006, 102(4):405–12.
Leone Roberti Maggiore U, Salvatore S, Alessandri F, Remorgida V, Origoni M, Candiani M, Venturini PL, Ferrero S: Pharmacokinetics and toxicity of antimuscarinic drugs for overactive bladder treatment in females. Expert Opin Drug Metab Toxicol 2012, 8(11):1387–408.
Macek J, Ptacek P, Klima J: Determination of solifenacin in human plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010, 878(31):3327–30.
Maruyama S, Tsukada H, Nishiyama S, Kakiuchi T, Fukumoto D, Oku N, Yamada S: In vivo quantitative autoradiographic analysis of brain muscarinic receptor occupancy by antimuscarinic agents for overactive bladder treatment. J Pharmacol Exp Ther 2008, 325(3):774–81.
Mistri HN, Jangid AG, Pudage A, Rathod DM, Shrivastav PS: Highly sensitive and rapid LC-ESI-MS/MS method for the simultaneous quantification of uroselective alpha1-blocker, alfuzosin and an antimuscarinic agent, solifenacin in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2008, 876(2):236–44. http://www.ncbi.nlm.nih.gov/pubmed/19010093
Morales-Olivas FJ, Estan L: Solifenacin pharmacology. Arch Esp Urol. 2010, 63(1):43–52.
Rami Reddy BV, Srinivasa Reddy B, Raman NVVSS, Reddy KS, Rambabu C: Development and Validation of a Specific Stability Indicating High Performance Liquid Chromatographic Methods for Related Compounds and Assay of Solifenacin Succinate. J Chem 2013, ᅟ: ᅟ. [http://dx.doi.org/10.1155/2013/412353] http://dx.doi.org/10.1155/2013/412353
Uchida T, Krauwinkel WJ, Mulder H, Smulders RA: Food does not affect the pharmacokinetics of solifenacin, a new muscarinic receptor antagonist: results of a randomized crossover trial. Br J Clin Pharmacol 2004, 58(1):4–7. http://www.ncbi.nlm.nih.gov/pubmed/15206986
Yamada S, Kuraoka S, Osano A, Ito Y: Characterization of bladder selectivity of antimuscarinic agents on the basis of in vivo drug-receptor binding. Int Neurourol J 2012, 16(3):107–15.
Yanagihara T, Aoki T, Soeishi Y, Iwatsubo T, Kamimura H: Determination of solifenacin succinate, a novel muscarinic receptor antagonist, and its major metabolite in rat plasma by semi-micro high performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2007, 859(2):241–5.
Acknowledgements
The authors wish to thank the support received from Indian Institute of Chemical Technology (IICT), Hyderabad, India, for providing literature survey and Anthem Bioscience Pvt., Ltd., Banglore, India, for carrying out this research work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SBP carried out the Protocal writing,design of the study, RPS and BRC participated in the Bioanalysis and Pharmacokinetic study, CKB drafted the manuscript and participate the technical guidence of the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Puttagunta, S.B., Shaik, R.P., Bannoth, C.K. et al. Bioanalytical method for quantification of Solifenacin in rat plasma by LC-MS/MS and its application to pharmacokinetic study. J Anal Sci Technol 5, 35 (2014). https://doi.org/10.1186/s40543-014-0035-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40543-014-0035-0