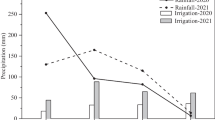
Abstract
Background
Climate change is increasingly impacting agriculture all over the world, with extended periods of drought, flooding, hail, winds and temperature extremes. To negate the effects of climate change, researchers are trying to find new agricultural production techniques, to save resources without losing yield quality and quantity. A study on Capsicum annuum L. 'Chili-AS Rot' and Capsicum chinense Jacq. 'Naga Morich' chilies, grown under field capacity, deficit irrigation (50% field capacity) and full and half dose of mineral fertilizer in peat soil substrate was conducted. Both cultivars were marked with the stable isotope 15N, to follow nitrogen translocation in chili plants under stress conditions.
Results
The yield and plant biomass, capsaicin synthase (CS), phenylalanine ammonia lyase (PAL), and peroxidase (POX), as well as total and individual capsaicinoids were studied. In 'Chili-AS Rot' the deficit irrigation with the full-fertilization (Diff) dose showed the highest yield and fruit number, compared to other treatments. In the 'Naga Morich' cultivar the deficit irrigation and half-fertilization (Dihf), showed the highest yield, fruit number and dry weight of chilies, compared to other treatments. In the cultivar 'Chili-AS Rot', deficit irrigation was found to reduce the utilization of N fertilizer when the plant (leaves, stems, and roots) and fruit were considered. The utilization of nitrogen fertilizer was 60 to 70% under deficit fertilization, regardless of the irrigation treatment. The half dose fertilized plants of 'Chili-AS Rot' had higher CS activity and PAL activity (from 50 to 80%) than fully fertilized plants, with deficit irrigation having about 10% higher enzymatic activity than field-capacity irrigated plants. In 'Naga Morich', irrigation treatment affected lower enzymatic activity than field-capacity irrigated plants.
Conclusions
The results indicate that deficit irrigation and fertilization can be used to maintain enzymatic activity and thus capsaicinoid content, which could reduce the economic cost of irrigation water and fertilizer. It was confirmed that the yield was better under deficit irrigation and fertilization for the more pungent 'Naga Morich'. The result of the study shows that when water and fertilizer use is reduced by up to 50%, chili yield losses are minimal or non-existent.
Graphical Abstract

Similar content being viewed by others
Introduction
Chilies are plants of the genus Capsicum with more than 30 known species, five of which, namely Capsicum annuum L., Capsicum baccatum L., Capsicum chinense Jacq., Capsicum frutescens L. and Capsicum pubescens Ruiz & Pav. are intensively cultivated throughout the world [1]. Generally, chilies are grown outdoors in warm climates and in greenhouses in cooler climates because they require higher temperatures to produce adequate yields [2]. With increasing climate change, especially drought, the demand for irrigation water and nutrients is changing. Studies are trying to find optimal solutions to declining water availability, with one possible solution being deficit irrigation, where farmers reduce irrigation with minimal yield loss [3]. With decreasing water use, there could be a potential problem of soil salinization from fertilizers. Soil salinization could be decreased with reduced fertilization, which in combination with deficit irrigation could result in minimal or no yield loss with reduced fertilizer and water use [4].
Plants under deficit irrigation experience a controlled, milder form of drought stress. Drought generally affects CO2 assimilation, photosynthesis, water use efficiency, enzyme activity, and plant metabolism in general. Due to the photorespiratory pathway, metabolite fluxes occur, leading to increased oxidative stress and thus increased formation of re-active oxygen species (ROS). ROS is the main reason for plant growth decline. A plant's adaptation to drought stress is genetic or phenotypic, with deeper root systems and smaller leaves. At the chemical level, a plant responds with different enzymatic activity and with more or less active metabolic pathways. Chili plants are known to respond with altered capsaicinoid content under drought stress conditions [5]. Deficit irrigation is used to stimulate plant response to drought stress, but with fewer negative effects that could result from uncontrollable drought stress.
Drought reduces nitrogen (N) uptake, transport, and redistribution [6]. Drought stress and the resulting reduction in soil moisture reduces plant nutrient supply through mineralization, reduces nutrient diffusion and mass flux in the soil, all of which contribute to lower plant growth and metabolic activity [7]. In addition, excessive fertilizer application reduces soil organic matter and soil enzyme and microbial activity [8].
To track the effects of deficit irrigation and reduced fertilizer application, stable isotopes can serve as valuable non-radioactive tracers of how plants interact and respond with the abiotic and biotic environment. Using the stable isotope of nitrogen, 15N, we can track the transport and translocation of nitrogen from fertilizer to plant tissue under stress conditions [9].
In our study, we attempted to determine how nitrogen is transferred using the stable isotope 15N in two chili cultivars grown under two irrigation conditions (field capacity and deficit irrigation) and two fertilization practices (full dose of fertilizer and 50% fertilizer). The yield and plant biomass were monitored. In addition, the metabolic response of the plants was monitored with an in-depth analysis of individual capsaicinoid levels and enzymatic activity. With regard to enzymatic activity, the enzymes capsaicin synthase (CS), phenylalanine ammonia lyase (PAL), and peroxidase (POX) were studied, all of which are critical for the synthesis and degradation of capsaicinoids.
Materials and methods
A pot experiment was conducted in Kamnik, Slovenia (46°13′33.88 "N; 14°36′39 "E) from May 20 to September 10, 2021 in a plastic greenhouse. In the experiment two chili cultivars were used, Capsicum annuum L. var. 'Chili-AS Rot' and Capsicum chinense Jacq. Var. 'Naga Morich' (Austrosaat). The experiment contained 40 plants (20 plants for each cultivar). A detailed experimental design is presented in Fig. 1. The pots were filled with peat soil (Klasmann N3). The substrate was a mix of white peat and black peat. The data of substrate were as follows: pH 5.5; salinity 1.3 g/L; N 180 mg/L; P 130 mg/L; K 230 mg/L; Mg 100 mg/L; S 130 mg/L.
Each pot was equipped with drip irrigation and the irrigation was activated based of the measurements of the T5 pressure transducer tensiometer (MeterGroup) and with the Theta probe (Delta-T, UK), using electronic valves connected to data loggers, which enabled continues measurements of the peat soil water tension and volumetric water content. Half of the experimental plants were irrigated at the peat substrate field capacity, and the other half of the chili plants at 50% of field capacity (deficit irrigation). The peat soil characteristic on which the irrigation regimes were based, were previously described by Zamljen, Zupanc and Slatnar [5]. The field capacity irrigation was maintained between − 15 kPa and − 25 kPa and deficit irrigation between − 50 and − 60 kPa. If the values dropped below the set levels, the irrigation started automatically, due to the signal from the tensiometers, to the electronic valves.
The chili plants were additionally fully fertilized using a full dose of 200 mg/L N (200 ppm nitrogen) and half dose at 100 mg/L N (100 ppm nitrogen), every 14 days. For the fertilization a water-soluble fertilizer containing macro- and microelements (Poly-Feed™; 16:8:32) was used. The fertilizer composition was as follows: macronutrients as total N (16%), nitric N (N-NO3 4.2%), ammonia N (1.8%), P (as P2O5 8%), K (as K2O 32%) and micronutrients Fe (1000 ppm), Mn (500 ppm), B (200 ppm), Zn (150 ppm), Cu (110 ppm) and Mo (70 ppm). To each fertilization the stable isotope 15N was added to the mix, with a relative content of 3.66 ± 0.02 at. % 15N.
With the above parameters four treatments were established for each cultivar: (i) field capacity irrigation and full dose of fertilizer (FCff); (ii) field capacity irrigation and half dose of fertilizer (FChf); (iii) deficit irrigation and full dose of fertilizer (Diff); (iv) deficit irrigation and half dose of fertilizer (DIhf).
Sampling of plant material
Both chili cultivars fruits were picked when they fully ripened, with red color, correct firmness, and glossy look, as reported by Villa-Rivera and Ochoa-Alejo [10].
The fruits were divided into three fruit parts (pericarp, placenta, seeds). Also, each plant was divided into three plant parts, namely leaves, stems and roots. The samples were lyophilized, ground to powder and stored at − 20 °C for isotopic and capsaicinoid studies and at − 80 °C for enzyme studies.
Total N analysis
The analysis of total N content was carried out in the Infrastructural Center for Soil Science and Environmental Protection, Department of Soil Science and Environmental Protection, Biotechnical faculty, laboratory following the SIST ISO 13878:1998 standard. The lyophilized samples (300 mg) were dry burned at 900 °C (Elementar Vario MAX). For the detection of total N, a thermal conductivity detector (TCD) (VarioMax CNS, Elementar Analysen systeme GmbH) was used and the data were expressed in percentages (%).
N isotope analysis
Between 2 and 7 mg of dried powder was used for N isotope analysis, depending on the N concentration in the sample. Samples were weighed into 5 × 8 mm tin capsules (Sercon). A Europa 20–20 isotope ratio mass spectrometer coupled to the ANCA SL preparation module for solid and liquid samples (Sercon Ltd., Crewe, U.K.) was used. The results were calibrated with certified reference materials (CRM) USGS-32 and IAEA-311 with 0.432 and 2.03 at. % of 15N, respectively. Results were reported as an atomic % of 15N. Further details on isotopic analysis were described by Zamljen et al. [11].
Formulas
Nitrogen fertilizer parameters in plants were calculated based on formulas from FAO/IAEA. [12] and were as follows:
-
Fraction of fertilizer N in the plant:
-
Total amount of N contained in the crop:
-
Fertilizer N uptake by crop:
-
Fraction of the fertilizer taken up by the plant:
Enzyme assays
Phenylalanine ammonia lyase assay
The PAL (EC 4.3.1.24) enzyme activity assay was performed as previously described by Phimchan, et al. [13] and Zamljen, et al. [14]. 0.5 g of fresh fruit was extracted with 3 mL 0.1 M boric acid and 0.4% sodium ascorbate (pH 8.5). The extraction solution was centrifuged at 12,000 × g for 30 min (5810 R; Eppendorf). For the reaction 10 µL 10 mM L-phenylalanine, 80 µL crude extract and 110 µL extraction buffer was used. For the blank sample 10 µL 10 mM L-phenylalanine and 190 µL extraction buffer was used. The samples were then incubated at 37 °C for 1 h and the reaction was terminated using 20 µL acetic acid and 400 µL MeOH.
The identification and quantification of trans-cinnamic acid was the same as reported by Zamljen, Medic, Hudina, Veberic and Slatnar [14], and the data were presented in nmol/s* g fresh weight (FW).
Capsaicin synthase assay
The CS (EC 2.3.2.35) enzyme assay was performed as reported Phimchan, Chanthai, Bosland and Techawongstien [13] and Zamljen, Medic, Hudina, Veberic and Slatnar [14], with some modifications. The fresh tissue (0.5 g) was extracted with 3 mL 100 mM Tris–HCl buffer (pH 6.8). The reaction mixture consisted of 0.4 M ATP, 0.4 M MgCl2, 0.4 M 8-methyl-6-nonenoic acid (each 5 µL), 300 µL enzyme extract, 100 µL 0.4 M Tris–HCl buffer (pH 6.8) and 10 µL 0.2 M vanillylamine. The samples were incubated for 1.5 h at 37 °C. The reaction was terminated using 40 µL acetic acid and 800 µL MeOH.
The identification and quantification of capsaicin was done as reported by Zamljen, Medic, Hudina, Veberic and Slatnar [14] and the data were presented in nmol/s* g FW.
Peroxidase assay
The POX (EC 1.11.1.7) activity assay was performed as described by Cebulj, et al. [15]. The buffer for the extraction of the enzyme contained 0.12% Tris, 0.2% EDTA, and 0.38% borax. 0.5 g of fresh tissue was ground to powder and extracted with 3 mL extraction buffer.
The reaction mixture contained 10 µL o-dianisidine, 50 µL enzyme extract and 1050 µL H2O2–KPi buffer. The blank sample contained 10 µL o-dianisidine and 1100 µL H2O2–KPi buffer.
The peroxidase activity was determined spectrophotometrically (Genesys 10S; Thermo Scientific, Waltham, MA, USA). Further details on the determination and calculation of the peroxidase activity was done as reported by Zamljen, Medic, Hudina, Veberic and Slatnar [14]. The data were presented as ΔA/min.
Capsaicinoid extraction and analysis
The extraction of capsaicinoids was as follows: 0.05 g of lyophilized powder was extracted with 80% methanol and 3% formic acid. Samples were placed in a cooled ultrasonic bath (0 °C) for 1 h and then centrifuged at 10,000 rpm for 5 min and filtered through a 0.25 µm polyamide filter (Chromafil AO-20/25, Macherey–Nagel, Düren, Germany). The quantification of individual capsaicinoids was done as reported by Zamljen, et al. [16] using aa UHPLC PDA Thermo Scientific Dionex UltiMate 3000 HPLC (Thermo Scientific) system, combined with a TSQ Quantum Access Max quadrupole mass spectrometer (MS) (Thermo Fischer Scientific Institute, Waltham, MA, USA). The data are expressed as g/kg FW.
Statistical analysis
Statistical analysis was performed using the R statistical environment. A two-factor analysis (irrigation and fertilization) was performed to test for statistical differences between treatments. Cultivar was not considered in the analysis (no comparisons were made) because previous studies have already reported that there are strong statistical differences between different chili cultivars, as reported by Zamljen, Zupanc and Slatnar [5]. The analysis was performed using a multi-way ANOVA to check if there were differences between treatments and a possible interaction. If an interaction was detected, a contrast analysis was performed, followed by a Duncan test.
Results
Irrigation data
The average amount of water supplied to each plant of 'Chili-AS Rot' during the experiment was 302.6 mm for field capacity and 185.0 mm for deficit irrigation. The second cultivar 'Naga Morich' required less irrigation water at 248.4 mm and 166.4 mm for field capacity and deficit irrigation, respectively.
Average soil water content was similar for both cultivars, with 'Chili-AS Rot' having 0.5% and 1.3% higher soil water content than 'Naga Morich' in the field capacity and deficit irrigation treatments, respectively. The average soil water tension was − 12.6 kPa and − 51.2 kPa for 'Chili-AS Rot' for the field capacity and deficit irrigation treatments, respectively. The second cultivar 'Naga Morich' had similar values of − 12.0 kPa and − 54.0 kPa for field capacity and deficit irrigation treatments, respectively (Table 1).
Chili yield and plant biomass yield
The yield of both chili cultivars and the yield of plant biomass are evaluated in Table 2. The yield of 'Chili-AS Rot' was between 18.2 and 26.7% higher than the other treatments. The number of fruits in 'Chili-AS Rot' was the highest in deficit irrigated plants. The total plant FW of the 'Chili-AS Rot' cultivar, was not statistically significant among treatments. On the other hand, the total plant DW of the cultivar 'Chili-AS Rot' was about 15.5% higher in the DIff treatment than in the other three treatments.
The yield of the more pungent cultivar 'Naga Morich' was much lower, due to smaller fruit size. The DIhf showed the best results, with an 8.8–25.6% higher yield, 19.1–36.5% higher fruit number per plant and 24.9–27.6% higher fruit DW when compared to the other treatments. The total plant FW of 'Naga Morich' was the highest in the optimal FCff treatment and the lowest in the DIhf.
N accumulation in chili plants and fruits under deficit irrigation and fertilization
Nitrogen yield (N yield), nitrogen yield from fertilizer (fertilizer N yield), and nitrogen utilization from fertilizer (% fertilizer N utilization) for both cultivars, irrigation treatments, and fertilizer treatments are shown in Fig. 2. 'Chili-AS Rot' cultivar N yield was higher with deficit irrigation than with field capacity irrigation (Fig. 2A). The half-fertilizer treatment had slightly lower N yield in both irrigation treatments. Fertilizer N yield was lower for 'Chili-AS Rot' under deficit irrigation. In addition, half-fertilization decreased fertilizer N yield in both irrigation treatments (Fig. 2B). A similar pattern was seen in fertilizer N utilization (Fig. 2C), with fully fertilized fruits of 'Chili-AS Rot' showing higher N utilization than half-fertilized fruits. Utilization of N from fertilizer was about 50% lower in both irrigation treatments when only half-fertilizer was added.
Nitrogen parameters [N yield (A), fertilizer N yield (B) and % fertilizer N utilization (C)] in 'Chili-AS Rot' and 'Naga Morich' fruits under two irrigation treatments and two fertilization treatments.  Data with different lower-case letters (a to d) are significantly different (multi-way ANOVA; Duncan test). FCff field capacity irrigation and full dose of fertilizer, FChf field capacity irrigation and half dose of fertilizer, Diff deficit irrigation and full dose of fertilizer, DIhf deficit irrigation and half dose of fertilizer
Data with different lower-case letters (a to d) are significantly different (multi-way ANOVA; Duncan test). FCff field capacity irrigation and full dose of fertilizer, FChf field capacity irrigation and half dose of fertilizer, Diff deficit irrigation and full dose of fertilizer, DIhf deficit irrigation and half dose of fertilizer
In 'Naga Morich', all three N parameters were higher with full fertilizer (regardless of irrigation regime) than with half-fertilizer. N yield under field capacity irrigation was 1.30 mg/plant lower when half N was added than under full-fertilization. Under deficit irrigation, N yield was 4.36 mg/plant lower when half-fertilizer was added (compared to full-fertilization). N yield and percent N utilization of fertilizer were approximately between 55 and 65% lower in the half-fertilizer treatments regardless of irrigation treatment in 'Naga Morich' fruits.
Plant N parameters are shown in Fig. 3 for 'Chili-AS Rot' and 'Naga Morich'. 'Chili-AS Rot' plants had the highest N yield under Diff at 37.57 mg/plant and the lowest N yield at FCff treatment with 24.84 mg/plant. Interestingly, nitrogen yield and utilization were highest in the full-fertilization and lowest in the deficit irrigation treated plants of 'Chili-AS Rot'.
Nitrogen parameters [N yield (A), fertilizer N yield (B) and % fertilizer N utilization (C)] in 'Chili-AS Rot' and 'Naga Morich' plants under two irrigation treatments and two fertilization treatments.  Data with different lower-case letters (a to d) are significantly different (multi-way ANOVA; Duncan test). FCff field capacity irrigation and full dose of fertilizer, FChf field capacity irrigation and half dose of fertilizer, Diff deficit irrigation and full dose of fertilizer, DIhf deficit irrigation and half dose of fertilizer
Data with different lower-case letters (a to d) are significantly different (multi-way ANOVA; Duncan test). FCff field capacity irrigation and full dose of fertilizer, FChf field capacity irrigation and half dose of fertilizer, Diff deficit irrigation and full dose of fertilizer, DIhf deficit irrigation and half dose of fertilizer
N yield of 'Naga Morich' ranged from 32.59 mg to 36.46 mg/plant. The FCff treatment had the lowest N yield and Diff treatment had the highest yield. In general, 'Naga Morich' had better fertilizer utilization in the full-fertilization treatment, regardless of the irrigation regime.
Enzymatic activity in deficit irrigated and fertilized chilies
Enzymatic activity and total capsaicinoid content are shown in Fig. 4A, B. In 'Chili-AS Rot' the CS activity was higher in half dose fertilized plants. In addition, the DIhf had the highest CS activity of 16.08 nmol/s*g FW compared to the other treatments. The activity of PAL was higher in both FChf and DIhf with 57.0 nmol/s*g FW (+ 77.0%) and 57.3 nmol/s*g FW (+ 57.9%), respectively, compared to the full dose fertilizer treatments. No differences were observed in POX activity for 'Chili-AS Rot'. Total capsaicinoid content was higher in Diff and Dihf 'Chili-AS Rot' plants at 41.65 mg/kg FW (+ 596.1%) and 21.31 mg/kg FW (+ 123.4%), respectively, compared to the FCff and FChf treatments.
CS activity (A), PAL activity (B), POX activity (C) and total capsaicinoids content (D) in 'Chili-AS Rot' and 'Naga Morich' under two irrigation treatments and two fertilization treatments.  Data with different lower-case letters (a to d) are significantly different (multi-way ANOVA; Duncan test). FCff field capacity irrigation and full dose of fertilizer, FChf field capacity irrigation and half dose of fertilizer, Diff deficit irrigation and full dose of fertilizer, DIhf deficit irrigation and half dose of fertilizer
Data with different lower-case letters (a to d) are significantly different (multi-way ANOVA; Duncan test). FCff field capacity irrigation and full dose of fertilizer, FChf field capacity irrigation and half dose of fertilizer, Diff deficit irrigation and full dose of fertilizer, DIhf deficit irrigation and half dose of fertilizer
In 'Naga Morich', CS activity was highest in FCff treatment (414.3 nmol/s*g FW) and lowest in Diff treatment (192.9 nmol/s*g FW). A similar pattern was observed in the activity of PAL, although the treatments with half dose of fertilizer had lower activity of PAL than the two treatments with full dose of fertilizer. In 'Naga Morich', no differences in POX activity were observed in any of the treatments. Total capsaicinoid content was higher in FCff and DIff than in the FCff and FChf at 15.75 mg/kg FW (+ 3.7%) and 22.26 mg/kg FW (+ 4.8%), respectively.
Individual capsaicinoid analysis results
The content of individual capsaicinoids is evaluated in Table 3. Capsaicin and dihydrocapsaicin represented the majority of capsaicinoids in both cultivars. In 'Chili-AS Rot' deficit irrigated plants contained higher levels of individual capsaicinoids than field-capacity irrigated plants. In deficit irrigation, plants treated with full N fertilization had higher contents of capsaicin, dihydrocapsaicin and homocapsaicin than half-fertilized plants. On the other hand, half-fertilization had higher content of individual capsaicinoids compared to full-fertilization at field capacity, ranging from 134 to 239% depending on the capsaicinoid studied.
In 'Naga Morich' cultivar, the differences between treatments were smaller than in 'Chili-AS Rot' cultivar. Capsaicin content was higher in full dose of fertilizer regardless of irrigation treatment, ranging from 3.9 to 5.9%. In general, Diff 'Naga Morich' plants had the highest capsaicin and dihydrocapsaicin contents compared to other treatments.
Discussion
The response of cultivars 'Chili-AS Rot' and 'Naga Morich' to two irrigation and two fertilization treatments was studied using stable isotope 15N. Yield, fruit number, and fruit DW was positively affected by deficit irrigation in both cultivars, which is contrary to the reports of Yang, et al. [17]. As described by Yang, et al. [17], the timing of application of deficit irrigation and fertilization is critical to achieve minimum yield loss and maximum yield quality.
In 'Chili-AS Rot', it was found that deficit irrigation decreased the utilization of nitrogen fertilizer when the plant (leaves, stems and roots) and fruits were observed. In addition, half dose of fertilizer decreased N parameters, although N yield was not much lower than full dose of fertilizer. N fertilizer yield and N utilization were lower when less fertilizer was added, but when 50% fertilizer was added, utilization was still 60–70% compared to 100% fertilization. A similar pattern in N fertilization was also observed in 'Naga Morich'. A similar result was reported by da Silva, et al. [18] and Rawal, et al. [19] where lower fertilizer concentration resulted in similar yield quantity and quality. The inedible parts of the plant (leaves, stems, and roots) and the edible fruits contained similar N yield and N utilization from fertilizer, indicating that the plant translocate a significant amount of N to the fruits. There are several metabolic pathways in fruits, as they are the reproductive organ of the plant. One of the most important in chilies is the capsaicinoid biosynthetic pathway [20]. The translocation itself occurs with the help of four amino acids, namely glutamate, glutamine, aspartate, and asparagine (GS -GOGAT pathway) [11] from the source to the sink. The nitrogen from these four amino acids is then incorporated into various metabolic pathways, one of which is the capsaicinoid biosynthetic pathway. The major enzymes of the capsaicinoid biosynthetic pathway are the PAL enzyme and the CS enzyme [13]. Half dose fertilized plants of 'Chili-AS Rot' showed higher CS activity and PAL activity regardless of irrigation treatment. In 'Naga Morich', irrigation treatment also affected enzyme activity. Phimchan, Chanthai, Bosland and Techawongstien [13] reported that enzymatic activity depends on drought stress and the higher the intensity of stress, the higher the enzymatic activity, which was the case for 'Chili-AS Rot', but for 'Naga Morich', deficit irrigation was found to lower the enzymatic activity.
As reported by Tanaskovik, et al. [21] and Parkash, et al. [22]. Deficit irrigation has less effect on bell pepper quality than fertilization with nitrogen. A similar conclusion can be drawn in our case. The content of individual and total capsaicinoids was higher in ‘Chili-AS Rot’ under deficit irrigation and did not differ from the optimally irrigated plants in the hotter 'Naga Morich'. Similar results were previously reported by Phimchan, et al. [23]. The lack of effect of irrigation on capsaicinoid content of 'Naga Morich' could be explained by genotype and genotype–environment interaction as reported by Zamljen, et al. [24]. The different effects on plant metabolism may contribute in different ways and with different intensities. The effects of fertilization, deficit irrigation, and genotype all contribute to the final total and individual capsaicinoid content [25]. All three effects were detected in our study, and there was a large difference between the two cultivars (representing genotype), type of irrigation, and type of fertilization. In addition, cultivars with high pungency are generally more genetically stable to environmental conditions than cultivars with lower pungency, as reported by Gurung, Techawongstien, Suriharn and Techawongstien [25], due to smaller fruits, lower number of seeds, and smaller leaves. The effect of N fertilization is also indirectly related to genotype, as different bell pepper species and cultivars convert different amounts of nitrogen depending on their genetic capabilities [26]
Conclusion
In two chili cultivars, namely 'Chili-AS Rot' and 'Naga Morich', the yield, the enzymatic and metabolic response were tested under deficit irrigation and deficit fertilization conditions using the isotope 15N technique. Deficit irrigation negatively affected the activity of CS and PAL in most cases. It also affected capsaicinoid content, which was higher in the less spicy cultivar 'Chili-AS Rot', while it mostly did not change in the spicier cultivar 'Naga Morich'. The deficit fertilization had a greater effect on enzymatic activity and capsaicinoid content in both cultivars. Interestingly, deficit fertilization improved enzymatic activity and yield regardless of irrigation treatment. Fertilizer utilization was better at deficit fertilization, and consequently enzymatic activity and yield was better, which is important from an economic point of view.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Al Othman ZA, Ahmed YB, Habila MA, Ghafar AA. Determination of capsaicin and dihydrocapsaicin in Capsicum fruit samples using high performance liquid chromatography. Molecules. 2011;16:8919–29. https://doi.org/10.3390/molecules16108919.
Khaitov B, Umurzokov M, Cho K-M, Lee Y-J, Park K, Sung J. Importance and production of chilli pepper; heat tolerance and efficient nutrient use under climate change conditions. Korean J Agric Sci. 2019. https://doi.org/10.7744/kjoas.20190059.
Fereres E, Soriano MA. Deficit irrigation for reducing agricultural water use. J Exp Bot. 2006;58:147–59. https://doi.org/10.1093/jxb/erl165.
Yu F, Feng S, Du W, Wang D, Guo Z, Xing S, Jin Z, Cao Y, Xu T. A Study of nitrogen deficiency inversion in rice leaves based on the hyperspectral reflectance differential. Front Plant Sci. 2020. https://doi.org/10.3389/fpls.2020.573272.
Zamljen T, Zupanc V, Slatnar A. Influence of irrigation on yield and primary and secondary metabolites in two chilies species, Capsicum annuum L. and Capsicum chinense Jacq. Agric Water Manag. 2020;234:106104. https://doi.org/10.1016/j.agwat.2020.106104.
Sardans J, Peñuelas J. The role of plants in the effects of global change on nutrient availability and stoichiometry in the plant-soil system. Plant Physiol. 2012;160:1741–61. https://doi.org/10.1104/pp.112.208785.
He M, Dijkstra FA. Drought effect on plant nitrogen and phosphorus: a meta-analysis. New Phytol. 2014;204:924–31. https://doi.org/10.1111/nph.12952.
Jia Q, Kamran M, Ali S, Sun L, Zhang P, Ren X, Jia Z. Deficit irrigation and fertilization strategies to improve soil quality and alfalfa yield in arid and semi-arid areas of northern China. PeerJ. 2018;6:e4410–e4410. https://doi.org/10.7717/peerj.4410.
Dawson TE, Mambelli S, Plamboeck AH, Templer PH, Tu KP. Stable isotopes in plant. Ecology. 2002;33:507–59. https://doi.org/10.1146/annurev.ecolsys.33.020602.095451.
Villa-Rivera MG, Ochoa-Alejo N. Transcriptional regulation of ripening in chili pepper fruits (Capsicum spp.). Int J Mol Sci. 2021;22:12151.
Zamljen T, Lojen S, Slatnar A, Zupanc V. Effect of deficit irrigation on nitrogen accumulation and capsaicinoid content in Capsicum plants using the isotope 15N. Agric Water Manag. 2022;260:107304. https://doi.org/10.1016/j.agwat.2021.107304.
FAO, IAEA. Use of isotope and radiation methods in soil and water management and crop nutrition. Vienna: International atomic energy agency; 2001.
Phimchan P, Chanthai S, Bosland PW, Techawongstien S. Enzymatic changes in phenylalanine Ammonia-lyase, Cinnamic-4-hydroxylase, capsaicin synthase, and peroxidase activities in Capsicum under drought stress. J Agric Food Chem. 2014;62:7057–62. https://doi.org/10.1021/jf4051717.
Zamljen T, Medic A, Hudina M, Veberic R, Slatnar A. Biostimulatory effects of amino acids on phenylalanine ammonia lyase, capsaicin synthase, and peroxidase activities in capsicum baccatum L. Biology. 2022;11:674.
Cebulj A, Halbwirth H, Mikulic-Petkovsek M, Veberic R, Slatnar A. The impact of scald development on phenylpropanoid metabolism based on phenol content, enzyme activity, and gene expression analysis. Hortic Environ Biotechnol. 2020;61:849–58. https://doi.org/10.1007/s13580-020-00268-0.
Zamljen T, Slatnar A, Hudina M, Veberic R, Medic A. Characterization and quantification of capsaicinoids and phenolic compounds in two types of chili olive oils using HPLC/MS. Food. 2022;11:2256.
Yang H, Liu H, Zheng J, Huang Q. Effects of regulated deficit irrigation on yield and water productivity of chili pepper (Capsicum annuum L.) in the arid environment of Northwest China. Irrigation Sci. 2018;36:61–74. https://doi.org/10.1007/s00271-017-0566-4.
da Silva JM, Fontes PCR, do Milagres CC, Garcia Junior E. Application of proximal optical sensors to assess nitrogen status and yield of bell pepper grown in slab. J Soil Sci Plant Nutr. 2021;21:229–37. https://doi.org/10.1007/s42729-020-00355-2.
Rawal N, Pande KR, Shrestha R, Vista SP. Nutrient use efficiency (NUE) of wheat (Triticum aestivum L.) as affected by NPK fertilization. PLoS One. 2022;17:e0262771. https://doi.org/10.1371/journal.pone.0262771.
Zhang J, Lv J, Xie J, Gan Y, Coulter JA, Yu J, Li J, Wang J, Zhang X. Nitrogen source affects the composition of metabolites in pepper (Capsicum annuum L.) and regulates the synthesis of capsaicinoids through the GOGAT-GS pathway. 2020. Foods (Basel, Switzerland). https://doi.org/10.3390/foods9020150.
Tanaskovik V, Chukaliev O, Moteva M, Jankulovska M, Markoski M, Spalevic V, Rusevski R, Bogevska Z, Davitkovska M. The influence of irrigation and fertilization regime on some phenological stages and earliness of pruned pepper. Agric For. 2015;61:7–17. https://doi.org/10.17707/AgricultForest.61.2.01.
Parkash V, Singh S, Deb SK, Ritchie GL, Wallace RW. Effect of deficit irrigation on physiology, plant growth, and fruit yield of cucumber cultivars. Plant Stress. 2021;1:100004. https://doi.org/10.1016/j.stress.2021.100004.
Phimchan P, Techawongstien S, Chanthai S, Bosland PW. Impact of Drought Stress on the Accumulation of Capsaicinoids in Capsicum Cultivars with Different Initial Capsaicinoid Levels. J HortScience horts. 2012;47:1204–9. https://doi.org/10.21273/hortsci.47.9.1204.
Zamljen T, Medic A, Hudina M, Veberic R, Slatnar A. Salt stress differentially affects the primary and secondary metabolism of peppers (Capsicum annuum L.) according to the genotype, fruit part, and salinity level. Plants. 2022;11:853.
Gurung T, Techawongstien S, Suriharn B, Techawongstien S. Stability analysis of yield and capsaicinoids content in chili (Capsicum spp.) grown across six environments. Euphytica. 2012;187:11–8. https://doi.org/10.1007/s10681-012-0672-6.
Pujarula V, Pusuluri M, Bollam S, Das RR, Ratnala R, Adapala G, Thuraga V, Rathore A, Srivastava RK, Gupta R. Genetic variation for nitrogen use efficiency traits in global diversity panel and parents of mapping populations in pearl millet. Front Plant Sci. 2021. https://doi.org/10.3389/fpls.2021.625915.
Acknowledgements
Not applicable.
Funding
This work is part of the Horticulture P4-0013-0481 and Agroecosystems P4-0085 programs supported by the Slovenian Research Agency and the infrastructural center IC RRC-AG (IO-0022-0481-001). Isotope analyses were supported by the SRA program P1-0143. This work was partially financed by IAEA TCP SLO5004 and IAEA CRP D1.50.18.
Author information
Authors and Affiliations
Contributions
TZ: experimental process, writing, metabolite extractions, statistical analysis; AS: reviewing of manuscript, conceptualization, methods; SL: isotope methods and reviewing of manuscript; VZ: conceptualization, methods, reviewing of manuscript, financial support.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zamljen, T., Lojen, S., Zupanc, V. et al. Determination of the yield, enzymatic and metabolic response of two Capsicum spp. cultivars to deficit irrigation and fertilization using the stable isotope 15N. Chem. Biol. Technol. Agric. 10, 129 (2023). https://doi.org/10.1186/s40538-023-00501-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40538-023-00501-9