Abstract
Background
The inefficient utilization of plant residues leads to serious environmental pollution and loss of plant nutrition. Nevertheless, the herbal residues including valuable mugwort have been rarely explored. Ensiling has been considered as a promising technique to reuse plant residues. Thus, this study investigated the effect of mugwort residues (M) and Lactiplantibacillus pentosus (LAB) on the fermentation quality, bacterial diversity, and metabolites of alfalfa silage after 60 days of ensiling.
Results
The results showed that compared with control, adding LAB, M and their combination significantly decreased pH (P < 0.05). Among all treatments, LAB + M had the lowest pH, butyric acid and ammonia nitrogen (NH3-N) concentrations (P < 0.05). Besides, lactic acid concentration of LAB + M treatment was increased nearly by 3 times compared with control. A shift on the bacterial profile was clearly observed, of which Lactiplantibacillus pentosus abundance increased to beyond 90% of total bacteria in LAB + M and all additives decreased Enterobacter hormaechei abundance than control (P < 0.05). Meanwhile, metabolite analysis indicated that mugwort residues addition enhanced the metabolites of apiin and apigenin 8-C-[xylosyl-(1- > 2)-galactoside] relevant to flavonoids (P < 0.05).
Conclusion
The addition of mugwort residues combined with Lactiplantibacillus pentosus significantly improved fermentation quality with the high relative abundance of Lactiplantibacillus pentosus. Moreover, mugwort residues addition could contribute to the upregulation of specific metabolites such as flavonoids, which would provide a new insight for facilitating fermentation with herbal residues.
Graphical Abstract

Similar content being viewed by others
Introduction
Large amounts of plant residues are produced worldwide from agriculture and the herb industry [1]. Most of them have not been efficiently utilized, causing serious problems, such as air pollution, ground water pollution, and global warming [2,3,4]. Nowadays, millions of tons of herbal residues are produced per annum in China alone [5]. However, these resources are inexpensive and still rich in nutrition, there has been growing interest in recycling them [6, 7].
Mugwort (Artemisia argyi L.), belonging to perennial plant, is well known as traditional Chinese herb medicine used for treating diseases such as diarrhea, hemostasis and inflammation [8]. Mugwort are supposed to be the treasure trove of bioactive constituents including volatile oils, flavonoids, triterpenes, and polysaccharide [9, 10]. Some reports found that the mugwort extracts (alkaloids, amino acids, flavonoids, phenol, quinines, tannins and terpenoids) had inhibitory effects on proliferation of undesirable microorganisms (such as Escherichia coli, Salmonella typhi, Enterobacter aerogenes, Proteus vulgaris, Pseudomonas aeruginosa and Shigella flexneri) in the fermented products and intestinal tract [11,12,13]. More specifically, volatile oil substances have strong inhibitory effects on Staphylococcus aureus, Escherichia coli, Aspergillus niger, Penicillium, and flavonoids and polysaccharides have scavenging effects on superoxide anion free radicals and hydroxyl free radicals [11]. Their antibacterial mechanism might be due to the bioactive substances in mugwort leading to the cell content flowing out, subsequently damaging their physiological and biochemical indexes [9]. Even after extracting specific ingredients, large amounts of stalk or by-products are produced. More importantly, mugwort residues still contain relatively abundant bioactive substances. It will be of great interest to utilize the antimicrobial property of mugwort residues. However, most of mugwort residues are still wasteful. Therefore, developing a cost-effective and sustainable method is urgent for better utilization of mugwort residues.
Ensiling has been considered as a promising technique to reuse plant residues [5, 6]. Among the forage resource of silage, alfalfa (Medicago sativa L.) has been widely considered as an important protein forage around the world [14]. However, the high moisture made it difficult to ensile and easy to spoil [15]. Fermentation is a microbial-driven process, and the silage quality mainly depends on species and activities of microbial community involved in ensiling process [5, 16]. Previous research has proved that astragalus and hawthorn residues could enhance the fermentation performance through restraining the proliferation of protein-degrading bacteria including Enterobacter and Clostridium during ensiling process of alfalfa [6]. Besides, our studies indicated that lactic acid bacteria inoculants could improve the fermentation quality by modifying the microbial community of silage [17, 18]. Hence, the combined addition of mugwort residues and lactic acid bacteria might yield an effect in silage quality.
This study hypothesized that adding mugwort residues and lactic acid bacteria could improve silage quality via modulating ensiling microbiota. The fermentation products and bacterial community were assessed after ensiling. Additionally, the metabolites were also analyzed to elucidate the potential mechanism of silage fermentation. The results will provide a promising way for reusing plant residues rich in bioactive substances.
Materials and methods
Experimental design and sample collection
Alfalfa (Medicago sativa) was planted at a farm located in Tongliao city, Inner Mongolia (44°10′N, 120°53′E), and harvested in early bloom stage on August 17, 2020. The raw alfalfa were manually harvested with a pruning shear (KOMAX, Zhenmei Co., Ltd., Zhejiang, China) at approximately 60–70 cm high. Fresh alfalfa and mugwort residues (supplied by Anyang Institute of Technology, Henan, China) were cut into segments at a length 20 mm with a fodder chopper. After that, 6 kg of chopped fresh forage were packed into plastic bags (22 cm × 32 cm; Cangzhou Hualiang Packaging and Decoration Co. Ltd., Dongguan, China), and every bag was loaded by 500 g. The experimental treatments were designed as the followings: LAB, with lactic acid bacteria (Lactiplantibacillus pentosus, preserved in the laboratory with good ability to ferment high moisture silage) at 1.0 × 106 colony forming units (cfu)/g of fresh matter (FM); M, with 8% mugwort residues (460 g alfalfa + 40 g mugwort residues per bag); LAB + M, LAB mixed with 8% mugwort residues (460 g alfalfa + 40 g mugwort residues per bag); CK, with an equal amount of sterilized water sprayed on fresh alfalfa as control. A total of 12 bags (4 treatments × 3 replicates) were vacuum-sealed and kept at room temperature (about 25 ℃). Bags were sampled to analyze fermentation products, microbial community, and metabolic activity after 60 days of ensiling.
Chemical composition and fermentation characteristics
For chemical quantification analysis, fresh materials were dried at 65 °C for 48 h in a forced-air oven to estimate the dry matter (DM) content. The contents of crude protein (CP), water-soluble carbohydrate (WSC), neutral detergent fiber (NDF), and acid detergent fiber (ADF) were determined by Association of Official Analytical Chemists [19].
For fermentation analysis, each silage sample (40 g) was mixed with 360 mL sterilized water for 1 h. Before determining fermentation products, the liquid was firstly filtered by 0.22 µm filter membrane. The pH values were measured by a pH meter (PHS-3C, INESA Scientific Instrument, Shanghai, China). The NH3-N concentrations were measured with the method of phenol–hypochloric acid colorimetry [20]. High-performance liquid chromatography was employed to detect organic acid concentrations including lactic acid, acetic acid, propionic acid, and butyric acid. The detector wavelength was 210 nm, the eluent was 3 mmol L−1 HClO4 with the flow rate of 10 mL min−1 and temperature of 50 °C. The method of microbial population (lactic acid bacteria, coliform bacteria and yeast) by plate culture were analyzed according to methods from Guo et al. [21].
Bacterial community analysis
To perform single molecule real-time (SMRT) sequencing, the 16S rRNA gene was amplified, and PCR amplification was performed under the conditions according to previous report [21]. PCR amplification with PacBio Sequel platform was sequenced. For processing raw sequencing data, PacBio sequencing data were demultiplexed using mothur (version 1.36.1); low-quality data were filtered via vsearch (version 1.11.1). The high-quality reads were clustered in OTUs at 97% sequence similarity using UCLUST. Further analyses including alpha diversity were calculated in QIIME. PCoA was performed with Vegan (version.3.5.3) using Bray–Curtis dissimilarities. Representative sequences were taxonomically annotated using the BLAST algorithm with SILVA (Release 138) database. The correlation analyses and production of heat map graphics were carried out in R (version. 3.2.5).
Metabolite analysis
Two milliliters of extract including internal standard (1000:2) was added to 100 mg of sample, then vortex 30 s. After that, the mixed sample was crushed with porcelain beads for 10 min at 45 Hz and was ultrasound for 10 min (ice-water bath), then was stored at − 20 °C for 1 h. The sample was centrifuged at 4 °C, 12000 rpm for 15 min. Then carefully removed 500 μL of supernatant into EP tube and dried the extract in a vacuum concentrator. Added 160 μL of extract (acetonitrile to water ratio was 1:1) to the dried sample, then vortex for 30 s and sonicate in an ice-water bath for 10 min. Then put samples at 4 °C, 12000 rpm for 15 min, carefully removed 120 μL of supernatant into a 2-mL injection bottle. Then 10 μL of each sample was mixed into QC samples for on-board detection. UHPLC–QTOF-MS non-target metabolomic detection method was used [22].
Raw data got from with MassLynx V4.2 were processed by Progenesis QI software, and identified on account of the online METLIN database of Progenesis QI software. Meanwhile, theoretical fragments were identified, and the mass deviations were all within 100 ppm. Statistical analysis used KEGG database and HMDB database for annotating metabolites. Principal component analysis was carried out using Simca. A metabolic model was established by PLS-DA, and metabolites with VIP > 1 and P < 0.05 and fold change (FC) > 1 between the two groups were considered as significantly differential metabolites. Evenn was employed to build a Venn diagram of significantly differential metabolites in different comparison groups. Metabolite markers were identified with analyzing the S-plot plots generated by Simca software (Umetrics SIMCA, 14.1.0.2047).
Statistical analysis
Statistical analysis of fermentation characteristics was carried out in the Statistical Package for the Social Sciences (SPSS version 24.0, SPSS Inc., Chicago, IL, USA) by Duncan's multiple range method to determine the significant difference between means per treatment. One-way analysis of variance was employed to study the impact of treatment. The significance was employed at P < 0.05 probability level.
Results
The characteristics of raw material
The characteristics of raw materials are listed in Additional file 1: Table S1. The contents of DM, NDF and ADF of alfalfa were 199.00 g/kg, 331.88 g/kg DM and 176.56 g/kg DM, respectively. The WSC contents in both alfalfa and mugwort residues were in the range of 30 to 40 g/kg DM. The CP content in alfalfa (237.02 g/kg DM) was higher compared to mugwort residues (120.64 g/kg DM) (P < 0.05).
Fermentation characteristics
As shown in Table 1, the treatment had significant effects on pH, concentrations of lactic acid, acetic acid, butyric acid, NH3-N and counts of coliform bacteria and yeast (P < 0.01). Compared with CK, the treated silages showed lower pH (P < 0.05). M treatment had higher lactic acid concentration, lower butyric acid concentration and undetected yeast than CK and LAB treatment (P < 0.01). Among all treatments, the highest lactic acid concentration and the lowest pH, butyric acid concentration, NH3-N concentration and coliform bacteria count were observed in LAB + M (P < 0.05).
Variation of the bacterial diversity
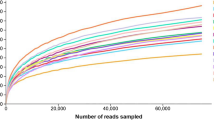
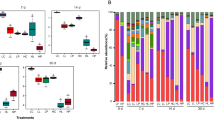
The Shannon index and principal co-ordinates analysis (PCoA) analysis of bacterial community are listed in Fig. 1. As shown in Fig. 1a, all treated silages reduced the Shannon indices of bacterial community compared with CK (P < 0.05). The lower Shannon indices were noticed in LAB and LAB + M treatments than M treatment (P < 0.05). The variance of bacterial community was observed by PCoA on the OTU level is shown in Fig. 1b. The component 1 and component 2 could explain 74.71% and 10.14% of the total variance, respectively. The different treated samples were well-separated from CK, suggesting the bacterial community was greatly influenced by the mugwort residues and Lactiplantibacillus pentosus addition.
Major bacteria across all samples at the species level are listed in Fig. 2. Overall, Lactiplantibacillus pentosus (29.62–94.07%) and Enterobacter hormaechei (3.86–36.36%) became the predominant species in all silages, followed by Enterococcus mundtii and Loigolactobacillus coryniformis with 0.04–9.07% and 0.24–1.95%, respectively. Compared with CK, all additives decreased Enterobacter hormaechei abundance (P < 0.05), and LAB and LAB + M treatments showed higher Lactiplantibacillus pentosus abundance and lower Enterococcus mundtii abundance (P < 0.05). Besides, M addition enhanced Enterococcus mundtii abundance and Loigolactobacillus coryniformis abundance compared with CK (P < 0.05).
Correction networks
The correlation networks among species are shown in Fig. 3a. Lactiplantibacillus pentosus was negatively correlated with Enterobacter hormaechei, Enterococcus faecalis, Ligilactobacillus acidipiscis, Enterococcus mundtii and Enterococcus flavescens. Spearman correlation heatmap of dominant species and fermentation parameters is shown in Fig. 3b. Only Lactiplantibacillus pentosus had a significantly positive relationship with lactic acid and negative relationships with NH3-N, acetic acid, butyric acid, and coliform bacteria (P < 0.05). Enterobacter hormaechei, Enterococcus flavescens and Enterococcus faecalis were negatively correlated with lactic acid and positively correlated with acetic acid, butyric acid, and coliform bacteria (P < 0.05). Besides, Enterococcus flavescens and Enterococcus faecalis were positively correlated with NH3-N (P < 0.05).
Metabolomic profiles of silage
Untargeted metabolomic analysis led to the identification of a total of 1590 metabolites including different classes, viz carboxylic acids and derivatives, fatty acyls, and phenolics in this study (Additional file 2: Table S2). As shown in Fig. 4, there were 59 metabolites significantly different between M treatment and LAB + M treatment (P < 0.05). Besides, 36, 38, and 35 significantly different metabolites detected in CK_vs_M, CK_vs_LAB + M, and LAB_vs_LAB + M, respectively (P < 0.05). The numbers of significantly differential metabolites unique to CK_vs_M, LAB_vs_LAB + M, and M_vs_LAB + M were all around 14, and CK_vs_LAB + M had only 6 significantly differential metabolites. The four comparison groups had 1 identical significantly differential metabolite. Similarly, Fig. 5 shows that the principal component analysis of metabolites provided clear separation between different treatments, in which the metabolites were clustered into four quadrants. The discrepancy patterns of significantly changing metabolites between group are shown in Fig. 6. Metabolites farther away from the center might be the maker constituents between groups, and then annotated when they were significantly different metabolites between groups. Compared with CK, 3 metabolites in the M treatment were significantly higher (P < 0.05), such as Phe Cys Met, apiin and apigenin 8-C-[xylosyl-(1- > 2)-galactoside], and 3 metabolites were significantly lower (P < 0.05), such as PC(18:1(11Z)/16:1(9Z)), PE(21:0/20:5(5Z,8Z,11Z,14Z,17Z)) and PC(16:0/22:5(7Z,10Z,13Z,16Z,19Z))[U]. Compared with M treatment, 4 metabolites in the LAB + M treatment were significantly higher (P < 0.05), such as 6,10,14-trimethyl-5,9,13-pentadecatrien-2-one, cis-9,10-epoxystearic acid, farnesyl acetone and DG(16:0/17:2(9Z,12Z)/0:0)[iso2], and 5 metabolites were significantly lower (P < 0.05), such as 6-hydroxysphingosine, glycocholic acid, Phe Cys Met, apigenin 8-C-[xylosyl-(1- > 2)-galactoside] and soraphen O. Compared with LAB treatment, 3 metabolites in the LAB + M treatment were significantly higher (P < 0.05), such as 2-methylbenzoic acid, apiin and harderoporphyrin, and 4 metabolites were significantly lower (P < 0.05), such as Phe Cys Met, Tyr Met Ala, soraphen O and soyasaponin bg.
Discussion
Although mugwort residues are abundant, efficient utilization remains a significant challenge. Anaerobic fermentation has recently emerged as a viable strategy to mitigate plant byproduct waste [5, 23]. Hence, ensiling presents a promising solution to reuse mugwort residues. In this study, it is hypothesized that alfalfa silage quality could be improved via modulating microbiota and metabolites with mugwort residues and Lactiplantibacillus pentosus addition. This investigation offers new prospects for exploiting herbal residues efficiently.
The characteristics of raw material
Before ensiling, the low contents of NDF and ADF of alfalfa might be influenced by environmental factors such as climate, genetic factors and harvest time [24]. The CP content in mugwort residues was similar with prior study [25], while that was relatively higher in alfalfa [26]. The DM content of fresh alfalfa observed in this study was lower than previous reports [18, 27], probably due to the early harvest time, which might propagate harmful bacteria during ensiling [19]. The WSC content of raw material is crucial for determining the silage fermentation [16]. However, the insufficient contents of WSC both in alfalfa and mugwort residues might not ensure acceptable silage quality in this study. Therefore, based on the low DM and WSC contents, the addition of high-efficiency fermentation lactic acid bacteria was necessary to expedite the fermentation process and enhance the quality of the silage.
Fermentation characteristics
The detection of fermentation characteristics can provide valuable insight into silage quality. In general, pH is considered as the key factor for evaluating fermentation degree. In this study, the treated silages exhibited lower pH than the CK, indicating that the addition of M and LAB might be feasible ways to improve the quality of alfalfa silage. However, the various treated silages performed differently in terms of ensiling effects on alfalfa. As we know, the pH decrease was mainly determined by organic acid accumulation especially lactic acid during ensiling process [28]. In this study, the M treatment showed higher lactic acid concentration compared to LAB treatment (P < 0.01). That result was similar with the previous report, in which the herbal residues such as Myristica fragrans H., Citri reticulata Pericarpium, Angelica sinensis O., Citrus aurantium L., Atractylodes lancea T., Astragalus membranaceus F., Codonopsis pilosula F., and Anemarrhenae rhizoma improved silage quality through enhancing lactic acid concentration [5]. Although the exact reason could not be classified, the different impact of bioactive substance of mugwort residues such as flavonoids on microbial function, such as promoting the growth of lactic acid bacteria and lactic acid production, might be one of the possible reasons [8]. Among all silages, LAB + M treatment had the lowest pH and the highest lactic acid concentration, indicating that the compound additive facilitated the fermentation processing of alfalfa. The occurrence of butyric acid usually indicates protein loss [29]. The experimental results revealed that adding M reduced butyric acid concentration compared with LAB treatment (P < 0.01). Furthermore, the combination of M and LAB exhibited lower butyric acid concentration than either of M or LAB addition alone (P < 0.01). It was possible that the bioactive constituents of mugwort residues had a synergistic effect on the inhibition of butyric acid along with LAB addition. The concentration of NH3-N in silage can be an indicator for reflecting the status of protein degradation, and is influenced by the undesirable microorganisms activities [29]. Compared with the CK, the NH3-N concentration of the LAB + M treatment was significantly lower (P < 0.01), which was in accord with the decline of coliform bacteria count, suggesting that the addition of M and LAB could effectively reduce protein degradation.
Variation of the bacterial diversity
Microbes play a crucial role in ensiling process and can be employed as evaluation indicator of fermentation quality. In this study, the addition of LAB and M resulted in a reduction of Shannon indices compared to CK, indicating decreased bacterial diversity [21]. Specifically, the lowest bacterial diversity was noticed in LAB + M treatments in all silages. This decline in bacterial diversity could result from the bioactive substances in M or antibacterial compounds such as hydrogen peroxide and organic acids produced by LAB that inhibiting the growth of the undesirable bacteria not tolerant to them [30]. Therefore, further exploration of the bacterial community is necessary to fully understand the mechanisms of regulation by these additives.
Lactiplantibacillus pentosus is a facultative heterofermentative microbe, and its fermentation pathway shifts from homofermentation to heterofermentation only in the absence of glucose [30, 31]. In the present study, Lactiplantibacillus pentosus addition enhanced the relative abundance of this microbe (LAB and LAB + M) compared to CK. This finding was consistent with the results reported by [32], who found that Lactiplantibacillus pentosus inoculum could increase substantially and dominated in silages. However, it was the combination of Lactiplantibacillus pentosus and mugwort residues (LAB + M) that could contribute to a considerable increase in lactic acid concentration than CK, which might be due to their positive synergistic effect. Published study had found that the presence of Enterobacter could increase ammonia and biogenic amines concentrations in silage through the deamination and decarboxylation, resulting in nutrition loss [33]. In the present study, the relative abundance of Enterobacter hormaechei followed the series CK > M > LAB > LAB + M at the end of ensiling day. Therefore, the decrease of Enterobacter hormaechei abundance might contribute to the reduced concentrations of butyric acid and NH3-N.
Enterococcus mundtii belongs to non-motile, yellow-pigmented enterococcus, and they are likely to be involved in homolactic glucose metabolism [34]. Enterococcus mundtii were often detected in oat silage, corn stover silage, paddy rice silage and Leymus chinensis silage [35,36,37,38]. More importantly, previous research found that Enterococcus mundtii could produce bacteriocins that were supposed to contribute to preserving fermented plant products [39]. However, Enterococcus mundtii were less effective in enhancing silage utilization by promoting forage digestibility and reducing ruminal CH4 emission than Lactiplantibacillus plantarum [40]. From the above information, the exact function of Enterococcus mundtii is still not inconclusive.
Loigolactobacillus coryniformis has been identified in various types of silages, such as Elymus nutans, Italian ryegrass and tomato pomace silages [41,42,43,44]. During ensiling process, Loigolactobacillus coryniformis can efficiently ferment glycerol to 3-hydroxypropionaldehyde, which serves as an antimicrobial compound [45]. Loigolactobacillus coryniformis is valuable for ensiling as it can inhibit butyric fermentation, reduce mold contamination, and improve silage quality [46, 47]. Therefore, the much-increased abundances of Loigolactobacillus coryniformis resulting from the addition of mugwort residues (M) might contribute to the lower butyric acid concentration and enhanced fermentation quality in this study.
Correction networks
Previous research has shown that Lactiplantibacillus pentosus inoculation could efficiently improve the fermentation quality and nutrient preservation of alfalfa silage [32, 48]. This could explain the analysis of Spearman correlation, in which Lactiplantibacillus pentosus might be the major species leading to the increase of lactic acid concentration and the decrease of butyric acid concentration. The higher concentration of lactic acid can effectively inhibit the growth of harmful microorganism and protein decomposition that caused NH3-N production [49]. Therefore, Lactiplantibacillus pentosus also showed negative correlation with coliform bacteria and NH3-N. It was worth noting that Ligilactobacillus acidipiscis had a negative relationship with Lactiplantibacillus pentosus in this study. Considering great discrepancy in their relative abundance, it was likely that Lactiplantibacillus pentosus had a competitive edge over Ligilactobacillus acidipiscis for sugar substance over during ensiling process. Enterobacter is an important competitor of lactic acid bacteria for available substrate in silage, and it can ferment lactic acid to acetic acid and other products, thus subsequently causing nutrition loss [29]. Hence, Enterobacter hormaechei was negatively correlated with lactic acid and positively correlated with acetic acid. Enterococcus flavescens and Enterococcus faecalis showed negative correlation with lactic acid and positive correlation with butyric acid, NH3-N and coliform bacteria, which implied that the presence of these species may reduce the quality of silage, so these species would be specifically studied in the future.
Metabolomic profiles of silage
To obtain a more comprehensive understanding of the metabolites in silage, an untargeted metabolomic approach was employed to profile the metabolites and differences among treatments. The flavonoids, such as apiin and apigenin 8-C-[xylosyl-(1- > 2)-galactoside], showed significant up regulation in M treatment than CK (P < 0.05). Apiin is one of the main forms of apigenin that belongs to flavonoids and apigenin was effectively anti-inflammatory, antineoplastic and has antioxidant scavenging ability [50,51,52]. Previous report found that apigetrin and apiin isolated from Sedum caeruleum could against a range of microorganisms, including Escherichia coli, Staphylococcus aureus, Pseudomonas, Klebsiella and other harmful bacteria [53]. Therefore, mugwort residues addition might enhance the metabolites of apiin and apigenin 8-C-[xylosyl-(1- > 2)-galactoside] relevant to flavonoids in silages by altering bacterial community in this study. Besides, it should be noted that apigenin 8-C-[xylosyl-(1- > 2)-galactoside] showed a decreased trend in LAB + M treatment. The reason for the decrease in flavonoids was not clear but might be attributed to the lower pH value. Interestingly, regarding LAB_vs_LAB + M, 2-methylbenzoic acid and apiin in LAB + M treatment showed increased regulation, in which 2-methylbenzoic acid is involved in biosynthesis of secondary metabolites. These results indicated that combined LAB + M addition might enhance the metabolic pathway of secondary metabolites such as flavonoids than LAB inoculation.
These findings verified our hypothesis that the addition of mugwort residues combined with Lactiplantibacillus pentosus significantly could improve alfalfa silage quality via modulating ensiling microbiota and metabolites.
Conclusions
In summary, this study demonstrated that a significant improvement in the fermentation quality of alfalfa silage through the addition of mugwort residues in combination with Lactiplantibacillus pentosus. This improvement was supported by higher concentrations of lactic acid, lower concentrations of butyric acid and NH3-N, as well as a higher relative abundance of Lactiplantibacillus pentosus. Besides, the differences in metabolic profiles between groups were also observed, and mugwort residues addition could contribute to the upregulation of specific metabolites such as flavonoids.
Availability of data and materials
The raw sequences of 16S rRNA gene were deposited in the NCBI database to be available to the public (PRJNA896374). All data are presented in the manuscript.
Change history
20 December 2023
A Correction to this paper has been published: https://doi.org/10.1186/s40538-023-00521-5
References
Negi T, Kumar Y, Sirohi R, Singh S, Tarafdar A, Pareek S, Awasthi MK, Sagar NA. Advances in bioconversion of spent tea leaves to value-added products. Bioresour Technol. 2021;346:126409. https://doi.org/10.1016/j.biortech.2021.126409.
Zhao ZC. Research progress on comprehensive utilization of extracting residues from traditional Chinese medicine. Animal Husbandry Feed Sci. 2019;40(2):47–52.
Zeng X, Shao RY, Wang F, Dong PW, Yu J, Xu GW. Industrial demonstration plant for the gasification of herb residue by fluidized bed two-stage process. Bioresour Technol. 2016;206:93–8. https://doi.org/10.1016/j.biortech.2016.01.075.
Khayum N, Anbarasu S, Murugan S. Biogas potential from spent tea waste: A laboratory scale investigation of co-digestion with cow manure. Energy. 2018;165:760–8. https://doi.org/10.1016/j.energy.2018.09.163.
Li XM, Chen F, Wang XK, Xiong Y, Liu ZY, Lin YL, Ni KK, Yang FY. Innovative utilization of herbal residues: exploring the diversity of mechanisms beneficial to regulate anaerobic fermentation of alfalfa. Bioresour Technol. 2022. https://doi.org/10.1016/j.biortech.2022.127429.
Ni KK, Wang XK, Lu Y, Guo LN, Li XM, Yang FY. Exploring the silage quality of alfalfa ensiled with the residues of astragalus and hawthorn. Bioresour Technol. 2020;297:122249. https://doi.org/10.1016/j.biortech.2019.122249.
Zhang RQ, Lin DX, Zhang L, Zhan RT, Wang SD, Wang K. Molecular and biochemical analyses of a novel trifunctional endoxylanase/endoglucanase/feruloyl esterase from the human colonic bacterium bacteroides intestinalis DSM 17393. J Agric Food Chem. 2022;70(13):4044–56. https://doi.org/10.1021/acs.jafc.2c01019.
Wang WW, Tan ZF, Gu LB, Ma H, Wang ZY, Wang L, Wu GF, Qin GY, Wang YP, Pang HL. Variation of microbial community and fermentation quality in corn silage treated with lactic acid bacteria and Artemisia argyi during aerobic exposure. Toxins. 2022;14(5):349. https://doi.org/10.3390/toxins14050349.
Ahmed M, Ji M, Qin P, Gu Z, Liu Y, Sikandar A, Iqbal MF, Javeed A, Shafi J, Du Y. Determination of phytochemicals, antioxidant activity and biochemical composition of Chinese Mugwort (Artemisia Argyi L.) leaf extract from northeast China. Appl Ecol Environ Res. 2019;17(6):15349–62. https://doi.org/10.15666/aeer/1706_1534915362.
Zhang Y, Kang LP, Teng ZQ, Zhan ZL, Nan TG, Zhou AX, Guo LP. Comparison of volatile constituents in two types of mugwort leaves (produced in Qichun and Nanyang) using the headspace GC-MS. J Acupuncture Tuina Sci. 2016;14(3):164–9.
Ahameethunisa AR, Hopper W. Antibacterial activity of Artemisia nilagirica leaf extracts against clinical and phytopathogenic bacteria. BMC Complement Altern Med. 2010;10(1):1–6. https://doi.org/10.1186/1472-6882-10-6.
Nam Y, Choi M, Hwang H, Lee MG, Kwon BM, Lee WH, Suk K. Natural flavone jaceosidin is a neuroinflammation inhibitor. Phytother Res. 2013;27(3):404–11. https://doi.org/10.1002/ptr.4737.
Nuerbiye A, Rena K, Yang L, Jinhui W. Study on chemical constituents and antifungal activity of volatile oil in Artemisia argyi Levl. et Vant. J Xinjiang Medical University. 2017; 40:1195–1198+ 1202.
Li X, Brummer EC. Applied genetics and genomics in alfalfa breeding. Agronomy. 2012;2(1):40–61. https://doi.org/10.3390/agronomy2010040.
Cai YM, Benno Y, Ogawa M, Ohmomo S, Kumai S, Nakase T. Influence of Lactobacillus spp. from an inoculant and of Weissella and Leuconostoc spp. from forage crops on silage fermentation. Appl Environ Microbiol. 1998;64(8):2982–7.
Wang Y, Wang C, Zhou W, Yang FY, Chen XY, Zhang Q. Effects of wilting and Lactobacillus plantarum addition on the fermentation quality and microbial community of Moringa oleifera leaf silage. Front Microbiol. 2018;9:1817. https://doi.org/10.3389/fmicb.2018.01817.
Wang Y, Zhou HZ, Gao Y, Wang NW, Liu H, Yang FY, Ni KK. Ensiling vine tea (Ampelopsis grossedentata) residue with Lactobacillus plantarum inoculant as an animal unconventional fodder. J Integr Agr. 2022;22(4):1172–83. https://doi.org/10.1016/j.jia.2022.10.001.
Guo LN, Yao DD, Li DX, Lin YL, Bureenok S, Ni KK, Yang FY. Effects of lactic acid bacteria isolated from rumen fluid and feces of dairy cows on fermentation quality, microbial community, and in vitro digestibility of alfalfa silage. Front Microbiol. 2020; 10. https://doi.org/10.3389/fmicb.2019.02998
AOAC Official methods of analysis. Artington. Virginia, USA: Association of Official Analytical Chemists; 1920. p. 2.
Broderick GA, Kang JH. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J Dairy Sci. 1980;63(1):64–75.
Guo LN, Wang XK, Lin YL, Yang XP, Ni KK, Yang FY. Microorganisms that are critical for the fermentation quality of paper mulberry silage. Food Energy Secur. 2021;10(4):e304. https://doi.org/10.1002/fes3.304.
Wang JL, Zhang T, Shen XT, Liu J, Zhao DL, Sun YW, Wang L, Liu YJ, Gong XY, Liu YX, Zhu ZJ, Xue FZ. Serum metabolomics for early diagnosis of esophageal squamous cell carcinoma by UHPLC-QTOF/MS. Metabolomics. 2016;12(7):1–10. https://doi.org/10.1007/s11306-016-1050-5.
Huang FQ, Wang TW, Zhang JQ, Tahir M, Sun JH, Liu YY, Yun FF, Xia TQ, Teng KL, Wang JW, Zhong J. Exploring the bacterial community succession and metabolic profiles of Lonicera japonica Thunb. residues during anaerobic fermentation. Bioresour Technol. 2023;367:128264. https://doi.org/10.1016/j.biortech.2022.128264.
Wang Y, Zhou W, Wang C, Yang FY, Chen XY, Zhang Q. Effect on the ensilage performance and microbial community of adding Neolamarckia cadamba leaves to corn stalks. Microb Biotechnol. 2020;13(5):1502–14. https://doi.org/10.1111/1751-7915.13588.
Ha G, Lee Y, Kim N, Shon G, Rho C, Jeong H, et al. Nutritional chemical composition in the different parts of Artemisia argyi H. J Agric Life Sci. 2012;46:155–64.
Li R, Jiang D, Zheng ML, Tian PJ, Zheng MH, Xu CC. Microbial community dynamics during alfalfa silage with or without clostridial fermentation. Sci Rep. 2020;10(1):1–14.
Zheng ML, Niu DZ, Zuo SS, Mao PC, Meng L, Xu CC. The effect of cultivar, wilting and storage period on fermentation and the clostridial community of alfalfa silage. Ital J Anim Sci. 2018;17(2):336–46. https://doi.org/10.1080/1828051X.2017.1364984.
Kung LM, Shaver RD, Grant RJ, Schmidt RJ. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J Dairy Sci. 2018;101(5):4020–33. https://doi.org/10.3168/jds.2017-13909.
Ni KK, Wang FF, Zhu BG, Yang JX, Zhou GA, Pan Y, Tao Y, Zhong J. Effects of lactic acid bacteria and molasses additives on the microbial community and fermentation quality of soybean silage. Bioresour Technol. 2017;238:706–15. https://doi.org/10.1016/j.biortech.2017.04.055.
Bustos G, Moldes AB, Cruz JM, Domínguez JM. Influence of the metabolism pathway on lactic acid production from hemicellulosic trimming vine shoots hydrolyzates using Lactobacillus pentosus. Biotechnol Prog. 2005;21(3):793–8. https://doi.org/10.1021/bp049603v.
Bintsis T. Lactic acid bacteria as starter cultures: an update in their metabolism and genetics. AIMS microbiology. 2018;4(4):665. https://doi.org/10.3934/microbiol.2018.4.665.
Liu F, Bai J, Huang WK, Li FH, Ke WC, Zhang YX, Xie DM, Zhang B, Guo XS. Characterization of a novel beta-cypermethrin-degrading strain of Lactobacillus pentosus 3–27 and its effects on bioremediation and the bacterial community of contaminated alfalfa silage. J Hazard Mater. 2022;423:127101. https://doi.org/10.1016/j.jhazmat.2021.127101.
Pedroso AF, Adesogan AT, Queiroz OCM, Williams SK. Control of Escherichia coli O157: H7 in corn silage with or without various inoculants: Efficacy and mode of action. J Dairy Sci. 2010;93(3):1098–104. https://doi.org/10.3168/jds.2009-2433.
Repizo GD, Espariz M, Blancato VS, Suárez CA, Esteban L, Magni C. Genomic comparative analysis of the environmental Enterococcus mundtii against enterococcal representative species. BMC Genomics. 2014;15(1):1–13. https://doi.org/10.1186/1471-2164-15-489.
Li X, Chen F, Wang XK, Sun L, Guo LN, Xiong Y, Wang Y, Zhou HZ, Jia SG, Yang FY, Ni KK. Impacts of low temperature and ensiling period on the bacterial community of oat silage by SMRT. Microorganisms. 2021;9(2):274. https://doi.org/10.3390/microorganisms9020274.
Li DX, Ni KK, Pang HL, Wang YP, Cai YM, Jin QS. Identification and antimicrobial activity detection of lactic acid bacteria isolated from corn stover silage. Asian-Australas J Anim Sci. 2015;28(5):620. https://doi.org/10.5713/ajas.14.0439.
Ni KK, Wang YP, Li DX, Cai YM, Pang HL. Characterization, identification and application of lactic acid bacteria isolated from forage paddy rice silage. PLoS ONE. 2015;10(3):e0121967. https://doi.org/10.1371/journal.pone.0121967.
Zhang Q, Yu Z. Characterization, identification and application of lactic acid bacteria isolated from Leymus chinensis silage. Grassl Sci. 2017;63(2):111–7. https://doi.org/10.1111/grs.12156.
Klein G. Taxonomy, ecology and antibiotic resistance of enterococci from food and the gastro-intestinal tract. Int J Food Microbiol. 2003;88(2–3):123–31. https://doi.org/10.1016/S0168-1605(03)00175-2.
Huo WJ, Wang XY, Wei ZX, Zhang HX, Liu Q, Zhang SL, Wang C, Chen L, Xu QF, Guo G. Effect of lactic acid bacteria on the ensiling characteristics and in vitro ruminal fermentation parameters of alfalfa silage. Ital J Anim Sci. 2021;20(1):623–31. https://doi.org/10.1080/1828051X.2021.1906167.
Ding ZT, Bai J, Xu DM, Li FH, Zhang YX, Guo XS. Microbial community dynamics and natural fermentation profiles of ensiled alpine grass Elymus nutans prepared from different regions of the qinghai-tibetan plateau. Front Microbiol. 2020;11:855. https://doi.org/10.3389/fmicb.2020.00855.
Tohno M, Kobayashi H, Nomura M, Kitahara M, Ohkuma M, Uegaki R, Cai YM. Genotypic and phenotypic characterization of lactic acid bacteria isolated from Italian ryegrass silage. Anim Sci J. 2012;83(2):111–20. https://doi.org/10.1111/j.1740-0929.2011.00923.x.
Wu JJ, Du RP, Gao M, Sui YQ, Xiu L, Wang X. Naturally occurring lactic acid bacteria isolated from tomato pomace silage. Asian-Australas J Anim Sci. 2014;27(5):648. https://doi.org/10.5713/ajas.2013.13670.
Tohno M, Kitahara M, Irisawa T, Masuda T, Uegaki R, Ohkuma M, Tajima K. Description of Lactobacillus iwatensis sp. nov, isolated from orchardgrass (Dactylis glomerata L.) silage, and Lactobacillus backii sp. nov. Int J Syst Evol Microbiol. 2013;63(Pt 10):3854–60. https://doi.org/10.1099/ijs.0.051920-0.
Tanaka O, Komatsu T, Oshibe A, Cai YM, Miyazaki S, Nakanishi K. Production of 3-hydroxypropionaldehyde in silage inoculated with Lactobacillus coryniformis plus glycerol. Biosci Biotechnol Biochem. 2009;73(7):1494–9.
Yan YH, Li XM, Guan H, Huang LK, Ma X, Peng Y, Li Z, Nie G, Zhou JQ, Yang WY, Cai YM, Zhang XQ. Microbial community and fermentation characteristic of Italian ryegrass silage prepared with corn stover and lactic acid bacteria. Bioresour Technol. 2019;279:166–73. https://doi.org/10.1016/j.biortech.2019.01.107.
Tanaka O, Otani R. Effect of inoculation with Lactobacillus coryniformis and glycerol in ensiling round bales of wholecrop rice. Bulletin of the NARO, Agric Res Tohoku Region. 2017;119:43–57.
Huo WJ, Zhang YJ, Zhang LY, Shen C, Chen L, Liu Q, Zhang SL, Wang C, Guo G. Effect of lactobacilli inoculation on protein and carbohydrate fractions, ensiling characteristics and bacterial community of alfalfa silage. Front Microbiol. 2022. https://doi.org/10.3389/fmicb.2022.1070175.
Du ZM, Sun L, Lin YL, Yang FY, Cai YM. The use of PacBio SMRT technology to explore the microbial network and fermentation characteristics of woody silage prepared with exogenous carbohydrate additives. J Appl Microbiol. 2021;131(5):2193–211. https://doi.org/10.1111/jam.15124.
Tanaka T, Hirano T, Kawai M, Higa S, Arimitsu J, Kuwahara Y, Ohkawara T, Hagihara K, Yamadori T, Shima Y, Ogata A, Kawase I, Tanaka T. Flavonoids and related compounds as anti-allergic substances. Allergol Int. 2007;56(2):113–23.
Park CH, Min SY, Yu HW, Kim K, Kim S, Lee HJ, Kim JH, Park YJ. Effects of Apigenin on RBL-2H3, RAW264.7, and HaCaT cells: anti-allergic, anti-inflammatory, and skin-protective activities. Int J Mol Sci. 2020;21(13):4620. https://doi.org/10.3390/ijms21134620.
Qi YY, Ding ZX, Yao YS, Ren FF, Yin M, Yang SB, Chen AP. Apigenin induces apoptosis and counteracts cisplatin-induced chemoresistance via Mcl-1 in ovarian cancer cells. Exp Ther Med. 2020;20(2):1329–36. https://doi.org/10.3892/etm.2020.8880.
Bensouici C, Kabouche A, Karioti A, Ozturk ME, Duru ME, Bilia AR, Kabouche Z. Compounds from Sedum caeruleum with antioxidant, anticholinesterase, and antibacterial activities. Pharm Biol. 2015. https://doi.org/10.3109/13880209.2015.1028078.
Acknowledgements
Not applicable.
Funding
This work was funded by the National Natural Science Foundation of China (32001402, 31971767), the Key Scientific Research Project of Colleges and Universities in Henan Province (23A230003), and the Doctoral Scientific Research Start-up Foundation from Henan University of Technology (31401489).
Author information
Authors and Affiliations
Contributions
KN and LG designed the study. LG, XW, XL, HZ performed the experiments. LG and HC wrote the manuscript. LG, HC, YX and GX conducted the statistical and bioinformatics analysis. KN and FY contributed to conceptualization and funding acquisition, and were involved in the revision of the manuscript. All the authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors listed have read the complete manuscript and have approved submission of the paper.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: A fund number 31971767 has been added to the Funding note.
Supplementary Information
Additional file 1: Table S1.
The chemical compositions of alfalfa and mugwort residues before ensiling.
Additional file 2: Table S2.
Original metabolites of silages.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Guo, L., Wang, X., Chen, H. et al. Exploring the fermentation quality, bacterial community and metabolites of alfalfa ensiled with mugwort residues and Lactiplantibacillus pentosus. Chem. Biol. Technol. Agric. 10, 107 (2023). https://doi.org/10.1186/s40538-023-00472-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40538-023-00472-x