Abstract
Laurus nobilis (LN) has been used throughout the years as a food flavoring and in traditional medicine. The LN leaves have various biological activities, such as antioxidant, wound healing, antibacterial, analgesic, and anti-inflammatory activities. However, oxidative stress, cancer, diabetes, microbial infections, and inflammatory diseases are closely linked. The objective of this research is to characterize Laurus nobilis (LN) aromatic oil (AO) and evaluate its antioxidant, antidiabetic, antiobesity, antimicrobial, and antimutagenic bioactivities. The AO constituents were characterized using gas chromatography–mass spectrometry (GC–MS). The antimicrobial activity was performed using a microdilution assay against six common microbial species. Free radicals, a porcine pancreatic lipase, α-amylase, and α-glucosidase inhibitory assays were conducted utilizing reference biomedical methods. The cytotoxic effect of LNAO was established on a variety of cancer and normal cell lines using the MTS assay. The anti-inflammatory activity of LNAO was evaluated using the Cayman COX activity kit. The results indicate about 99% of the total oil is composed of 36 compounds, the characterized AO metabolites showed content of many oxygenated terpenoids with 1,8-Cineole and Terpinyl acetate as a major component with a percentage of (40.39 and 15.07, respectively. The plant AO showed potent antioxidant activity (IC50 = 2.2 ± 1.38) and has moderate anti-amylase (IC50 = 60.25 ± 1.25), anti-glucosidase (IC50 = 131.82 ± 0.1), and antilipase (IC50 = 83.17 ± 0.06) activities. Moreover, LNAO showed potent antimicrobial activity against Staphylococcus aureus, Escherichia coli, Klebsiella pneumonia, Proteus vulgaris (MICs = 1.56 µg/mL), methicillin-resistant Staphylococcus aureus (MRSA) (MIC = 3.125 µg/mL) and Candida albicans (MIC = 0.195 µg/mL). The cytotoxicity results demonstrated that at a concentration of 1 mg/mL, LNAO has potent breast cancer (MCF-7), and hepatocellular carcinoma (Hep 3B) cancer cells inhibitory activities of 98% and 95%, respectively. Importantly, we are the first to show that LNAO significantly hinders hepatocellular carcinoma spheroids’ formation capacity in a 3D model. These results show that LNAO is a promising natural source with powerful antioxidant, antidiabetic, anticancer, and antimicrobial activities that could be exploited in the future to treat a variety of diseases.
Graphical Abstract

Similar content being viewed by others
Introduction
Communities have used complementary and alternative medicine since ancient times, particularly the aromatherapy, which employs aromatic oils (AOs) to treat a variety of illnesses [1]. Aromatherapy is an ancient healing practice that dates back roughly 6000 years and was widely practiced in many civilizations like Egypt, India, and China [2]. Recently, the reputation of natural AOs has grown in the pharmaceutical, cosmetic, medical, food, and fragrance industries. AOs are derived from numerous plant parts, including: leaves, flowers, fruits, bark, roots, and seeds [3]. In actuality, AOs comprise a mixture of unsaturated and saturated hydrocarbon derivatives, such as oxides, alcohol, phenols, aldehydes, esters, and terpenes, which can produce aromas [4]. In addition, plants and their derivatives, such as essential oils and extracts, are used as medicine in the global healing system. However, it is exceedingly difficult to obtain drugs made from plants. As a result, scientists are doing research on plants and other natural resources in an effort to identify a feasible, medicinally active material [5].
Until now, data indicated that plant-based medication was more acceptable to people than synthetic medicine. The explanations for such use are the cultures and beliefs of people; the awareness of herbal medicine’s safety; the availability and affordability of herbal remedies; the delays in medical counseling checkups; and the belief in medicinal plants' superior efficacy to conventional medicine, particularly in the event of the latter's failure [6].
The genomic integrity of the cell is maintained by an equilibrium between pro-oxidants of the cell constituents [7]. If this equilibrium is disturbed; the cellular signaling pathways are altered, leading to uncontrolled cell proliferation and cancer, while macrophage polarization results in the formation of atherogenic plaques [7, 8]. Excessive oxidative stress leads to the generation of reactive oxygen species (ROS), which have been associated with a range of deadly illnesses, including diabetes, cancer, chronic inflammation, neurological, infectious, and cardiovascular disorders [9, 10].
In addition, oxidative stress and free radical-induced complications from diabetes mellitus include retinopathy, nephropathy, neuropathy, and coronary artery disease [11]. Moreover, in microbial infections, oxidative stress results, at the very least in part, from altered metabolic pathways. This type of stress has also been linked to the destruction of organs and the development of inflammation and cancer [12].
The involvement of oxidative stress in the etiology of obesity and its related risk factors has been proven by animal, clinical, and epidemiological studies. By promoting the deposition of white adipose tissue and increasing food intake, oxidative stress may cause overweight and obesity [13]. ROS has been identified to play a role in the regulation of body weight by exerting different impacts on the neurons of the hypothalamus that regulate appetite [14]. Besides, obesity produces systemic oxidative stress by numerous biochemical mechanisms, including polyol and hexosamine, protein kinase C (PKC) activation, glyceraldehyde auto-oxidation, oxidative phosphorylation, and superoxide production from NADPH oxidases (NOX) pathways [15]. In addition to hyperleptinemia, tissue dysfunction, inadequate antioxidant defense, chronic inflammation, and postprandial ROS production also contribute to obesity’s oxidative stress [16].
The rapid increase in antimicrobial resistance complicates the treatment of life-threatening diseases and endangers the foundation of modern healthcare [17]. Antimicrobial medications were and are still used inappropriately. In many developing countries, individuals can get antibiotics without a prescription and follow the correct treatment guidelines [18].
Cancer is undoubtedly among the most fatal diseases in society, as it affects everyone regardless of their socioeconomic standing, age, or place of origin. After cardiovascular disease, cancer is one of the leading causes of death in rich societies. According to estimates, over 20 million new instances of cancer were identified worldwide in 2020, and as many as 10 million individuals perished from the disease [19].
Chronic inflammatory diseases are the leading global cause of death. According to the World Health Organization, chronic diseases pose the biggest risk to human health (WHO). Over the next 30 years, the prevalence of illnesses associated with chronic inflammation is projected to increase steadily in the United States. Chronic inflammatory illnesses kill three out of every five individuals globally [20].
Over one billion people worldwide are obese, including 650 million adults, 340 million adolescents, and 39 million children. The WHO estimates that by 2025, approximately 167 million people (adults and children) will experience a decrease in their health due to their overweight or obesity. Obesity is a condition that affects virtually every system of the body. It impacts the cardiovascular, hepatobiliary, renal, musculoskeletal, and reproductive systems. It causes various noncommunicable diseases (NCDs), such as type 2 diabetes, cardiovascular disease, hypertension, stroke, cancer, and mental health issues [21].
Diabetes is a chronic metabolic illness characterized by elevated blood glucose (or blood sugar) levels, which over time cause serious damage to the heart, blood vessels, eyes, kidneys, and nerves. Diabetes affects over 422 million people worldwide, with the majority residing in low- and middle-income countries; it is directly responsible for 1.5 million deaths annually. Both the number of cases and the prevalence of diabetes have increased during the previous two decades [22].
Numerous and diverse plant species are known to possess therapeutic potential. There are around 70,000 plant species utilized for therapeutic purposes, ranging from algae to trees. The National Cancer Institute (NCI) has investigated roughly 35,000 plant species for possible anticancer antitumor properties. Approximately 3000 plant species demonstrate repeatable antitumor efficacy [23].
Laurus nobilis L. (LN) is a perennial shrub that belongs to the family Lauraceae. It has been used throughout the years as a food flavoring and in traditional medicine, because it contains a variety of bioactive and flavoring components [24]. However, several studies have proved the effect of LN aromatic oil (AO) as an antimicrobial, wound healing, antioxidant, anticonvulsant, antimutagenic, and immuno-stimulant [24,25,26,27,28,29]. The LNAO could be considered a natural supplement or antioxidant in cosmetics and medicine [30, 31].
Despite various ethnomedical applications and important pharmacological activities of LNAO, the biological and phytochemical potentials of this plant species found in Palestine have not been studied. In addition, as we previously reported, oxidative stress, cancer, diabetes, microbial infections, and inflammatory diseases are closely linked. Therefore, this paper deals with the extraction, characterizations of the chemical constituents, antioxidant, antimicrobial, antilipase, anti-α-amylase, anti-α-glucosidase, cytotoxic and anti-tumorigenic effects of the AO of LN leaves collected from Palestine.
Materials and methods
Preparation and extraction of LNAO
The LN plant's leaves were gathered in March 2022 in the Palestinian city of Qalqilya. Before being pounded into a coarse powder, the green, fresh leaves were dried for 2 weeks in the shade at a normal temperature and humidity. Pharmacognosist Dr. Nidal Jaradat carried out the study’s characterization at the Department of Pharmacy, Faculty of Medicine and Health Sciences, An-Najah National University, Palestine. The plant specimen was preserved using the voucher specification code (Pharm-PCT-1366). The LN plant's AO was isolated as stated previously by Jaradat et al. [32]. In a summary, 500 g of dried, powdered leaves were hydrodistilled in a Clevenger-style equipment for 4 h to produce AO, which was then dehydrated with anhydrous sodium sulfate. The dried plant sample had an average extracted AO yield of 1.55%. Up until it was employed in the studies, the AO was kept in an amber flask at a temperature of 5 °C.
GC–MS analysis
A Perkin Elmer Clarus 500 GC gas chromatograph with a Perkin Elmer Clarus 560 mass spectrometer was used for the GC–MS analysis to identify the major constituents of LNAO. Perkin Elmer Elite-5 fused-silica capillary column (30 m × 0.25 mm, film thickness 0.25 m) was utilized to perform the chromatographic separation. The column temperature was scheduled to rise by 4 °C every minute from 50 °C for 5 min to 280 °C. Helium was used as a carrier gas at a constant flow rate of 1 mL/min. The oven temperature was set at 250 °C. An exact volume of 0.2 µl of plain oil was injected in split mode with a split ratio of 1:50. The National Institute of Standards and Technology's MS Data Centre reference spectra library were compared to the obtained mass spectra of the chemical components of the tested AO, and their Kovats and retention indices were compared to values reported in the literature [33,34,35].
Antioxidant assay
A solution of AO (1 mg/mL) in methanol was serially diluted with methanol to generate concentrations of 2, 5, 10, 20, 30, 50, and 80 µg/mL for measurement of LNAO antioxidant inhibition effect. The DPPH (2,2-diphenyl-1-picrylhydrazyl) reagent (Sigma, USA) was then dissolved in methanol (0.002%w/v) and combined in a 1:1 ratio with the previously generated working concentration. The same methods were done for Trolox (Sigma-Aldrich, Denmark) as a positive control. All of the solutions were maintained at room temperature in a dark chamber for 30 min. Their absorbance values were then measured using a UV–visible spectrophotometer at a wavelength of 517 nm. The inhibition potentials of LNAO and Trolox against DPPH were calculated using the following equation:
where absblank is the blank absorbance and abssample is the absorbance of the samples. The antioxidant half-maximal inhibitory concentration (IC50) of LNAO and Trolox was assessed using the BioDataFit program [36].
Antimicrobial activity
The antibacterial activity of LNAO was determined as previously described in our research work [37]. The bactericidal effect of the AO was performed utilizing five common bacterial species derived from the American Type Culture Collection (ATCC), including: Klebsiella pneumonia (13883), Escherichia coli (25922), Pro-teus vulgaris (8427), Staphylococcus aureus (25923), and Pseudomonas aeruginosa (9027). Methicillin-resistant Staphylococcus aureus (MRSA) that was used in this investigation which was isolated from An-Najah National University Hospital. LNAO's antifungal activity was also tested against Candida albicans (90028). The Minimal Inhibitory Concentration (MIC) of the LNAO was evaluated using broth microdilution, whereas the Minimal Lethal Concentration (MLC) was determined by subculturing into the surface of the agar plates.
The AO was first dissolved in DMSO at a 200 µg/mL concentration. In sterile Mueller–Hinton Broth, twofold serial microdilutions were performed 10 times (10 wells) (MHB). The dilutions were performed in 96-well plates under aseptic conditions, with the abovementioned 10 wells containing a gradient of LNAO concentrations (50 µg/mL to 0.1 µg/mL), mixed with prepared bacterial solutions. The other two wells were utilized as controls; one was a positive growth control containing only media and bacteria, while the other was a negative growth control containing only media. Then, micro-well plates were incubated at 37 °C for 18–24 h. The exact process was performed for the used fungal strain, C. albicans, in which the media used was RPMI, and the incubation time was 48 h. The lowest concentration of the AO that inhibits microorganism growth is considered MIC. To calculate the MIC values, those micro-wells with no growth were subcultured into the surface of the agar plate, and the lowest concentration with no microbial growth was considered as the MLC. Doxycycline and Ciprofloxacin were used as positive controls, while Miconazole was used as positive control for antifungal activity [37].
Porcine pancreatic lipase inhibitory assay
The pancreatic lipase inhibitory test was performed in a similar methodology described by Zheng et al. with slight modifications [39]. Orlistat medication is an anti-obesity and anti-lipase medicine and was used as a positive control. A 500 mg/mL AO stock solution was mixed in DMSO: methanol (1:9), and five separate dilutions were made to make a final concentration of (10, 50, 100, 500, and 700 µg/mL). A freshly prepared 1 mg/mL stock solution of porcine pancreatic lipase was dispersed in Tris–HCl buffer. PNPB (Sigma-Aldrich, Germany) solution was prepared by dissolving 20.9 mg in 2 mL of acetonitrile. In 5 distinct working test tubes, 0.1 mL of porcine pancreatic lipase (1 mg/mL) and 0.2 mL of the AO from each concentration series were combined. The mixture was completed to1 mL with Tri-HCl solution and was incubated at 37 °C for 15 min. After that, 0.1 mL of p-nitrophenyl butyrate solution was added to each test tube, and the mixture was incubated at 37 °C for 30 min. A UV–Vis spectrophotometer was used to measure the hydrolysis of PNPB into p-nitrophenolate ions at 410 nm to estimate pancreatic lipase activity. The same steps were taken with the positive control sample (Orlistat) (Sigma-Aldrich, Germany). Using the following equation, the inhibitory percentage of anti-lipase activity was calculated:
where AB is the recorded absorbance of the blank solution and Ats is the recorded absorbance of the tested sample solution.
α-Amylase inhibition assay
The α-amylase inhibitory activity of LNAO was tested according to the standard method reported by Nyambe-Silavwe et al. with slight changes [38]. The AO was dissolved in DMSO (Riedel-de-Haen, Germany) and then diluted to 1000 µg/mL with a buffer ((Na2HPO4/NaH2PO4 (0.02 M), NaCl (0.006 M) at pH 6.9). A series of concentrations of 10, 50, 70, 100, and 500 µg/mL were created. 0.2 mL of porcine pancreatic -amylase enzyme solution (Sigma-Aldrich, USA) was combined with 0.2 mL of the AO and incubated at 30 °C for 10 min. The mixture was then incubated for at least 3 min after adding 0.2 mL of newly produced starch solution (1%). The reaction was stopped using 0.2 mL of dinitrosalicylic acid (DNSA) (AlfaAesar, UK), the mixture was then diluted with 5 mL of distilled water and heated in a water bath at 90 °C for 10 min. The mixture was allowed to cool to room temperature and then its absorbance at 540 nm was measured. Following the same above steps; a blank was prepared by replacing the LNAO with 0.2 mL of the buffer.
Aacarbose (Sigma-Aldrich, USA) was used as appositive control following the same process as described above. The inhibitory activity of -amylase was determined using the following equation:
where Ab is the absorbance of the blank and AS is the absorbance of the tested sample or control.
α-Glucosidase inhibitory activity assay
LNAO’s-glucosidase inhibitory activity was tested using a standard methodology followed by Ademiluyi et al. with a minor modification [39]. A mixture of 50 μL of phosphate buffer (100 mM, pH 6.8), 10 μL α-glucosidase (1 U/mL) (Sigma-Aldrich, USA), and 20 μL LNAO to have a serial concentration of (100, 200, 300, 400 and 500 µg/mL) which were added in 5 distinct test tubes. After 15 min at 37 °C, 20 μL of pre-incubated 5 mM PNPG (Sigma-Aldrich, USA) was added to each test tube. The reaction mixtures were incubated for 20 min at 37 °C. The process was stopped by adding 50 μL of aqueous Na2CO3 solution (0.1 M). The absorbance of the emitted p-nitrophenol was measured by a UV/Vis spectrophotometer at 405 nm. The positive control was acarbose at similar quantities to the plant AO. The inhibition percentage was calculated using the following equation [40]:
where Ab is the absorbance of the blank and AS is the absorbance of the tested sample or control.
Cytotoxicity method
Cells from the following types of cancer were grown in RPMI 1640 medium: breast cancer (MCF-7), hepatocellular carcinoma (Hep 3B & Hep G2), skin tumor (B16-F1), colorectal adenocarcinoma (COLO 205, Caco-2), cervical adenocarcinoma (HeLa), human hepatic stellate (LX-2), and human epithelial kidney (HEK-2 HeLa cells were grown at 37 °C in a humidified environment with a 5% CO2 atmosphere, and 5 × 103 cells were then seeded into each well of a 96-well plate. After 24 h, cells were exposed to various concentrations of the tested AO (10, 50, 100, 500, and 700 µg/mL) with Doxorubicin as a positive control for 48 h. The Cell-Tilter 96® Aqueous One Solution Cell Proliferation (MTS) bioassay (Promega Corporation, Madison, WI) was used to test the cell viability in accordance with the instructions on the package. Furthermore, 100 µL of medium and 20 µL of MTS solution were added to each well, and the plates were then incubated for 2 h 37 °C. The absorbance of the plates was read using a UV–Vis spectrophotometer at 490 nm [41, 42].
Cyclooxygenase inhibitory effect
The AO cyclooxygenase inhibition activity was tested using Cayman COX inhibitor screening test. The test procedure followed Cayman Chemical Manufacturer's Guidelines. The kit has human recombinant COX-2 and bovine COX-1 which convert arachidonic acid (AA) to PGH2. LNAO's 50% inhibitory concentration (IC50) on COX-1/COX-2 selectivity was evaluated at two prepared concentrations (50 and 350 g/mL). All the test we performed in triplicate. The inhibition of the extracted plant was calculated from a standard curve consisting of eight different standard doses of prostaglandin, a non-specific binding sample, and a maximum binding sample. The multiple regression equation of the best-fit line generated by the kit was employed. The IC50 concentration was computed using the percentage of inhibition of the tested concentration [43].
Liver cancer spheroids production test
The ability of spheroids to develop was investigated in round-bottom wells with extremely low attachment conditions. In this experiment, 4 × 103 Hep3B liver cancer cells were planted per well for 24 h at 37 °C and 5% CO2 in the presence of increasing doses of Laurus Nobilis essential oil. Doxorubicin, an anti-cancer drug, was employed as a positive control (100 mg/mL). Images of the formed spheroids/clusters were obtained at 0 h and 24 h using an inverted microscope. ImageJ was used to examine the spheroids' images.
Statistical analysis
All tests were done in triplicate; the results were expressed as means ( ±) standard deviation (SD). A p value < 0.05 was considered statistically significant when analysis of variance (ANOVA) and t test were employed.
Results and discussion
Chemical composition of aromatic oil
A GC–MS combination was used to determine the chemical composition of the LN plant's leaf-extracted AO on both a qualitative and quantitative level (Additional file 1: Figure S1). The identities and amounts of the chemical components of AO isolated from LN are presented in Table 1.
About 99% of the total oil is constituted of 36 compounds and most of the characterized AO metabolites are oxygenated terpenoids. As can be seen from Table 1, 1,8-cineole is the major component (40.388%), followed by terpinyl acetate (15.067%), and sabinene (10.350%).
A study conducted by El-Sawi et al. reported that 1,8-cineole (50.38%), α-terpenyl acetate (19.97%), 4-trepinol (6.48%), and sabinene (4.82%) were the major ingredient of LNAO from Egypt [26]. In addition, 1,8-cineole (31.9%), sabinene (12.2%), and linalool (10.2%) are the main components of LNAO from Italy [44]. Besides, 1.8-cineole (52.43%), α-terpinyl acetate (8.96%), and sabinene (6.13%) were the abundant molecules identified in the LNAO from Morocco [25].
DPPH-free radicals, porcine pancreatic lipase, α-amylase, and α-glucosidase inhibitory activities
Obesity is gaining acceptance as a serious primary health burden that impairs the quality of life because of its associated complications, including cancer, infertility, renal dysfunction, hepatic disorders, sleep problems, asthma, diabetes, and cardiovascular diseases [45]. There is a strong correlation between obesity, oxidative stress, diabetes, cancer, inflammatory and infectious diseases. As well as each of the mentioned diseases can lead directly to other diseases [45].
Antioxidant activity of natural and synthetic products can be evaluated using the DPPH (2,2-diphenyl-1-picrylhydrazyl) technique, which is an accurate, simple, cheap, rapid technique for evaluating the potential of diverse substances to serve as free radical scavengers or hydrogen donors [46].
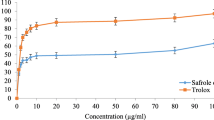
Figure 1 demonstrates the in-vitro DPPH scavenging ability of the LNAO and positive control drug Trolox. In fact, Trolox (6-hydroxy-2,5,7,8-tetramethyl chromane-2carboxylic acid) is a water-soluble antioxidant that was produced in 1974 as a vitamin E derivative and has been utilized as a standard antioxidant in assays of antioxidant properties [47]. According to this colorimetric assay, LNAO has potent free radical scavenging activity and at the concentration of 80 µg/mL, the AO exhibited 93.22 ± 1.7 percentage compared with Trolox, which at the same concentration has 92.65 ± 0.01% DPPH inhibitory activity. Table 2 results are presented as IC50 values; the lower IC50 doses indicated stronger antioxidant activity. However, DPPH inhibitory activity experiment in the present work demonstrates that the LNAO has potent free radical scavenging power, even more than Trolox, with IC50 doses of 2.2 ± 1.38 and 2.88 ± 0.57, respectively which means that the DPPH radical scavenging activity of the LNAO was higher than the activity of the positive control.α-Amylase inhibitors suppress the action of pancreatic and salivary amylase in vivo and in-vitro. They can impair the animal’s complex carbohydrate metabolism when given in high amounts in supplements or diet, which may be of great importance in the treatment of diabetes or obesity.
In an array to explore the antidiabetic activity, LNAO was screened for the α-amylase inhibitory property. Initial screening showed that the AO has strong α-amylase inhibitory potential compared with the antidiabetic drug Acarbose, that is, 58.67 ± 0.00% and 72.54 ± 1.37% at 500 µg/mL, respectively (Fig. 2). Furthermore, the AO showed a concentration-dependent increase in percent inhibition of α-amylase activity with IC50 values (60.25 ± 1.25 and 28.18 ± 1.22 µg/mL, respectively) (Table 2).
Porcine pancreatic α-amylase inhibitory property of Laurus nobilis aromatic oil and Acarbose α-Amylase and α-glucosidase inhibitors suppress the action of pancreatic and salivary amylase in vivo and in-vitro. They can impair the animal’s carbohydrate metabolism when given in high amounts in supplements or diet, which may be of great importance in the treatment of diabetes or obesity
It is well known that inhibiting intestinal α-glucosidase activity causes delayed digestion of monosaccharides, resulting in less postprandial hyperglycemia. In Fig. 3, the LNAO suppressed the α-glucosidase in a dose-dependent manner. Actually, at the starting treatment (100 µg/mL), the LNAO inhibited the effect of α -glucosidase by 53.22 ± 0.2%, compared with Acarbose, which inhibited at the same concentration the action of α-glucosidase by 65.5 ± 0.5%. At 500 µg/mL dose, the LNAO inhibited the action of α-glucosidase by 72.54 ± 0.15% compared with Acarbose, which inhibited the action of α-glucosidase at the same dose by 85.1 ± 0.6%. The plant AO showed (Table 2) a moderate α-glucosidase inhibitory activity matched with Acarbose (IC50 = 131.82 ± 0.1 and 41.68 ± 0.34 µg/mL, respectively). α-Glucosidase antagonists slow the rapid increase in blood sugar levels that diabetes people commonly suffer following snacking by delaying gastrointestinal carbohydrate digestion. However, none of the α-glucosidase inhibitors that are now available for clinical usage are free from serious side effects [48].
Besides hyperglycemia associated with high consumptions of simple and complex types of carbohydrates, diabetic patients are usually highly characterized by high levels of triglycerides in the blood which is highly associated with increased consumption of a high-fat diet and modern lifestyle [49].
Reducing the absorption of free fatty acids by inhibiting the action of pancreatic lipase decreases hyperlipidemia and hyperglycemia associated with obesity and diabetes [50].
Figure 4 shows that the LNAO has a moderate antilipase effect compared with Orlistat. However, at a concentration of 500 µg/mL, LNAO and Orlistat inhibited the lipase enzyme with 62.64 ± 0.2 and 97.3 ± 0.58%, respectively. From this figure, the IC50 values of the tested sample were calculated and were 83.17 ± 0.06 and 12.88 ± 0.94 µg/mL, respectively (Table 2).
A study established by [26] found that LNAO has DPPH free radical scavenging activity with an IC50 value of 0.52 mg/mL.
Antibacterial and antifungal activities
Table 3 depicts the results of the antimicrobial activity of LNAO against Gram-negative and Gram-positive bacteria and fungal strains. The results revealed that LNAO has strong antibacterial and antifungal activities against the screened Gram-positive, Gram-negative, and fungal strains (Table 3). The highest antibacterial activity was noticed on S. aureus, E. coli, K. pneumonia, and P. vulgaris with MIC and MLC values of 1.56 µg/mL. Besides, compared with Doxycycline and Ciprofloxacin antibiotics, the LNAO showed powerful anti-MRSA with MIC and MLC values of 3.125 µg/mL for each. In addition, against S. aureus, the antibacterial activity of the LNAO showed similar effective potential as in Ciprofloxacin and less effective than Doxycycline with MICs of 1.56, 1.56, and 0.1 µg/mL, respectively. While LNAO MLC against S. aureus showed promising potential activity that is equal to Ciprofloxacin and more potent than Doxycycline with MLCs values of 1.56, 1.56, and 12.5 µg/mL, respectively. Moreover, the LNAO demonstrated high activity against E. coli and K. pneumoniae than Doxycycline with MLCs values of 1.56, vs 50 µg/mL, respectively, while having the same MIC values as 1.56 µg/mL. In the same context, in comparison with Ciprofloxacin, LNAO has less potency against E. coli, P. vulgaris, P. aeruginosa, and K. pneumoniae. Interestingly, the activity of our extract against P. aeruginosa is valuable in comparison with Doxycycline, with MIC and MLC values of 6.25, 6.25, 12.5, and 50 µg/mL, respectively. Indeed, the LNAO has strong activity against C. albicans compared with the commercial anti-yeast drug Miconazole with MIC values of 0.195 and 0.39 µg/mL, respectively, while has more potent MLCs (1.95 and 12.5, respectively).
These results go beyond previous reports, showing that LNAO has potential antibacterial activity against S. aureus, E. coli, and P. aeruginosa with MICs of 0.4, 0.8, and 0.4 µm/mL, respectively [44].
When comparing our results to those of older studies, it must be pointed out that LNAO from Palestine has more potential anticandidal activity than LNAO from Brazil and has MIC and MLC values of 250 and 500 µg/mL, respectively, while LNAO from Palestine has MIC and MLC values of 0.195 µg/mL [51].
The current study outcomes are directly in line with previous findings which reported the antimicrobial strength of LNAO collected from various locales [25, 52, 53].
Cyclooxygenase activity
For ages, people treated various inflammatory illnesses utilizing medicinal plants due to the presence of secondary metabolites like polyphenols, terpenes, aromatic oils and tannins. It was demonstrated that those molecules can regulate the innate and adaptive immune response by inhibiting or stimulating cell signaling pathways involved in the inflammatory response [54].
The anti-inflammatory activity of the LNAO plant from Palestine was the first time ever to be evaluated using an in-vitro Cayman kit test. The inhibition activity against cyclooxygenase (COX) coenzymes was evaluated and the result indicates almost an equivalent activity for both COX coenzymes (Fig. 5). However, the results showed slightly higher activity against COX 1. The plant extract at a concentration of 40 µg/mL showed more than 80% inhibition for both COX coenzymes. This result indicates lower potency compared to the selective COX2 Celecoxib drug which has IC50s = 22.9 and 0.05 μM for COX-1 and COX-2, respectively)[55]. However, LNAO showed much more potent COX inhibition activity compared to Ibuprofen as shown in previously published research, where it has 50% inhibition at a concentration of 1 μg/mL and 46 μg/ml for COX1 and COX2 enzymes, respectively [56]. Moreover, the COX activity of LNAO was much more potent against COX 2 compared to a recently published work by our research team of Artemisia arborescens essential oil (IC50 = 81.7 µg/mL) [57].
Cytotoxicity
The LNAO was tested against various cancer cell lines including: hepatocellular carcinoma (Hep 3B & Hep G2), breast cancer (MCF-7), skin tumor (B16-F1), cervical adenocarcinoma (HeLa), colorectal adenocarcinoma (COLO 205, Caco-2), and Human epithelial kidney (HEK-293 T) and human hepatic stellate (LX-2) cells. The results indicate variation in the inhibition activity according to cancer cell line type. The results illustrate moderate to potent activity against HeLa MCF-7, HepG2 and Hep3B cancer cells. The detailed IC50 results are shown in Table 4.
The cytotoxicity results clearly demonstrated the potent inhibition activity of LNAO against MCF-7 and HeLa cancer cells. Moreover, a higher concentration of the plant AO (1 mg/mL) caused 98% and 95 inhibitions of MCF-7 and Hep 3B cancer cells, respectively (Fig. 6). The percentage inhibition showed a significant increase in cell inhibition at a higher dose for MCF-7 and Hep 3B compared to other tested cell lines (Fig. 7).
The cytotoxicity test of this research comes in support of similar research done on the same plant by other research groups showing antitumor activity in HPV16-transgenic mice [58]. Kalaldeh et al. did a cytotoxicity evaluation of plants on breast cancer cells only; in this research paper, we examined nine cancer cell lines making this research broader and more extensive work to evaluate the anticancer activity of L. nobilis aromatic extract [59].
LNAO blocks three-dimensional spheroids’ formation in a dose-dependent manner
Multicellular 3D spheroid models mimic the microenvironment of real tumors. In addition, they offer a platform for the identification of cancer therapies as well as a deeper comprehension of cellular and external interactions. The formation of 3D cancer spheroids reflects cancer cells’ capacity to form tumors. In this context, we examined the capacity of Hep3B liver cancer cells to form spheroids in presence of different concentrations of LNAO. In Control, non-treated cells aggregated to form a round 3D spheroid (Fig. 8). The increasing concentrations of LNAO showed a dose-dependent hindering effect on spheroid formation capacity as 500 µg/mL and 1000 µg/mL have significantly hindered spheroid formation (Fig. 8A–D). Alternatively, cells were aggregated as a very large 2D cluster with irregular shapes, while a similar result was observed in the positive control condition (Doxorubicin). It has been recently reported that sensitivity to certain anti-tumor drugs was reduced in a regrowth test of hepatocellular carcinoma in a 3D model [60]. In addition, 3D spheroid formation capacity was shown to be prevented in ovarian cancer 3D models [61]. The effect of LNAO has been studied on MCF-7 spheroids and results showed an inhibitory effect on cell viability (63). Altogether, those results suggest strongly that LNAO possesses tumor formation-blocking abilities against hepatocellular carcinoma.
LNOA blocks three-dimensional spheroids’ formation in a dose-dependent manner. Illustrative images of Hep3B liver cancer cells after 24 h of incubation in presence of (0, 31.25, 62.5, 125, 250, 500, and 1000 µg/mL of LNAO or 100 µg/mL of Doxorubicin (used as a positive control) to test their spheroids formation capacity (8 A). Analytic figures include Cluster area relative to a negative control (8 B), cluster perimeter relative to the negative control (8 C), and cluster circularity "value of 1 = round cluster, the value of 0 = elongated cluster" (8 D). Control condition refers to non-treated cells. Scale bar = 10 μm
Conclusions
The current research is the first of its kind to explore the biological and phytochemical potentials of this plant species found in Palestine. The current results revealed the presence of many phytochemicals in the aromatic oil (AO) extracts of Laurus nobilis. Obviously, the Laurus nobilis aromatic oil showed a very potent antioxidant activity with more inhibition activity against the positive control Trolox (IC50 = 2.2 ± 1.38). The results also showed that the extracts have potential cytotoxic activity against MCF-7 and Hep 3B cancer cells with inhibition activity of 98% and 95%, respectively. Besides, AO LN showed many other inhibitory activities against amylase, glucosidase, and lipase enzymes; indicating a promising therapeutic effect for diabetic and obese patients. Moreover, Laurus nobilis aromatic oil showed a potent antimicrobial effect against S. aureus, E. coli, P. aeruginosa, K. pneumonia, MRSA (MIC = 3.125 µg/mL), and C. albicans (MIC = 0.195 µg/mL) and was more effective than the positive controls used: Doxycycline, Ciprofloxacin, and Miconazole. Of note, Laurus nobilis aromatic oil proved to have an inhibitory impact on liver cancer spheroids’ formation capacity in a three-dimensional model that mimics the primary tumor. These findings indicate that the AO LN collected from Palestine is a promising natural source of potent biological activity as an antioxidant, antidiabetic, antimicrobial, and anti-tumorigenic agent. In fact, it can be used in future pharmaceutical formulations and as a treatment strategy for oxidative stress, diabetes, obesity, cancer, and microbial infectious diseases.
Availability of data and materials
All data are contained within the article.
Abbreviations
- LN:
-
Laurus nobilis
- AO:
-
Aromatic oil
- GC–MS:
-
Gas chromatography–mass spectrometry
- MRSA:
-
Methicillin-resistant Staphylococcus aureus
- MCF-7:
-
Breast cancer
- Hep 3B:
-
Hepatocellular carcinoma
- 3D:
-
Three dimensional
- PKC:
-
Protein kinase C
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate hydrogen
- WHO:
-
World Health Organization
- NCDs:
-
Noncommunicable diseases
- NCI:
-
The national cancer institute
- IC50:
-
The half maximal inhibitory concentration
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- UV:
-
Ultraviolet
- ATCC:
-
American Type Culture Collection
- DMSO:
-
Dimethyl sulfoxide
- RPMI:
-
Roswell Park Memorial Institute
- MIC:
-
Minimum inhibitory concentration
- AB:
-
Absorbance of the blank
- Ats:
-
Recorded absorbance of the tested sample solution
- DNSA:
-
Dinitrosalicylic acid
- PNPG:
-
4-Nitrophenyl-β-D-glucopyranoside
- HEK:
-
Human epithelial kidney
- MTS:
-
3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- COX:
-
Cyclooxygenase
- ANOVA:
-
Analysis of variance
- SD:
-
Standard deviation
References
Lis-Balchin M. Aromatherapy science: a guide for healthcare professionals. London: Pharmaceutical press; 2006.
Baldwin AL, Chea I. Effect of aromatherapy on equine heart rate variability. J Equine Vet Sci. 2018;68:46–50.
Andrade M, Ribeiro-Santos R, Silva AS. Essential oils from plants: industrial applications and biotechnological production. In: Malik S, editor. Exploring Plant Cells for the Production of Compounds of Interest. Cham: Springer International Publishing; 2021. p. 145–70.
El Hadi MAM, Zhang F-J, Wu F-F, Zhou C-H, Tao J. Advances in fruit aroma volatile research. Molecules. 2013;18(7):8200–29.
Veeresham C. Natural products derived from plants as a source of drugs. J Adv Pharm Technol Res. 2012;3(4):200.
Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2014;4:177.
Jain KA, Mehra KN, Swarnakar KN. Role of antioxidants for the treatment of cardiovascular diseases: challenges and opportunities. Curr Pharm Des. 2015;21(30):4441–55.
Khosravi M, Poursaleh A, Ghasempour G, Farhad S, Najafi M. The effects of oxidative stress on the development of atherosclerosis. Biol Chem. 2019;400(6):711–32.
Hajam YA, Rani R, Ganie SY, Sheikh TA, Javaid D, Qadri SS, et al. Oxidative stress in human pathology and aging: molecular mechanisms and perspectives. Cells. 2022;11(3):552.
Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49(11):1603–16.
Asmat U, Abad K, Ismail K. Diabetes mellitus and oxidative stress—A concise review. Saudi Pharm J. 2016;24(5):547–53.
Ivanov AV, Bartosch B, Isaguliants MG. Oxidative stress in infection and consequent disease. Oxid Med Cell Longev. 2017. https://doi.org/10.1155/2017/3496043.
Savini I, Catani MV, Evangelista D, Gasperi V, Avigliano L. Obesity-associated oxidative stress: strategies finalized to improve redox state. Int J Mol Sci. 2013;14(5):10497–538.
Horvath TL, Andrews ZB, Diano S. Fuel utilization by hypothalamic neurons: roles for ROS. Trends Endocrinol Metab. 2009;20(2):78–87.
Serra D, Mera P, Malandrino MI, Mir JF, Herrero L. Mitochondrial fatty acid oxidation in obesity. Antioxid Redox Signal. 2013;19(3):269–84.
Manna P, Jain SK. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: causes and therapeutic strategies. Metab Syndr Relat Disord. 2015;13(10):423–44.
Toner E, Adalja A, Gronvall GK, Cicero A, Inglesby TV. Antimicrobial resistance is a global health emergency. Health Secur. 2015;13(3):153–5.
Cantón R, Akova M, Langfeld K, Torumkuney D. Relevance of the consensus principles for appropriate antibiotic prescribing in 2022. J Antimicrob Chemother. 2022;77:i2–9.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Pahwa R, Goyal A, Jialal I. Chronic inflammation. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
World Health Organization. World Obesity Day 2022—Accelerating action to stop obesity: WHO; 2022 [Retrieved July 16, 2022]. Available from: https://www.who.int/news/item/04-03-2022-world-obesity-day-2022-accelerating-action-to-stop-obesity.
Diabetes. World Health Organization. Diabetes Geneva: WHO; 2022 [Retrieved July 16, 2022]. Available from: https://www.who.int/health-topics/diabetes#tab=tab_1.
Pan S-Y, Litscher G, Gao S-H, Zhou S-F, Yu Z-L, Chen H-Q, et al. Historical perspective of traditional indigenous medical practices: the current renaissance and conservation of herbal resources. Evid Based Complement Altern Med. 2014;2014: 525340.
Batool S, Khera RA, Hanif MA, Ayub MA. Bay Leaf Medicinal Plants of South Asia. Amsterdam: Elsevier; 2020.
Derwich E, Benziane Z, Boukir A, Mohamed S, Abdellah B. Chemical composition and antibacterial activity of leaves essential oil of Laurus nobilis from Morocco. Aust j basic appl sci. 2009;3(4):3818–24.
El-Sawi S, Ibrahim M, Ali A. In vitro cytotoxic, antioxidant and antimicrobial activities of essential oil of leaves of Laurus nobilis L grown in Egypt and its chemical composition. Med Arom Plant Sci Biotechnol. 2009;3(1):16–23.
Fernandez-Andrade CM, da Rosa MF, Borges F, Iwanaga CC, DgA C, Martins CVB, et al. Chemical composition and antifungal activity of essential oil and fractions extracted from the leaves of Laurus nobilis L. cultivated in Southern Brazil. J Med Plants Res. 2016;10(48):865–71.
Ivanović J, Mišić D, Ristić M, Pešić O, Žižović I. Supercritical CO2 extract and essential oil of bay (Laurus nobilis L.): Chemical composition and antibacterial activity. J Serbian Chem Soc. 2010;75(3):395–404.
Caputo L, Nazzaro F, Souza LF, Aliberti L, De Martino L, Fratianni F, et al. Laurus nobilis: composition of essential oil and its biological activities. Molecules. 2017. https://doi.org/10.3390/molecules22060930.
Georgiev E, Stoyanova A. A guide for the specialist in aromatic industry. Bulgaria: Plovdiv; 2006.
Zolfaghari B, Samsam-Shariat SH, Ghannadi A. Chemical composition of volatile oils from the endocarp and hulls of Persian bay laurel fruit: A fragrant herb used in traditional Iranian medicine. J Rep Pharm Sci. 2013;2(31):1–4.
Jaradat N, Qneibi M, Hawash M, Al-Maharik N, Qadi M, Abualhasan MN, et al. Assessing Artemisia arborescens essential oil compositions, antimicrobial, cytotoxic, anti-inflammatory, and neuroprotective effects gathered from two geographic locations in Palestine. Ind Crops Prod. 2022;176: 114360.
Vinaixa M, Schymanski EL, Neumann S, Navarro M, Salek RM, Yanes O. Mass spectral databases for LC/MS-and GC/MS-based metabolomics: state of the field and future prospects. TrAC - Trends Anal Chem. 2016;78:23–35.
Wei X, Koo I, Kim S, Zhang X. Compound identification in GC-MS by simultaneously evaluating the mass spectrum and retention index. Analyst. 2014;139(10):2507–14.
Jaradat N, Al-Lahham S, Abualhasan MN, Bakri A, Zaide H, Hammad J, et al. Chemical constituents, antioxidant, cyclooxygenase inhibitor, and cytotoxic activities of Teucrium pruinosum boiss. Essential oil Biomed Res Int. 2018;2018:4034689.
Jaradat NA, Shawahna R, Hussein F, Al-Lahham S. Analysis of the antioxidant potential in aerial parts of Trigonella arabica and Trigonella berythea grown widely in Palestine: a comparative study. Eur J Integr Med. 2016;8(5):623–30.
Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: A review. J Pharm Anal. 2016;6(2):71–9.
Nyambe-Silavwe H, Villa-Rodriguez JA, Ifie I, Holmes M, Aydin E, Jensen JM, et al. Inhibition of human α-amylase by dietary polyphenols. J Funct Foods. 2015;19:723–32.
Ademiluyi AO, Oboh G. Soybean phenolic-rich extracts inhibit key-enzymes linked to type 2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin I converting enzyme) in vitro. Exp Toxicol Pathol. 2013;65(3):305–9.
Jaradat NA, Al-lahham S, Zaid AN, Hussein F, Issa L, Abualhasan MN, et al. Carlina curetum plant phytoconstituents, enzymes inhibitory and cytotoxic activity on cervical epithelial carcinoma and colon cancer cell lines. Eur J Integr Med. 2019;30:93–100.
Jaradat N, Al-Lahham S, Abualhasan MN, Bakri A, Zaide H, Hammad J, et al. Chemical constituents, antioxidant, cyclooxygenase inhibitor, and cytotoxic activities of Teucrium pruinosum boiss essential oil. Biomed Res Int. 2018. https://doi.org/10.1155/2018/4034689.
Hawash M, Eid AM, Jaradat N, Abualhasan M, Amer J, Zaid AN, et al. Synthesis and biological evaluation of benzodioxole derivatives as potential anticancer and antioxidant agents. Heterocycl Comm. 2020;26(1):157–67.
Ayerbe N, Routier S, Gillaizeau I, Maiereanu C, Caignard D-H, Pierré A, et al. Synthesis and biological evaluation of novel benzodioxinocarbazoles (BDCZs) as potential anticancer agents. Bioorg Med Chem Lett. 2010;20(15):4670–4.
Caputo L, Nazzaro F, Souza LF, Aliberti L, De Martino L, Fratianni F, et al. Laurus nobilis: Composition of essential oil and its biological activities. Molecules. 2017;22(6):930.
Manna P, Jain SK. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: causes and therapeutic strategies. Metab Syndr Relat Diso. 2015;13(10):423–44.
Jaradat NA, Al-Masri M, Zaid AN, Hussein F, Al-Rimawi F, Mokh AA, et al. Phytochemical, antimicrobial and antioxidant preliminary screening of a traditional Palestinian medicinal plant, Ononis pubescens L. Eur J Integr Med. 2017;14:46–51.
Niki E, Noguchi N. Antioxidant action of vitamin E in vivo as assessed from its reaction products with multiple biological oxidants. Free Radic Res. 2021;55(4):352–63.
Kurihara H, Ando J, Hatano M, Kawabata J. Sulfoquinovosyldiacylglycerol as an α-glucosidase inhibitor. Bioorganic Med Chem Lett. 1995;5(12):1241–4.
Jacobson TA, Miller M, Schaefer EJ. Hypertriglyceridemia and cardiovascular risk reduction. Clin Ther. 2007;29(5):763–77.
Toma A, Makonnen E, Mekonnen Y, Debella A, Addisakwattana S. Intestinal α-glucosidase and some pancreatic enzymes inhibitory effect of hydroalcholic extract of Moringa stenopetala leaves. BMC Complement Altern Med. 2014;14(1):1–5.
Peixoto LR, Rosalen PL, Ferreira GLS, Freires IA, de Carvalho FG, Castellano LR, et al. Antifungal activity, mode of action and anti-biofilm effects of Laurus nobilis Linnaeus essential oil against Candida spp. Arch Oral Biol. 2017;73:179–85.
Fidan H, Stefanova G, Kostova I, Stankov S, Damyanova S, Stoyanova A, et al. Chemical composition and antimicrobial activity of Laurus nobilis L essential oils from Bulgaria. Molecules. 2019;24(4):804.
Ramos C, Teixeira B, Batista I, Matos O, Serrano C, Neng N, et al. Antioxidant and antibacterial activity of essential oil and extracts of bay laurel Laurus nobilis Linnaeus (Lauraceae) from Portugal. Nat Prod Res. 2012;26(6):518–29.
Alhazmi HA, Najmi A, Javed SA, Sultana S, Al Bratty M, Makeen HA, et al. Medicinal plants and isolated molecules demonstrating immunomodulation activity as potential alternative therapies for viral diseases including COVID-19. Front Immunol. 2021. https://doi.org/10.3389/fimmu.2021.637553.
Uddin MJ, Rao PN, Knaus EE. Design and synthesis of novel celecoxib analogues as selective cyclooxygenase-2 (COX-2) inhibitors: replacement of the sulfonamide pharmacophore by a sulfonylazide bioisostere. Bioorg Med Chem. 2003;11(23):5273–80.
Assali M, Abualhasan M, Zohud N, Ghazal N. RP-HPLC method development and validation of synthesized codrug in combination with indomethacin, paracetamol, and famotidine. Int J Anal Chem. 2020. https://doi.org/10.1155/2020/1894907.
Medeiros-Fonseca B, Mestre VF, Colaço B, Pires MJ, Martins T, Gil da Costa RM, et al. Laurus nobilis (laurel) aqueous leaf extract’s toxicological and anti-tumor activities in HPV16-transgenic mice. Food Funct. 2018;9(8):4419–28.
Al-Kalaldeh JZ, Abu-Dahab R, Afifi FU. Volatile oil composition and antiproliferative activity of Laurus nobilis, Origanum syriacum, Origanum vulgare, and Salvia triloba against human breast adenocarcinoma cells. Nutr Res. 2010;30(4):271–8.
Ek F, Blom K, Selvin T, Rudfeldt J, Andersson C, Senkowski W, et al. Sorafenib and nitazoxanide disrupt mitochondrial function and inhibit regrowth capacity in three-dimensional models of hepatocellular and colorectal carcinoma. Sci Rep. 2022;12(1):8943.
Parashar D, Geethadevi A, Mittal S, McAlarnen LA, George J, Kadamberi IP, et al. Patient-derived ovarian cancer spheroids rely on PI3K-AKT signaling addiction for cancer stemness and chemoresistance. Cancers. 2022. https://doi.org/10.3390/cancers14102443.
Ciantar M. Effect of multiple treatment regimes of cytotoxic extracts and drugs on tumour cell spheroids (Bachelor dissertation). 2020.
Acknowledgements
The author would like to acknowledge An-Najah National University.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, NJ methodology, NJ software, MH, MAA, MQ, and SA. validation, MH, MAA, MQ, SS, NA and SA formal analysis, NA, NJ, MH, MAA, MQ and SA investigation, NJ, MAA, SA, AJ, HH, SS, SAl, AZ, AM, FH, LI resources, MA and NJ data curation, MH, MAA, MQ, SS, NA and SA writing—original draft preparation, NJ writing—review and editing, NJ MH MQ and SA visualization, NJ supervision, NJ project administration, NJ funding acquisition, NJ. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Figure S1: GC–MS chromatogram of Laurus nobilis aromatic oil.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jaradat, N., Abualhasan, M., Hawash, M. et al. Chromatography analysis, in light of vitro antioxidant, antidiabetic, antiobesity, anti-inflammatory, antimicrobial, anticancer, and three-dimensional cancer spheroids’ formation blocking activities of Laurus nobilis aromatic oil from Palestine. Chem. Biol. Technol. Agric. 10, 25 (2023). https://doi.org/10.1186/s40538-023-00396-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40538-023-00396-6