Abstract
Background
Essential oil of Rosa × damascena Herrm. is one of the most valuable and important raw materials for the flavor and fragrance industry. The cultivation of this plant has ancient origins, and Kashan was one of the first mountainous regions of Iran dealing with the cultivation of R. × damascena. In this study, both chemical composition and antimicrobial activity of different rose essential oils obtained from five mountainous areas of Kashan region (Maragh, Qamsar, Sadeh, Javinan, and Kamoo) has been investigated along with the influence of the environmental conditions on these properties.
Results
Results showed that yield and chemical composition of essential oils obtained from Rosa × damascena were significantly affected by the collection area. In particular, the yield of oils varied from ~0.08 to ~0.132% and citronellol (36.70-9.18%), geraniol (12.82-0.47%), nonadecane (22.73-10.36%), heneicosane (31.7-11.43%), and 1-nonadecene (6.03-3.93%) have been detected as main compounds in all the plants collected, but at different concentrations depending on the collection area. The best fragrance and the highest yield were found in the oil from Kamoo area. Similarly to the chemical composition, the antimicrobial activity of the essential oils was affected by their origin, and essential oil obtained from plants collected from Kamoo area disclosed the highest antibacterial and antifungal efficacy. Its inhibition halos were 17.33±0.58 mm against Aspergillus brasiliensis, 15.67±0.58 mm against Staphylococcus aureus, and 12.33±0. 58 mm against Streptococcus pyogenes. Essential oils of R. damascena were also effective against Gram-negative Pseudomonas aeruginosa and they had a MIC value of 62.50 μg/mL irrespective of the collection area (except the oil from Javinan area). On the contrary, the highest antifungal power against Candida albicans yeast was reached using the essential oil obtained from plants collected in Javinan region (MIC and MBC ~62.50 μg/mL).
Conclusions
Overall results underline the influence of environmental conditions of the different areas of Kashan region, on the chemical composition of and antimicrobial activity of the essential oils of Rosa × damascena. In addition, results disclosed that Kamoo seemed to be the most suitable area for the competitive cultivation of R. × damascena to the intensive production of aromatic flower oil and natural antimicrobial essential oils.
Similar content being viewed by others
Background
The genus Rosa is made up of over 365 taxa and 84 hybrids [67], but only a few of them such as Rosa × centifolia L., Rosa gallica L., and Rosa × damascena Herrm. can be used at industrial level due to their pleasant flavor and beneficial properties [36]. Among all, R. × damascena is one of the most important and cultivated species of Rosaceae family [47]. It is wildly known as Damask rose or Persian rose [35] while in Persia is called “Gol Mohammadi". The essential oil of R. damascena is widely used in perfumes, cosmetics, enriched foods, and pharmaceutical products (e.g., [3, 7, 48]. Due to the low essential oil content of R. × damascena and the lack of natural and synthetic alternatives, rose essential oil is one of the most expensive on the market [10]. The area of cultivation and the climate conditions are key parameters, which can positively affect the yield and chemical content of essential oils [5, 34, 41, 61, 72].
Rosa × damascena is largely cultivated because of its aromatic and beneficial properties [55]. The main producers of rose essential oil are Bulgaria, Turkey, Iran and India. Iran has been considered as one of the first countries in which R. × damascena has been cultivated and exploited at commercial level [55]. These plants are adapted to the local environmental conditions, thus producing essential oil different in quantity and quality, as a function of the genetic content as well as environmental factors. Therefore, the evaluation of genotypes from different areas of Iran should permits to select the varieties of R. × damascena more suitable for commercial cultivation due they rich and diverse content of essential oil. Just few studies have been performed to evaluate the content of essential oil of R. × damascena, its chemical composition and activity as a function of the environmental conditions. To this purpose, in the previous study, R. × damascena has been collected from different areas of the Kashan region of Iran [34, 42, 60]. On the contrary, different studies analyzed the chemical composition of rose oil and more than 400 volatile compounds have been identified in the different varieties [29]. Based on their properties they can be grouped into five major groups: hydrocarbons, alcohols, esters, aromatic ethers, and others (including aldehydes, oxides and norisoprenes) [32]. Citronellol, nonadecane, geraniol, heneicosane, nerol, 1-nonadecene, and trans-geraniol are the most important bioactives found in the essential oils of R. × damascena [27, 40, 57, 66]. Citronellol, nonadecane, and geraniol, have been detected as the major components of rose essential oils collected from the central region of Iran [56]. In other studies 1-nonadecene, hexatriacontane, n-tricosane and geraniol have been reported as the main components of the essential oil of the R. × damascena collected from northern parts of Iran [70] and nonadecane, heneicosane, docosane, citronellol, and 9-nonadecene were reported to be the most representative bioactives of the essential oil of R. × damascena collected from the southern of Iran [42]. Regarding the essential oil of R. × damascena collected from Kashan region, β-citronellol (14.88–47.43%), nonadecane (10.5–40.5%), geraniol (5.5–18%), and heneicosane (7–14%) were the main components [34].
According to their rich composition, some health promoting properties of rose oil such as antiviral [23, 37], antioxidant [1, 50, 63], anti-cancer [52], laxative [22], anti-inflammatory, and antiseptic have been previously reported [15, 43, 46]. Moreover, strong antimicrobial activity has been reported against different bacterial and fungal strains such as Propionibacterium acnes, Escherichia coli, Proteus vulgaris, Klebsiella pneumoniae, Candida albicans, Enterococcus faecalis, Staphylococcus aureus, among others [6, 9, 14, 62, 68, 74].
In the present study, the essential of R. × damascena has been prepared by plants collected from different areas of Kashan region, considering that it is one of the largest areas of rose essential oil production in Iran and in the world. The main constituents of rose essential oil, were identified and compared and their antimicrobial activity against 11 bacterial strains have been deeper investigated.
Materials and methods
Selected sites
To select the sampling regions, the mountainous areas of Kashan region where R. × damascena is usually cultivated were firstly identified by means of field surveys. Already existing farms were selected, where the plants were cultivated with the same conditions of irrigation (drip irrigation), used fertilizers and care factors. To minimize the local factors such as water quality, pruning methods, physical and chemical characteristics of soil and harvesting period, the areas with similar geographic characteristics have been selected (Table 1). They were Maragh, Qamsar, Sadeh, Javinan, and Kamoo, with a distance of about 40 km from each other.
Plant material
In the collection areas, plants having the same age were selected. The collection of R. × damascena has been performed at six o'clock in the morning on May 2019, which is the blooming period in each selected region. Three parts of the plants were randomly selected as collecting point (100 plants for each area). After collection the samples were transferred to the laboratory and kept at 4 °C for one hour. One sample of the whole plant from each region was also collected and pressed. The plant was identified and recorded in the University of Kashan herbarium with a code number.
Isolation of the essential oil
To extract the essential oil, three hundred grams of fresh flowers for each replicate of each area of Kashan region were weighed and used to extract the oil by water distillation using a Clevenger apparatus for 4 h. The essential oil was treated with sodium sulfate and stored in dark bottles at 4 °C until further use. Then the yield of essential oil of three replicates of each region was reported as mean ± standard deviation.
GC–MS analysis
The most abundant bioactives contained in the essential oils have been separated and identified by GC–MS using a chromatograph (Model 6890 Chromatography) coupled with an Agilent Mass Spectrometer (Model N-5973). A capillary column (HP-5MS) with 5% methylphenylsiloxane static phase (length 30 m, internal diameter 0.25 mm, layer static thickness 0.25 μm) and ionization energy of 70 eV was used. The temperature for the analyses was first set at 60 °C and then it was increased at a rate of 3 °C up to 246 °C. The injector and detector temperatures were maintained at 250 °C, the volume of the injected sample was 1 µl and the helium carrier gas was maintained at a flow rate of 1.5 ml/min. The identification of chemical components was based on the analysis of the chromatograms obtained for each oil measuring the retention indices (RI). Data were compared with that of standards of n-alkane mixtures (C8–C20) and mass spectral data reported in computer library (Wiley-14 and NIST-14 Mass Spectral Library), and in the literature [2].
Antimicrobial assays
Microbial strains
Eleven microorganisms, provided by the Iranian Research Organization for Science and Technology (IROST), have been used to evaluate the antimicrobial activity of the essential oils. Four Gram-positive bacteria such as Staphylococcus epidermidis (CIP 81.55), Staphylococcus aureus (ATCC 29,737), Streptococcus pyogenes (ATCC 19,615), and Bacillus subtilis (ATCC 6633) and five Gram-negative bacteria including Klebsiella pneumoniae (ATCC 10,031), Shigella dysenteriae (PTCC 1188), Pseudomonas aeruginosa (ATCC 27,853), Salmonella paratyphi-A serotype (ATCC 5702), and Escherichia coli (ATCC 10,536) were selected. Two fungal species such as Aspergillus brasiliensis (ATCC 16,404) and Candida albicans (ATCC 10,231) were also selected for the evaluation of the antifungal activity of the obtained essential oils. Bacterial strains were cultured overnight at 37 °C in agar nutrient (Merck, Germany) while yeasts were cultured at 30 °C for 48 h in Sabouraud dextrose agar (Merck, Germany).
Agar well diffusion method
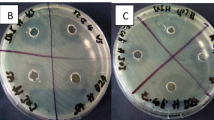
The procedure was performed according to Clinical and Laboratory Standards Institute method (CLSI, 2012). Plates (6 mm diameter and 4 mm thickness) containing the culture medium (Müller Hinton agar, Merck, Germany) were used for bacteria, while those containing sub-dextrose agar medium were used for fungi growth. Microbial suspensions (100 μl) were cultured with turbidity equivalent to half-McFarland under uniform conditions. The essential oil was dissolved in dimethyl-sulfoxide to reach a concentration of 300 μg/mL, then 10 μL of the diluted essential oil has been added to each plate. Plates were incubated at 37 °C for 24 h for bacterial strains and for 48 and 72 h at 30 °C for fungal strains and their antimicrobial activity was evaluated by measuring the diameter of the inhibition halo (in millimeters) according to the antibiogram rules. To evaluate the repeatability of the results, three replicates for each essential oil and each strain were performed. Dimethyl-sulfoxide was used as negative control. Gentamicin (10 µg/disk), and rifampicin (5 µg/disk) for bacteria and nystatin (100 I.U.) for yeast were used as positive controls under the same conditions of tested oils.
Determination of the minimum inhibitory concentration (MIC)
The minimum concentration able to inhibit the growth of bacteria (absence of turbidity) was calculated by means of the microdilution broth method. Essential oils (2000 μg/ml) were dissolved in a mixture of tryptic soy broth medium (Merck, Germany) and DMSO and then were diluted by using the same mixture to reach different concentrations (> 1000, 1000, 500, 250, 125, 62.50, 31.25 and < 15.63 μg/ml).
Sterile 96-well plates were filled with 95 µl of culture medium (Sabouraud dextrose agar containing 50% (v/v) of Tween 20), 5 µl of bacterial or fungal suspension with 0.5 McFarland dilution, and 100 µl of the essential oils at different concentrations. Plates were then incubated at 37 °C for 24 h for bacterial strains and for 48 h and 72 h at 30 °C for fungi. Untreated bacteria and yeasts were used as negative control, while gentamicin, rifampin (antibiotics) and nystatin (antifungal) treated microorganisms were selected as positive controls. The MIC was determined by means of the improvement of the opacity of the dispersion or its change in color. The agar dilution assay was used to determine the MICs for the fungal strains based on the protocol introduced by of Gul et al. (2002). 100 µl of essential oil at different concentrations (2000, 1000, 500, 250, 125, 62.5, 31.25 and 15.63 μg/mL) were added. Nystatin powder was used as the positive control, and the negative control was the plate with Sabouraud dextrose agar containing 50% (v/v) Tween 20 without any essential oil. The culture media were spot inoculated with 4 ml of spores (104 spores/mL). The inoculated plates were incubated at 30 °C for 72 h, the test was performed in triplicate for each essential oil, and the minimal concentration of the essential oil that inhibited the growth of the fungi was reported as the MIC.
Determination of minimal bactericidal and fungicidal concentrations (MBC and MFC)
To determine the minimum concentration able to kill the bacteria and fungi, the same microdilution method described in Sect. 2.5.3 was used. After 24 h of incubation of bacteria or fungi with oils at different concentrations, 5 µl of the content of each well were inoculated with nutrient agar (Merck, Germany) medium and incubated at 37 °C for 24 h for bacterial strains and for 48 and 72 h at 30 °C for fungal strains. After incubation, the colony-forming units were enumerated. The MBC and MFC were the lowest concentrations able to effectively reduce the growth of microorganisms (99.5%).
Statistical analysis
The statistical analysis was performed using SPSS 22 software. First, the normality of the statistical variables was investigated using a Kolmogorov–Smirnov test, and after ensuring the normality of the data, the variance of the data (essential oil and antimicrobial activity) was analyzed using F-test and a comparison of the means was performed using Duncan test with a probability level of 1% error.
Results
Essential oil yield
The yield of essential oil obtained from R. × damascena changed significantly depending on the area of collection (P ≤ 0.01) (Table 2). Results obtained by comparing the oil yield obtained from the different R. × damascena underlined that the highest was obtained using the plants from Kamoo area (0.1340 ± 0.0010%) and the lowest using the plants from Maragh area (0.0120 ± 0.0010%) (Table 3).
Essential oil chemical compositions
72 different compounds have been identified on the rose essential oils collected from the selected sites of Kashan region (Figs. 1, 2, 3, 4, 5). The highest number of compounds were found in the essential oil of R. × damascena collected in Qamsar (46 compounds) followed by the oil from Maragh (43 compounds), Sedeh (38 compounds), Javinan (34 compounds), and Kamoo (33 compounds). The relative composition of essential oil of R. × damascena collected in these five sites were 97.57, 100, 100, 99.87 and 99.06%, respectively (Table 4).
The main components of the five essential oil obtained from R. × damascena were nonterpenoids (others) and oxygenated monoterpenes, while the components found at lower amount were monoterpenes hydrocarbons and oxygenated sesquiterpenes irrespective of the area of plant collection.
The analysis of variance showed that there was a significant difference between the average amount of components obtained from the essential oil of R. × damascena collected from the different sites (P ≤ 0.01) (Table 4). As expected the amount of citronellol and geraniol was significantly affected by both the site of collection and the altitude above the sea level (Table 2). Regarding the highest amount of citronellol (36.70%) was detected in the oil of plants from Kamoo site (Fig. 6).
Heneicosane is an alkane compound contained in R. × damascena essential oils that was found in all the samples. Based on the results of analysis of variance, the grown area significantly affected (P ≤ 0.01) the amount of heneicosane and nonadecane produced by the different plants and found in the oils (Table 4). The highest and lowest percentages of these compounds were found in the oils of plants from Sedeh (31.7%) and Maragh (11.43%) sites (Fig. 7). In all the tested samples, irrespective of the area of plant collection, nonadecane was one of the most abundant compounds in R. × damascena essential oils. The highest amount was found in the oil of plants from Sedeh (22.73%) and the lowest in that of plants from Kamoo (10.36%) (Fig. 8), due to the different environmental factors. These environmental factors increase the synthesis of citronellol and geraniol, and decrease the production of heneicosane and nonadecane.
Antimicrobial activity
The antimicrobial activity of essential oils isolated from R. × damascena flowers collected from different sites of Kashan region was evaluated by measuring the inhibition halos, the MCI and the MBC (Tables 6, 7, 8). Analysis of variance confirmed difference statistically significant (P ≤ 0.01) between the inhibition halos of the five tested oils (Tables 5, Table 6). The MIC and MFC values of essential oil against A. brasiliensis were ranged from 500 and 1000 μg/mL, and were not statistically significant (Tables 7 and 8), indicating that the minimum concentration of essential oil needed to control and kill A. brasiliensis is very high and these oils are not suitable to inhibit this fungus. Similarly, any inhibition halo has been detected treating Candida albicans yeast with the rosa oil, irrespective of the area of plant collection. Differently, the inhibition halo provided by R. × damascena essential oil collected from Bulgaria, against C. albicans was ~ 20 mm. MIC and MFC values of R. × damascena essential oils varied from 62.50 to > 1000 μg/mL, and the highest inhibitory and antifungal values against C. albicans were obtained using the oil of rosa from Javinan area (MIC = 62.50 μg/mL), which was significantly high if compared with the MIC obtained with nystatin (~ 125 μg/mL).
Among Gram-positive bacteria, the largest inhibition halo was obtained against Staphylococcus aureus treated with essential oil of rosae from Kamoo area (15.67 mm), followed by the oil from Javinan area (14.67 ± 0.58 mm), and that from Qamsar area (10.67 mm). The antimicrobial power of essential oil was significantly lower than that observed by treating the same bacterial strain with rifampin (~ 21 mm) and gentamicin (~ 27 mm). However, the antimicrobial activity of essential oils may be connected to neral, geranial and phenylethyl alcohol content, which is higher in the essential oils obtained from these three regions. MIC value obtained treating S. aureus with R. × damascena essential oil was 500 μg/mL and the MBC value was 1000 μg/mL, irrespective of the collection area.
The activity of R. × damascena essential oil against Bacillus subtilis, a Gram-positive bacteria, was observed only for plants collected from Kamoo region (IZ = 10.67 ± 0. 58 mm) probably because of the higher content of citronellol. Although the MIC value obtained with R. × damascena essential oil was 250 μg/mL, its MBC value increased up to more than 1000 μg/mL irrespective of the area of collection (except Qamsar). This indicates that the killing power of R. × damascena essential oil against B. subtilis is much lower in comparison with its inhibitory power.
The only inhibition halo against S. epidermidis belonged to the R. × damascena essential oil collected from Qamsar region (9.00 ± 0.00 mm), probably due to the exclusive presence of the phytol compound. The highest effect of R. × damascena essential oil against S. epidermidis was reported in the essential oil obtained by using R. × damascena collected from Bulgaria (IH = 21.5 ± 1.0 mm and MIC = 256 μg/mL).
Among Gram-negative bacteria, inhibition halo has been observed only against Klebsiella pneumoniae. The highest inhibition halo has been obtained by treating K. pneumoniae with rose essential oil belonging to Kamoo (10.67 ± 0.58 mm) and Javinan (9.33 ± 0.58 mm), which is significantly similar to that obtained by using rifampin (~ 8 mm), that can be connected with the high content in oxygenated monoterpenes detected in the essential oils obtained from the two regions. MIC values were 250 μg/mL and MBC values ranged from 500 to over 1000 μg/mL, irrespective of the area of collection.
Inhibition halo against S. pyogenes bacteria observed by using essential oils obtained from Rosa × damascena collected from three of the selected areas, such as Kamoo (12.33 ± 0.58 mm), Javinan (12.00 ± 0.00 mm), and Qamsar (9.00 ± 0.00 mm) suggested a good activity of these essential oils. MIC values ranged from < 15.63 to 250 μg/mL. The lowest values (< 15.63 μg/mL) were obtained by using Rosa × damascena essential oil collected from Sedeh and Javinan, which showed the same MBC as well.
The MIC value detected by treating a Gram-negative bacteria Escherichia coli with Rosa × damascena essential oil collected from different areas of Kashan region, varied from 125 to 500. MIC value against Shigella dysenteriae and Salmonella paratyphi-A also ranged from 125 to 500 μg/mL, but any study on the effect of Rosa × damascena essential oil on these bacteria have been reported so far. Given that, the present study proved that despite the similarity of soil and cultivation condition, the amount, quality and biological properties of Rosa × damascena essential oil are significantly affected by environmental factors such as altitude above sea level and temperature.
Discussion
According to Tabaei-Aghdaei et al. [64] and Thakur et al. [66] results, which analyzed the effect of the environment on the yield of the plant collected in the central part of Iran and in western Himalayas, respectively. Similarly, the amount of secondary metabolites in the plants collected from different sites has been investigated, confirming the role of the environment in affecting the amount and accumulation of secondary metabolites, as also reported by others [16, 19, 73]. Indeed, the area in which the plant is cultivated may affect the process by which the bioactive are synthesized by means of temperature and humidity changes [31]. The Kamoo region is the highest (altitude of 2460 m above sea level), which can be considered the main reason for the significant increase of the yield, as radiation, especially UV-B, are generally improved at high altitudes [44, 44] for Tagetes minuta L. and Yavari and Shahgolzari [71] for Stachys inflata Benth. obtained similar results. Moreover, our results underlined that temperature gradient, due to altitude changes, is among the most important factors affecting plant life and production of bioactives, so that as altitude increase or decrease, factors such as temperature, relative humidity, wind speed, amount of available water and type and amount of radiations also change. The characteristic of plants may also be affected by changes in both ecosystem and habitat which can undergo many ecophysiological reactions and modification [65]. Increasing the altitude from the sea level intensifies the exposition of plants to light, which in turn led to the reduction of the plant’ height and an increases of the number of branches. Moreover, high intensity of light causes a general growth of branches and increase the number and thickening of lateral branches, full color and gloss of leaves, the amount of chlorophyll produced, the photosynthesis activity and the yield of the dry weight, while it decreases the stomatal respiration.
Razaee et al. [53] detected 15 compounds in the essential oil of R. × damascena collected from Qamsar area which are not in agreement with the present results. The results indicated that despite the same cultivation conditions, the number of compounds decreased as the altitude above the sea level also increased, probably because of the increased radiation, especially UV-B at high altitudes [26].
These results are in agreement with those obtained from Yassa et al. [70]. The highest percentage of oxygenated monoterpenes belonged to Kamoo (51.1%) and nonterpenoids to Sedeh (70.02%), probably because as previously reported the changes in altitude may affect the biosynthesis of terpenoids [58]. Differences in volatiles compounds extracted from plants cultivated in different sites have been detected confirming the influence of the environmental conditions on secondary metabolites production. A similar trend was observed by [39], which found that the amount of monoterpene compounds found in the essential oil of Helichrysum italicum (Roth) G.Don increased as the altitude also increased.
Similarly, differences in the amounts of essential oil constituents in Zanthoxylum armatum DC. populations were attributed to the variations in geographical location [13, 13]. The role of habitat as an effective factor affecting the accumulation of secondary metabolites has been confirmed, as the production of secondary metabolites appears to be influenced by the environmental conditions under which the plant has to grow. Therefore, identifying suitable conditions for the production of specific metabolites of plants can be an effective approach capable of improving their production [12]. Previous studies suggested that the main constituents responsible for the quality of the essential oil extracted from R. × damascena are citronellol and geraniol [18].
Similar results were obtained by [24] for Teucrium polium L. and by [33] for Salvia fruticosa Mill. confirming the influence of the area of collection on the chemical composition of essential oils.
The highest amount of geraniol was observed in Qamsar (12.82%) (Fig. 9), which is in agreement with the findings of Yassa et al. [70] found in northern Iran (15.5%) and inconsistent with the findings of [34]. The highest amount of this compound has been detected in India (30.2%), Turkey (22.19%) and Bulgaria (16.96%) [8, 68, 69].
Previous studies showed that the highest amount of citronellol was in western Himalayas (42.0%), and India (35.3%) [66, 66] and, confirming the importance of the geographic localization, harvesting time, plant and seasonal growth stages, and environmental factors affecting the chemical composition of essential oils probably because of their ability to induce genetic variation. Kamoo region has the highest altitude above sea level (2460 m), which may be the reason why the amount of citronellol is the highest in the essential oil. The study of Rhododendron aureum Georgi collected from different region of Russia characterized by different altitude above the sea level showed that the amount of the main component of the essential oils at the highest altitude was higher in comparison with the other regions [17, 49]. Therefore, it can be concluded that at higher altitudes, due to the reduced temperature and increased exposure to UV radiation, the synthesis of some compounds increases. Clearly, some exception can be also detected as Maragh region has the lowest altitude above sea level (1750 m), but it ranks second in terms of citronellol content. Despite the similarity of climatic conditions and the choice of similar cultivation conditions, it seems that additional ecological conditions other than the altitude may positively affect the production of these compounds. Overall results obtained comparing the average amount of citronellol and geraniol detected in essential oils obtained from R. × damascena showed that the lowest amount of both compounds belonged to Sedeh region (Figs. 5, 6). It seems that the regions with the 2000 m altitude above the sea level is not a good condition for plants to synthesize these two compounds. Changes in the altitude of the growing region can lead to changes in the production of active ingredients, so that each plant species has a desirable altitude, which in turn led the production of higher amount of the main bioactives. Therefore, according to the findings of this study, the optimal altitude for synthesizing both citronellol and geraniol is 2400 and 2000 m above sea level, respectively.
In the study by [42], heneicosane and nonadecane has been reported as the dominant constituents of R. × damascena essential oils (32.38%) in southern of Iran, which is consistent with the results obtained in the present study. Heneicosane was found in lower amounts in India (7.9%), Turkey (8.90%) and western Himalayas (10.6%) [11, 69, 66]. According to the study of [34], the amount of heneicosane and nonadecane was 33.89% in Qamsar, which is higher than that found in the present study. The reduction of the sea level altitude in the Maragh region appears to have had a significant effect on the reduction of the amount of nonterpenoids.
Together with citronellol and geraniol, neral is another important bioactive contained in the R. × damascena essential oil, responsible for the typical aroma of this plant. Neral has been found only in the Kamoo region (1.37%), which is in agreement with the findings of Yassa et al. [70]. Similar results in terms of the presence of some compounds only at the highest altitude have been reported by Rostaefar et al. [54] for Juniperus communis L. ssp. hemisphaerica (J.Presl and C.Presl) Nyman. The highest amount of neral in the R. × damascena essential oil has been found in India (9.6%) [69].
An aromatic antibiotic, phenylethyl alcohol, is also contained in the R. damascena essential oil and the highest amount has been found in the plants cultivated in Bulgaria (27.75%) [45]. In the present study only a small amount of phenylethyl alcohol was extracted from R. × damascena cultivated in Qamsar (0.70%) and Javinan (0.78%), which is in agreement with the results of Yassa et al. [70] and Loghmani-Khouzani et al. [34]. It appears that the environment in the areas of Iran selected have not the needed conditions for production/synthesis of this compound due to their climatic and ecological factors.
A similar trend was observed by [20] for Tagetes minuta L. The highest inhibition zone was for the R. × damascena essential oil collected from Kamoo region (17.33 ± 0.58 mm), which was significantly high if compared with the inhibition halo obtained treating the same yeast with nystatin (~ 30 mm). The highest antimicrobial activity of R. × damascena essential oil, irrespective of the area of collection, was against Aspergillus brasiliensis. The different antimicrobial activity obtained by testing the R. × damascena essential oils obtained from different areas of Iran may be connected with their different chemical composition and their complex chemical profiles [20, 21]. Citronellol, geraniol, neral and trans-rose oxide appear to be important contributors to the antimicrobial activity of the essential oils [38, 53, 59]. The antifungal activity of R. × damascena essential oil has not been studied in any area of Iran so far, thus this property has been investigated for the first time in this work in the different areas selected of Kashan. With the exception of Qamsar, the antifungal activity in the other selected regions was low as the inhibition halos were in the range of 10–14 mm, probably because of their chemical composition [51].
Jirovetz et al. [28] found a MIC value (600 μg/mL) of Bulgarian R. × damascena essential oil similar to those detected for the essential oil from Maragh and Qamsar, probably because of the predominance of some alkanes such as 1-nonadecene. Ali et al. [4] obtained similar results for Teucrium yemense Deflers characterized by high amount of γ-selinene, an effective antimicrobial compound against some microorganisms.
Indeed, previous studies have reported the antibacterial effect against S. epidermidis of phytol [25]. The MIC values varied from 250 to 500 μg/mL and the lowest belonged to Sedeh and Javinan regions. Results reported by Shohayeb et al. [62] showed a higher MIC (250 μg/mL) and MBC (500 μg/mL) values, which were significantly lower than those obtained by treating the S. pyogenes with R. × damascena essential oil obtained from different regions of Kashan. This antibacterial effect against S. pyogenes is probably due to the high amount of alkanes, such as heneicosane, eicosane, nonadecane, and 1-nonadecene, which characterize the R. × damascena essential oil of Sedeh and Javinan regions [30].
Conclusion
Rosa × damascena essential oil obtained by using the flowers of plants collected from different sites of Kashan regions varied significantly in terms of yield and chemical composition. Indeed, essential oil of Kamoo, which was located at the highest altitude of Kashan region, oxygenated monoterpenes (citronellol and geraniol) were the most abundant compounds capable of increasing the quality of the essential oil. Nonadecane, heneicosane, and 1-nonadecene were the main constituents of R. × damascena essential oil belonging from Sedeh and Javinan. Clearly, differences among essential oils are also associated with different biological and antimicrobial activity. Indeed, R. × damascena essential oil from Kamoo had the highest effect against A. brasiliensis, S. aureus, and K. pneumoniae, that from Javinan was effective against C. albicans yeast. In the light of overall results, the best region for the cultivation of R. × damascena capable of producing/synthesizing essential oil in high amount and with a good quality is Kamoo, which besides being used in cosmetic, aromatic, and food industries, can be considered a potential natural antimicrobial substance.
Availability of data and materials
Not applicable.
References
Achuthan CR, Babu BH, Padikkala J. Antioxidant and hepatoprotective effects of Rosa damascena. Pharm Biol. 2003;41:357–61.
Adams RP. Identification of Essential Oil Components by Gas chromatography/mass Spectroscopy. Illinois: Allured Publishing Corporation; 2007.
Ahmadi, K., 2005. Situation of Rosa damascena Mill. in Iran and the world. Ministry of Agriculture. Department of Horticulture, Tehran.
Ali N, Chhetri B, Dosoky N, Shari K, Al-Fahad A, Wessjohann L, Setzer W. Antimicrobial, antioxidant, and cytotoxic activities of Ocimum forskolei and Teucrium yemense (Lamiaceae) essential oils. Medicines. 2017;17:1–14.
Ames GR, Mathews WSA. The distillation of essential oils. Trop Sci. 1968;10:136–48.
Ardogan BC, Baydar H, Kaya S, Demirci M, Ozbasar D, Mum UE. Antimicrobial activity and chemical composition of some essential oils. Arch Pharmacal Res. 2002;25:860–4.
Babaei, A., 2007. Genetic variation analysis of different populations of Rosa damascena Mill. in Iran, using morphological and molecular markers. Ph.D. thesis. Horticultural Science of Tarbiat Modaress University.
Babu KGD, Kaul KV. Variation in essential oil composition of rose scented geranium (Pelargonium sp.). Flavour Frag J. 2005;20:222–31.
Basim E, Basim H. Antibacterial activity of Rosa damascena essential oil. Fitoterapia. 2003;74:394–6.
Baydar H, Baydar NG. The effects of harvest date, fermentation duration and Tween 20 treatment on essential oil content and composition of industrial oil rose (Rosa damascena Mill.). Ind Crop Prod. 2005;21:251–5.
Bayrak A, Akgul A. Volatile oil composition of Turkish rose (Rosa damascena). J Sci Food Agric. 1994;4:441–8.
Becerro MA, Paul VJ. Effects of depth and light on secondary metabolites production and cyanobacterial symbionts of the sponge Dysidea granulosa. Marin Eco Progress Series. 2004;280:115–28.
Bhatt V, Sharma S, Kumar N, Sharma U, Singh B. Chemical composition of essential oil among seven populations of Zanthoxylum armatum from Himachal Pradesh: chemotypic and seasonal variation. Nat Prod Commun. 2017;12:1643–6.
Boskabady MH, Shafei MN, Saberi Z, Amini S. Pharmaecological effects of Rosa damascene. Iran J Basic Medi Scie. 2011;14:213–8.
Dagli R, Avcu M, Metin M, Kiymaz S, Ciftci H. The effects of aromatherapy using rose oil (Rosa damascena Mill.) on preoperative anxiety: a prospective randomized clinical trial. Europ J Integr Medi. 2019;26:37–42.
Dorri MH, Hosseini SA, Lebaschi MH. Investigating the amount of hypericin in two natural sites of Hypericum perforatum in Golestan province. Iran Sci. Res Quar J Aro Herbs. 2009;24:117–25.
Engelmann U, Walther C, Bondarenko B, Funk P, Schläfke S. Efficacy, and safety of a combination of Sabal and Urtica extract in lower urinary tract symptoms. A randomized, double-blind study versus tamsulosin. Arizona Fors. 2006;56:222–9.
Farooqi AH, Abad Sharma S. Effect of growth retardants on flowering of Rosa damascena Mill. In: Sinha SK, Sane PV, Bhargava SC, Agrawal PK, Editors. proceeding of international congress of plant physiology. Society of Plant Physiology and Biochemistry, New Delhi; 1988. vol. 2, pp 1369–1372
Ghavam M, Azarnivand H, Akhbari M. Examining of the quality and quantity of active ingredients of Smirnova iranica Sabeti in different habitats. Health Biotech Biopharma. 2018;1:21–7.
Ghavam M, Manca ML, Manconi M, Bachetta G. Chemical composition and antimicrobial activity of essential oils obtained from leaves and flowers of Salvia hydrangea DC. ex Benth. Sci Rep. 2020;10:15647.
Ghavam M, Manconi M, Manco ML, Bachetta G. Extraction of essential oil from Dracocephalum kotschyi Boiss. (Lamiaceae), identification of two active compounds and evaluation of the antimicrobial properties. J Ethnopharmacol. 2021;267:1–26.
Gholamhoseinian A, Shahouzehi B, Sharififar F. Inhibitory effect of some plant extract on pancreatic lipase. Intern J Pharm. 2010;6:18–24.
Heydarirad G, Keyhanmehr AS, Mofid B, Nikfarjad H, Mosavat SH. Efficacy of aromatherapy with Rosa damascena in the improvement of sleep quality of cancer patients: A randomized controlled clinical trial. Complem Therap Clini Pract. 2019;35:67–61.
Hosseinzadegan R, Bakhshi Khaniki G. The effect of some ecological factors on the essential oil of Teucrium polium L. NCMBJ. 2014;4:65–70.
Inoue Y, Hada T, Shiraishi A, Hirose K, Hamashima H, Kobayashi SH. Biphasic effects of geranylgeraniol, teprenone, and phytol on the growth of Staphylococcus aureus. Am Soc Microbiol. 2005;49:1770–4.
Jakola L, Hohtola A. Effect of latitude on flavonoid biosynthesis in plants. Plant Cell Envi. 2010;33:1239–47.
Javed Naquvi K, Ansari M, Ali SH, Najmi AK. Volatile oil composition of Rosa damascena Mill. (Rosaceae). J Pharma Phytoch. 2014;2:177–81.
Jirovetz L, Eller G, Buchbauer G. Chemical composition, antimicrobial activities and odor descriptions of some essential oils with characteristic floralrosy scent and of their principal aroma compounds. Recent Res Dev Agron Hortic. 2006;2:1e12.
Koksal N, Aslancan H, Sadighazadi S, Kafkas E. Chemical investigation on Rose damascena Mill. volatiles; effects of storage and drying conditions. Acta Scie Polon. 2015;1:105–14.
Konovalova O, Gergel E, Herhel V. GC-MS analysis of bioactive components of Shepherdia argentea (Pursh) Nutt. From Ukrainian Flora Pharma Innov. 2013;2:7.
Laurel FR, Servio RP, Valerie BK, Gregory MJ, Ian CP. Direct and indirect effects of climate change on Hypericum perforatum L. (Hypericaceae). Oecolo. 1999;120:113–22.
Lavid N, Wang J, Shalit M, Guterman I, Bar E, Beuerle T, Menda N, Shafir S, Zamir D, Adam Z, Vainstein A, Weiss D, Pichersky E, Lewinsohn E. O-Methyl- transferases involved in the biosynthesis of volatile phenolic derivatives in rose petals. Plant Physio. 2002;129:1899–907.
Leontaritou P, Lamari FN, Papasotiropoulos V, Iatrou G. Morphological, genetic and essential oil variation of Greek sage (Salvia fruticosa Mill.) populations from Greece. Ind Crop Prod. 2020;150:112346.
Loghmani-Khouzani H, Fini OS, Safari J. Essential oil composition of Rosa damascena Mill. cultivated in Central Iran. Sci Iran. 2007;14:316–9.
Mabberley DJ. Mabberley’s plant-book: A portable dictionary of plants, their classification and uses. 3rd ed. Cambridge: Cambridge University Press; 2008. p. 1–1021.
Mahboubi M. Rosa damascena as holy ancient herb with novel applications. J Tradit Complement Med. 2016;6:10–6.
Mahmood N, Piacente S, Pizza C, Burke A, Khan AI, Hay AJ. The anti- HIV activity and mechanisms of action of pure compounds isolated from Rosa damascena. Biochem Biophys Res Commun. 1996;229:73–9.
Maksimović Z, Stojanović D, Šoštarić I, Dajić Z, Ristić M. Composition and radical-scavenging activity of Thymus glabrescens Willd. (Lamiaceae) essential oil. J Scie Food and Agric. 2008;11:2036–41.
Melito S, Sias A, Petretto GL, Chessa M, Pintore G, Porceddu A. Genetic and metabolite diversity of Sardinian populations of Helichrysum italicum. PLoS ONE. 2013;8:E79043.
Mirzaei M, Ahmadi N, Sefidkon F, Shojaeiyan A, Mazaheri A. Evaluation of some postharvest storage approaches on essential oil characteristics of fresh organic damask rose (Rosa damascena Mill.) flowers. Horticul. 2017;16:1–5.
Misra T, Sharma S, Singh A, Patra N. Influence of topographical and edaphic factors on rose. II. Flowering quality and quantity. Commun Soil Sci Plant Anal. 2002;33:2771–80.
Moein M, Ghasemi Y, Karami F, Tavallali H. Composition of the essential oil of Rosa damascena Mill. from South of Iran. Iran J Pharma Scie. 2010;1:59–62.
Mohebitabar S, Shirazi M, Bioos S, Rahimi R, Malekshahi F, Nejatbakhsh F. Therapeutic efficacy of rose oil: A comprehensive review of clinical evidence. Avic J Phytomed. 2017;3:06–213.
Moradi H, Ghavam M, Tavili A. Study of antioxidant activity and some herbal compounds of Dracocephalum kotschyi Boiss in different ages of growth. Biotech Rep. 2020;25:e00408.
Nedeltcheva-Antonova D, Stoicheva P, Antonov L. Chemical profiling of Bulgarian rose absolute (Rosa damascena Mill.) using gas chromatography–mass spectrometry and trimethylsilyl derivatives. Ind Crop Prod. 2017;108:36–43.
Nayebi N, Khalili N, Kamalinejad M, Emtiazy M. A systematic review of the efficacy and safety of Rosa damascena Mill. with an overview on its phytopharmacological properties. Complement Ther Med. 2017;34:129–40.
Niazi M, Hashempur MH, Taghizadeh M, Heydari M, Shariat A. Efficacy of topical Rose (Rosa damascena Mill.) oil for migraine headache: a randomized double-blinded placebo-controlled cross-over trial. Complement Ther Med. 2017;34:35–41.
Nikbakht, A., Kafi, M., 2004. A study on the relationship between Iranian people and Damask Rose (Rosa damascena Mill.) and its therapeutic and healing properties. In: 8th International Plant-People Symposium (IPPS), 4–6 June., Hyogo, Japan. pp 251–254.
Olennikov DN, Dudareva LV, Osipenko SN, Penzina TA. Chemical composition of Rhododendron aureum (gold rosebay) essential oil from Pribaikal’e (Russian Federation). J Serb Chem Soc. 2010;75:209–15.
Ozkan G, Sagdic O, Baydar NG, Baydar H. Antioxidant and antibacterial activities of Rosa damascena flower extracts. Food Sci Technol Int. 2004;10:277–81.
Popović-Djordjević J, Cengiz M, Ozer MS, Sarikurkcu C. Calamintha incana: essential oil composition and biological activity. Ind Crop Prod. 2019;128:162–6.
Ren W, Qiao Z, Wang H, Zhu L, Zhang L. Flavonoids: promising anticancer agents. Medic Rese Revi. 2003;4:519–34.
Rezaee M, Jaimand K, Tabaei-Aghdaei S, Brazandeh M, Meshkizadeh S. Comparative study essential oils of Rosa damascena Mill. from center and northwest of Iran. Iran J Med Aromat Plants Res. 2004;4:339–48.
Rostaefar A, Hassani A, Sefidkon F. Effect of altitude on essential oil composition in different gender of Juniperus communis ssp. hemisphaerica growing wild in Amol. Eco-phy J Med Plants. 2018;1:1–11.
Rusanov K, Kovacheva N, Atanassov A, Atanassov I. Rosa damascena Mill., the oil-bearing Damask rose: genetic resources, diversity and perspectives for molecular breeding. Floricul Ornam Biotech. 2009;3:14–20.
Sadraei H, Asghari G, Emami S. Inhibitory effect of Rosa damascena Mill flower essential oil geraniol and citronellol on rat ileum contraction. Res Pharm Sci. 2013;8:1e7.
Sarkic A, Stappen I. Essential oils and their single compounds in cosmetics-a critical review. Cosmetics. 2018;11:1–21.
Sanli A, Karadogan T. Geographical impact on essential oil composition of endemic Kundmannia anatolica Hub.-Mor. (Apiaceae). Afr J Tradit Complement Altern Med. 2017;14:131–7.
Sartoratto A, Machado ALM, Delarmelina C, Figueira GM, Duarte MCT, Rehder VLG. Composition and antimicrobial activity of essential oils from aromatic plants used in Brazil. Brazil J Mic. 2004;4:275–80.
Shahbazi K, Esmaeili A. Evaluation effective interaction essence compound in Rosa damascena genotypes. Int J Agr Crop Sci. 2012;24:1827–32.
Shawl A, Adams R. Rose oil in Kashmiri India. Perfum Flavor. 2009;34:2–5.
Shohayeb M, El-Sayed S, Abdel-Hameed., S. Bazaid, A., Maghrabi, I., . Antibacterial and antifungal activity of Rosa damascena Mill. essential oil, different extracts of rose petals. Glob J Pharm. 2014;1:1–7.
Slavov A, Vasileva I, Stefanov L, Stoyanova A. Valorization of wastes from the rose oil industry. Rev Environ Sci Biotechnol. 2017;16:309–25.
Tabaei-Aghdaei SR, Babaei A, Jaimand MK, Rezaee MB, Assareh MH, Naghavi MR. Morphological and oil content variations amongst Damask rose (Rosa damascena Mill.) landraces from different regions of Iran. Sci Hortic (Amsterdam). 2007;113:44–8.
Tajbakhsh, M., Ghiasi, M., 2008. Seeds ecology. 134 (Jihad Daneshgahi Publications, West Azerbaijan).
Thakur M, Sharma Sh, Sharma U, Kumar R. Study on effect of pruning time on growth, yield and quality of scented rose (Rosa damascena Mill) varieties under acidic conditions of western Himalayas. J Appl Rese Med Arom Plants. 2019;13:100202.
The Plant List (2013). Version 1.1. Published on the Internet; http://www.theplantlist.org/. Accessed 1 Jan 2021.
Ulusoy S, Boşgelmez-Tınaz G, Seçilmiş-Canbay H. Tocopherol, carotene, phenolic contents and antibacterial properties of rose essential oil, hydrosol and absolute. Curr Microbiol. 2009;59:554.
Verma RS, Padalia RC, Chauhan A, Singh A, Yadav AK. Volatile constituents of essential oil and rose water of damask rose (Rosa damascena Mill.) cultivars from North Indian hills. Nat Prodt Res. 2011;17:1577–84.
Yassa N, Masoomi F, Rohani Rankouhi SE, Hadjiakhoondi A. Chemical composition and antioxidant activity of the extract and essential oil of Rosa damascena from Iran, population of Guilan. DARU J Pharm Sci. 2009;3:175–80.
Yavari A, Shahgolzari S. Effect of some ecological factors on quality and quantity of effective ingredient of Stachys inflate at Touyserkan region. Agroecol J. 2016;1:77–85.
Yousefi B, Tabaei-Aghdaei SR, Darvish F, Assareh MH. Flower yield performance and stability of various Rosa damascena Mill. landraces under different ecological conditions. Sci Horti. 2009;121:333–9.
Zargoosh Z, Ghavam M, Bacchetta G, Tavili A. Effects of ecological factors on the antioxidant potential and total phenol content of Scrophularia striata Boiss. Sci Rep. 2019;9:16021.
Zu Y, Yu H, Liang L, Fu Y, Efferth T, Liu X, Wu N. Activities of ten essential oils towards Propionibacterium acnes and PC-3, A-549 and MCF- 7 cancer cells. Molecules. 2010;5:3200–10.
Acknowledgements
Not applicable.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
Mansureh Ghavam was the supervisor, designer of the hypotheses, and responsible and functor for all the steps (plant collection, laboratory, statistical analysis, data analysis, etc.) and wrote the text of the article. Afsaneh Afzali helped to interpret part of data and substantively revised the text. Maria Manconi interpreted part of data, substantively revised and edited English language. Gianluigi Bacchetta identified and approved the study plan and edited the text and wrote part of the text. Maria Letizia Manca helped with statistical analysis of data and corrected part of the text.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ghavam, M., Afzali, A., Manconi, M. et al. Variability in chemical composition and antimicrobial activity of essential oil of Rosa × damascena Herrm. from mountainous regions of Iran. Chem. Biol. Technol. Agric. 8, 22 (2021). https://doi.org/10.1186/s40538-021-00219-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40538-021-00219-6