Abstract
Background
Minimum tillage (MT) and organic farming (OF) are increasingly conducted in agricultural managements from the interest of optimizing soil conditions and developing sustainable agriculture. Our understanding of their effects on water-extractable organic matter (WEOM) is still insufficient.
Methods
To study the effects of MT and OF on WEOM, we analyzed soil materials sampled at two depths (0–8-cm-upper soil and 12–25-cm-deeper soil) from long-term field experiments using different farming and tillage methods. The content, composition, and quality of WEOM were examined.
Results
The results showed organic farming significantly decreased water-extractable organic carbon and nitrogen, but had positive effect on WEOM humic-like components revealed by parallel factor analysis with excitation–emission matrix, soil organic carbon (SOC), total nitrogen (TN), as well as SOC/TN. In addition, organic farming increased the aromaticity and condensation of WEOM as indicated by specific UV absorption and humification index. MT had no effect on WEOM both quantitatively and qualitatively but significantly decreased SOC and TN of the whole investigated soil profile. The depth effect was significant with strong stratification of WEOM, WEOM components as well as SOC and total N in upper soil. Moreover, the WEOM spectroscopic quality showed sharp differences between the upper and deeper soils.
Conclusions
The results indicated that in the combined presence both tillage management and farming management, farming management imposed more influence on WEOM than tillage, and organic farming may facilitate the transformation of WEOM and lead to formation of WEOM with high stability. MT significantly changed the distribution of SOC and WEOM in soil, profile but did not increase the contents of SOC and WEOM in the site of the present study. However, the presence of larger pool of WEOM in MT + OF treatment at upper soil is likely to fuel possibly greater microbial activity and more rapid nutrient cycling in soil which can be favorable practice with potential in improving soil conditions in view of developing a sustainable ecosystem in the studied site

The impacts of agricultural practices on soil water extractable organic matter
Similar content being viewed by others
Background
Agriculture is a potential source of negative impacts on ecosystems. Tillage is basically used to prepare soil for plant growth, while organic farming, as a holistic management practice in agriculture, is conducted for achieving the objectives of protecting human health, conserving natural resources, and preserving the quality of environment while being economically sustainable [1]. Minimum tillage and organic farming practices are reported as having a potential of retaining more soil organic matter and enhancing microbial biomass and activities [2–5]. Water-extractable organic matter (WEOM) is the soluble fraction of organic matter extracted from the soil under various laboratory conditions, the most active and mobile fraction of soil organic matter (SOM). Although it is only a small part of soil organic matter, it has a strong influence on several ecologically relevant processes in soil [6–8]. It is a potential carbon source for soil microorganisms, modulating soil microbial community via the changes of quality and quantity of carbon compounds and their bioavailability [9, 10].
Land-use changes and management activities influence the dynamics of WEOM [6]. Reduced tillage and organic farming as alternative practices of conventional tillage and farming were reported to increase WEOC content by reducing soil disturbance and the addition of organic matter [11–14], while negative or neutral effect also existed [15–18]. However, most of the previous studies focused less on the quality of WEOM. Actually, the concentration, composition, and structural complexity of WEOM affect soil chemical reactions; soil physicochemical and physical degradation in soil ecosystem; and WEOM bioavailability and biodegradability [19, 20]. The characterization of both concentration and composition will contribute to a better understanding of processes in soil and function of soil WEOM in agricultural system. Therefore, the study of WEOM can be of paramount importance to understand the dynamics of soil organic carbon pool and its implication in microbial activities, carbon fluxes, and climate change.
Characterization of WEOM has been performed using specific absorbance [8], the humification index [21], and fluorescence spectroscopy [22]. Furthermore, the advancement in fluorescence spectroscopy enables more detailed characterization on the chemical properties of fluorescing fraction of organic matter. Multidimensional fluorescence spectroscopy is a rapid, nondestructive, and highly sensitive method [7, 19]. More spectral information could be obtained from multidimensional fluorescence spectroscopy than from the traditional fluorescence approaches. The fluorescence index (FI) strongly correlated with the structural conjugation and aromaticity and was used to differentiate source of dissolved organic matter [23]. Biological/freshness index (BIX) or β/α index is an indicator of relative contribution of recently microbially produced dissolved organic matter [24, 25]. With the development of parallel factor analysis, it is also possible to decompose excitation–emission matrix (EEM) data into chemically meaningful components rather than only extract the information by peak picking method [7].
The availability of soils from long-term (21-year) field experiment in adjacent fields with the same soil formation offers an opportunity to evaluate management and tillage effects on SOM and WEOM. Further, the EEM combined with PARAFAC analysis provides a new insight into evaluating WEOM changes in response to tillage. Therefore, we hypothesized that (1) minimum tillage and organic farming increased content and changed the quality of WEOM, and (2) organic farming affects more on WEOM quality compared with minimum tillage.
Methods
Field experiment
Field experiments were carried out since 1992 at the Scheyern Research Farm (TERENO site, http://www.tereno.net) located 40 km north of Munich, Germany (48.50°N, 11.45°E). The altitude of the farm ranges between 445 and 500 masl. The mean annual precipitation and mean annual temperature are 803 mm and 7.4 °C, respectively. The central part of the research station was divided into two parts: organic and integrated, each striving for ecological and economical sustainability. Moreover, detailed studies on management-induced changes were carried out in plots subdivided into integrated and organic farming [26]. The soil types are sandy-to-loamy Cambisols, derived from tertiary sediments and partly covered by loess, and most of the soils have loamy texture [27, 28].
Plot experiments to study tillage-induced [Plow tillage (PL) and minimum tillage (MT)] and management-induced [Integrated farming (IF) and Organic farming (OF)] changes to the systems were set up in two adjacent fields, with the same soil formation and basic soil properties. The soils in both fields have a soil texture of silty loam (USDA), and the soil texture of top soil is composed of 22% sand, 58% silt, and 20% clay. The field trial was arranged in a randomized factor design with three replicate treatments for each factor. The tillage practices in the study represent two possible tillage intensities under the local soil and climatic conditions. PL as a conventional tillage system means to till the soil with moldboard plow (25–30 cm). MT in the study was cultivated soil with chisel mixing in the first 6–8 cm of soil.
For OF system, organic manure (as cattle manure 30 t ha−1, dry weight) was applied instead of synthetic N-fertilizer, and no pesticides were applied. The OF plots are 12 × 12 m in size, and the crop rotation is a seven-crop rotation: (1) Grass–clover–alfalfa (GCA) (Lolium perenne L. + Trifolium pratense L. + Medicago sativa L.), (2) potatoes (Solanum tuberosum L.) + mustard (Sinapis alba L.) as cover crop, (3) winter wheat (Triticum aestivum L.), (4) sunflower (Helianthus annuus L.) + GCA as cover crop, (5) GCA, (6) winter wheat, and (7) winter rye (Secale cereale L.) + GCA as cover crop. In IF system, nitrogen fertilization was conducted with UAN (50% urea N + 50% ammonium nitrate N) with a modified boom sprayer with tubes to conduct the solution directly to the soil surface. Pesticide and herbicide were used for pest and weed control. Fertilizer rates were fixed for the cultivated crops (135 kg N ha−1 for winter wheat, 105 kg N ha−1 for maize, and 100 kg N ha−1 for potato), irrespective of credits due to effects of previous crop cultivation or mineralization in the soil. The IF plots are 12 × 12 m in size, and the crop rotation was (1) potatoes + mustard as catch crop, (2) winter wheat, (3) maize (Zea mays L.) + mustard as catch crop, and (4) winter wheat.
Therefore, there are four treatments in the present study in total: (1) organic farming + plow tillage (OPL); (2) organic farming + minimum tillage (OMT); (3) integrated farming + plow tillage (IPL); (4) integrated farming + minimum tillage (IMT). The assignments of the treatments to the plots were kept constant since 1992.
Sampling and soil properties
Three replicates of composite samples (each a mixture from 5 cores taken randomly) of top soil were collected from plowing tillage and minimum tillage plots at two soil depths (0–8-cm-upper soil and 12–25-cm-deeper soil) in integrated and organic-farming systems in April 2013. Soil organic carbon (SOC) and total nitrogen were determined using a CN analyzer (EA3000 Eurovector) with an aliquot air-dried soil samples. Soil texture was determined by wet sieving and pipette method [29]. In brief, sand and silt fractions ≥20 µm were measured by sieving after treatment with H2O2, silt, and clay <20 µm and using the pipette procedure. The soil pH was measured in a soil suspension with 0.01 M CaCl2 solution (1:5, w/v).
WEOM extraction and spectroscopic characteristics
Soil WEOM was extracted according to the method of Zsolnay [30]. In brief, 2-mm mesh-sieved soil samples were centrifuged with 0.01 M CaCl2 at a ratio of 1:2 (soil: volume) using an overhead shaker for 10 min. After 10-min centrifugation at 3000g, the supernatant was filtered through 0.4 µm polycarbonate membrane filter. WEON was quantified by subtracting inorganic N from soluble total N. Soluble total N, NO3–N, and NH4–N were quantified using an automated continuous flow analyzer (Skalar).
Specific UV absorption (SUVA), obtained by dividing the absorption at 254 nm by WEOC concentration, provides the information about the aromatic structures of WEOM. Absorption was determined using 1 cm quartz cells with a Varian Cary 50 Bio UV–Visible spectrophotometer [22].
Both pH and molecular concentrations can influence fluorescence [21, 31]. Therefore, to avoid concentration artifacts, dilution was made to have WEOC absorbance <0.1 cm−1 at 254 nm [21], and then WEOC extracts were acidified until reaching a constant standard pH of 2 with 2 M HCl [8, 32]. At low pH, most metal complexes disassociate, which should minimize the quenching of fluorescence due to metal complexation [23]. The pH has been used for fluorescence spectra [8, 23, 32]. PARAFAC analysis was also used to decompose the EEMs measurements at a pH range of 2–12.5 [20, 33–35]. In present study, EEMs spectra and fluorescence spectra for humification index calculation were measured separately, and a round of whole measurement procedure including pH adjustment was finished within half an hour, and no precipitation was observed in the samples. All the fluorescence measurements for humification index were performed on a Varian Cary Eclispe fluorescence spectrophotometer using 1-cm quartz cells at 254-nm excitation. The fluorescence spectra were recorded (300–480 nm with 2-nm increment) at 21 ± 1 °C. Even though the optical density was adjusted by dilution in advance, the fluorescence emission spectra were corrected for “inner-filter effect” [36] according to Zsolnay [21] with a simplified formula that measured fluorescence emission multipliers, eA, where A is the absorbance in cm−1 at the 254-nm excitation wavelength [8, 37]. Humification index (HIX) indicating the complexity and condensation (H/C ratios) of WEOM was calculated as the ratio of integrated fluorescence emission peak at longer wavelength region (435–480 nm) over shorter wavelength region (300–345 nm) at the 254-nm excitation wavelength [21].
EEMs were obtained by scanning over excitation wavelengths from 250 to 450 nm with an increment of 5 nm and emission wavelength from 300 to 600 nm with an increment of 5 nm. The slit widths were set as follows: excitation slit: 10 nm, and emission slit: 20 nm. Fluorescence data were corrected for inner-filter effect using the absorption data as suggested by Lakowicz [38]:
where I corr and I obs are the corrected and uncorrected fluorescence intensities; and A ex and A em are the absorbance values at the excitation and emission wavelengths of the fluorescence intensity values, respectively. The correction is based on the assumption that the average path length of excitation and emission lights is 50% of the cuvette width, respectively. Although the correction factors of EEMs and fluorescence spectra for humification index calculation were different, this will not affect the results in a comparison study. First, EEMs spectra and fluorescence spectra for humification index calculation were measured separately, the correction for each was done in the same way, respectively. Second, the two correction formulas are both simplified from the same original formula and based on the same theory as reported by Gauthier et al. [36].
The fluorescence index (FI), which strongly correlated with the structural conjugation and aromaticity and was used to differentiate the sources of DOM, was calculated as the ratio of emission intensities at 470 and 520 nm at the fixed excitation wavelength of 370 nm [23]. Biological/freshness index (BIX) or β/α index, an indicator of the relative contribution of the recently microbially produced DOM, was calculated as the ratio of emission intensity at 380 nm (β) to the maximum emission intensity observed between 420 and 435 nm (α) for an excitation wavelength of 310 nm [24, 25].
PARAFAC modeling
PARAFAC provides a way to decompose a dataset of EEM into individual fluorescence components [39, 40]. An EEM can be reduced to trilinear terms and a residual array by
where, for the EEM fluorescence data, X ijk is the fluorescence intensity of the ith sample at the kth excitation and at the jth emission wavelength. a in is directly proportional to the concentration (here defined as scores) of the nth fluorophore in the ith sample. b jn and c kn are the estimates of emission and excitation spectra (loadings) of the nth fluorophore at the wavelengths j and k, respectively. F is the number of components, and ε ijk is the residual matrix of the model which represents unexplained variability by the model. For the full description about PARAFAC, reader can refer to Bro [39]. Components extracted by PARAFAC can be ascribed to specific species of organic matter present in liquid samples, but they are more likely to represent groups of organic compounds having similar fluorescence properties. The PARAFAC model identifies the number of components as well quantifies the scores for each component that is directly proportional to the component’s (fluorophore) concentration in the sample. This concentration can be converted into actual concentration if specific absorption coefficient or quantum yields associated with excitation and emission of each fluorophore are known [41]. The concentration scores of the PARAFAC components were expressed as maximum fluorescence intensity (F max) (R.U.) [42] for each modeled component in current study. F max gives estimates of the relative concentrations of each component; however, direct comparison of relative concentrations between different components depends on the magnitude of their quantum efficiencies as well as on their individual responses to quenching effects [43].
Before the application of PARAFAC model, zero emission intensities were assigned for excitation wavelengths (λ ex) greater than emission wavelengths (λ em). Raleigh scattering was minimized according to [19, 40]. An EEM of the 0.01 M CaCl2 solution was obtained and subtracted from the EEM of each sample in order to remove most of the Raman scatter peaks; any negative values produced by the subtraction were converted to missing values, and the fluorescence was normalized by dividing the integrated area (Ex 350 nm, Em 371–428 nm) under Raman scatter peak of the corresponding Milli-Q water of each set of measurement. This calibration procedure is universal, and the Raman signal of water can be used irrespective of the sample solvent and matrix, and no spectral changes will occur from applying this method [44] and the fluorescence intensities were reported in Raman units (R.U.). In total, the dataset contained 36 corrected and standardized EEMs. After an initial exploratory analysis (calculating its leverage using DOMFluor), one outlier was identified and removed from the dataset. Therefore, 35 EEMs were performed with PARAFAC analysis in the end. Split-half analysis and examination of residual error plots were applied to get the appropriate number of components. A series of PARAFAC models were generated with Matlab (2009a) by means of DOMfluor toolbox specifically developed for PARAFAC analysis of DOM fluorescence [45].
Statistics
The results presented in the tables are geometric means and expressed on dry-weight basis (for about 24 h at 105 °C). The significance of treatment effects was tested by a two-way ANOVA using tillage and farming management as independent factors and soil depth as repeated measure. ANOVA and Pearson correlation coefficients were calculated using the vegan package of R [46].
Results
Soil total organic matter and water-extractable organic matter
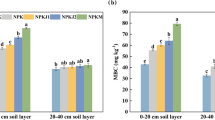
The contents of SOC and total N as well as the SOC/total N and WEOC/WEON were significantly higher in organic farming in comparison with integrated farming system, while the contents WEOC, WEON, NO3–N as well as NH4–N were lower (Table 1). The contents of WEOC and WEON as well as the SOC/total N and WEOC/WEON ratios did not differ between the tillage treatments, while MT significantly decreased the contents of SOC and total N but increased those of NO3–N and NH4–N. The accumulation of SOC, total N, WEOC, WEON, NO3–N and NH4–N in the 0–8 cm layer was significant in comparison with 12–25 cm layer, usually leading to significant tillage × soil–depth interactions, except WEON and NH4–N. The ratio of SOC/total N was significantly higher, while WEOC/WEON ratio was significantly lower at 0–8 cm in comparison with 12–25 cm. The significant interactions of farming × tillage on SOC (Fig. 1), but also those on total N, SOC/total N, WEON, NO3–N, and NH4–N were caused by the much stronger tillage effects in the organic farming than in the integrated system.
Spectroscopic properties of WEOM
SUVA and HIX were significantly higher, while BIX was significantly lower in organic farming in comparison with integrated farming (Table 2). The FI did not differ between farming systems. Tillage had no effect on any WEOM spectroscopic property. The SUVA values were significantly higher at 0–8 cm than at 12–25 cm, which was especially true for the organic-farming system (Fig. 2). The HIX values were significantly lower at 0–8 cm than at 12–25 cm, which was again most pronounced in the organic-farming system (Fig. 3). In contrast, the BIX values did not change with depth and farming system (Table 2).
PARAFAC components
Three EEM-PARAFAC components (C1, C2, C3), which account for 99.28% of the fluorescence variability, were extracted from the dataset (Fig. 4). The C1 component had two excitation maxima at 240 and 325 nm and one emission maxima at 435 nm, similar to the humic-like substance identified by [47–49]. The C2 component had two excitation maxima at <240 and 260 nm and one emission maxima at 360 nm, similar to the component 5 in [50–52] or peak N in [53] and C4 component in [49]. The C2 component is characterized by tryptophan-like substance associated with biological production, and one shoulder could be found on the emission spectrum due to different fluorescence characteristics [54]. The C3 component had one excitation maxima at 265 nm and one emission maxima at 505 nm. The C3 component is similar to humic-like substance reported before [41, 49, 53, 54].
Concentrations of three components as indicated by the concentration scores followed an order of C1 > C2 > C3 (Table 2). The two humic-like substances (C1 and C3) were significantly higher in organic farming in comparison with integrated farming, while the tryptophan-like substance (C2) did not differ between farming systems. The effect of tillage on the three EEM-PARAFAC components was insignificant. The three EEM-PARAFAC components were significantly higher at 0–8 cm than those at 12–25 cm. All three EEM-PARAFAC components were significantly correlated to SOC and total N at both depths (Table 3), although the relationships were always closer at 12–25 cm. This was also true for the relationships to WEOC, which were already insignificant for C1 and C2 at 0–8 cm. The relationships to WEON were only significant for C1 and C2 at 12–25 cm.
Discussion
Soil organic carbon and nitrogen
In the present study, our results showed MT and organic farming significantly increased SOC and TN in upper soil (Table 1). The results agreed with the results of several previous studies [11, 12, 55]. Higher contents of SOC and TN in MT at upper soil could be attributed to the fact that MT reduces the mineralization of SOM [14] and improves the physical protection of SOM via reducing the disruption of soil aggregates and avoiding physical access of SOM by microbes [56] compared with conventional tillage. Furthermore, crop residues accumulate in the upper layer of soil, while plowing tillage incorporates crop residues into the whole tillage horizon of soil more uniformly resulting in dilution effects of C and N [57, 58]. When the two soil depths were considered, the effect of MT was negative, this being perhaps because of dilution effect in deeper soil part. The results agreed with the findings of Luo et al. [59] that no-tillage changed distribution of C in the soil profile significantly, but did not increase the total SOC, as the increase of SOC in surface soil compensates for the loss of SOC in deeper soil. In terms of organic farming, similar findings [60, 61] were reported, although some inconsistent findings occurred where no obvious improvement of SOC was observed [2, 62]. The difference between studies can be attributed to the difference in the climatic conditions of the studied sites. In the study of Parras-Alcantara et al. [62], their trial site (Los Pedroches Valley) is characterized by cold winters and warm, dry summers with temperatures ranging from −2 to 40 °C (extreme temperatures) and an average annual rainfall of 600 mm, while the climate of our study is milder with mean precipitation of 803 mm and mean temperature of 7.4 °C. Therefore, different degradations and turnovers of organic matter might occur in the two study sites. Further, their soils were sandier. This supports the hypothesis that the tillage-induced changes of soil are soil and site specific [63]. The beneficial effect of organic farming could be associated with the continuous inputs of organic matter by manure, plants, and legume catch-crop-based crop rotations [60]. It is also recognized that the addition of organic manure increases the soil water repellency, thus increasing the aggregate stability [64] and carbon sequestration [65]. In addition, the diverse microbial community under organic farming facilitates the degradation of plant materials, thus increasing the SOM from the decayed plant debris [64].
WEOM components
WEOM is believed to originate from plant roots, litter, and soil humus and is a labile substrate for microbial support and transformations [11]. In the present study, organic farming significantly decreased the contents of WEOC and MT resulting in significant accumulation of WEOC in upper soil. The decreased WEOM in organic-farming treatments could be attributed to the sampling time. Bausenwein et al. [9] reported significant effect of sampling time on WEOC, and the higher WEOC was observed in October in the same research station as ours but with samples from different fields. Our samples were collected in 2013 after a long winter when the snow ended in May. In addition, we also observed increased microbial biomass compared with integrated farming [3]. Therefore, due to more WEOC consumption than its production, the production and consumption of WEOC became unbalanced. Organic farming significantly decreased the content of WEON. One explanation could be that microbial communities in organic farming are more N-depleted, and the N fertilization in integrated farming changed the microbial community’s structure thus resulting in the alteration of organic N mineralization [66]; this agreed with Embacher et al.’s report that the fertilizer itself is not the direct source of WEOM but affects WEOM via the biota [32]. The increased WEOC in upper soil under MT treatments was consistent with other findings [11, 12], although some authors also reported tillage had no significant effect at any soil depth [15]. The reason behind this might be that, on the one hand, the MT reduced the mineralization of WEOM [13], and that, on the other hand, the diverse microbial community in MT [67] increased the degradations of SOM, and the plant residues accumulated in upper soil contributed to WEOM.
Organic farming significantly increased the components 1 and 3 (humic-like substance components). This is in line with the result of Zhang et al. [16] who observed that the application of manure increased the humic-like substance identified by EEM-PARAFAC; but contrary to the result reported by Ohno et al. [68] that tillage and cropping management did not affect the humic-like substance components 1 and 5 and amino acid-like component 4. But they found N sources (NH4NO3 and poultry litter) significantly affected the identified components of WEOM. In the present study, organic farming caused significantly lower NO3–N in comparison with integrated farming. Thus, the increased humic-like substance in organic-farming treatments indicated that WEOM in organic farming was highly microbial processed which can also be reflected by SUVA and HIX of WEOM (Table 2). Besides, EEM-PARAFAC components of upper soil were significantly higher than those of deeper soil. This can be explained by the accumulation and degradation of accumulated organic matter by MT in top soil which can be proved by the close association of EEM-PARAFAC components with SOC and total N (Table 3). Compared with humic-like substance, C2 as tryptophan-like substance with simpler chemical structure can be degraded more easily by microbes and will return to background level; this can be a reason for the tillage and organic-farming practices having insignificant effects on C2.
Spectroscopic properties of WEOM and relations to soil characteristics
In comparison with tillage effect, farming practices are the main factor that influenced the quality of WEOM. Increased values of SUVA and HIX in organic farming in the present study implied that WEOM in organic farming is more aromatic and condensed compared with that in integrated farming. This might be because of the altered diverse microbial community’s structure in organic farming [69], which might promote the degradation of WEOM in comparison with integrated farming. What’s more, the different crop rotations which affected WEOM by crop root exudates, and the return of crop residues might be another reason to explain the different SUVA and HIX values of WEOM. Our result suggested that complex crop rotations release WEOM with higher aromaticity and humification. This is opposite to that of Xu et al. [18] who reported the inclusion of a perennial crop alfalfa in a crop rotation, which releases WEOM with lower aromaticity and lower extent of humification. This may be attributed to simpler crop rotations in their study and different crop species they planted compared with our study. Although FI values of WEOM at upper and deeper soils were statistically different, according to McKnight et al. [23], FI value of WEOM around 1.4 indicates WEOM derives from terrestrial source, while FI around 1.9 indicates WEOM derives from microbial source. Therefore, FI values in the present study were around 1.6 indicating the influence of both terrestrial and microbial source on WEOM. The lower BIX (freshness index) in organic farming indicated that less recently microbial-derived organic matter contributed to WEOM in organic farming, while the higher BIX in MT implied that more freshly derived WEOM contributed to WEOM in MT.
Conclusions
In the present agricultural long-term field study, soil WEOM responded diversely to the long-term applications of MT and OF. MT resulted in negative depth gradients of soil properties and SOC, total N as well as WEOM and its components accumulated in upper soil. In contrast, MT had no effect on the quality and WEOM components. OF had significant effects on SOM and WEOM. Specifically, OF increased SOC, total N content as well as the humic-like components and the complexity of WEOM, while the content of WEOM was lowered by OF. The results indicated that the minimum tillage changed the distribution of SOM in soil profile rather than increasing SOM in the field-trial region of the present study, and organic farming dominated the quality change of WEOM. The combined organic farming plus minimum tillage would be a better option of agricultural practice to improve soil conditions as indicated by the improved soil WEOM at upper soil and is likely to fuel possibly greater microbial activity, favoring the development of nutrient cycling in view of sustainable agricultural practice at the site of present study. Minimum tillage may be considered in the concerned landscape as a more acceptable way in realizing the balance between the yield loss and improved soil conservation [70].
Abbreviations
- MT:
-
minimum tillage
- PL:
-
plowing tillage
- OF:
-
organic farming
- IF:
-
integrated farming
- WEOM:
-
water-extractable organic matter
- WEOC:
-
water-extractable organic carbon
- WEON:
-
water-extractable organic nitrogen
- EEM:
-
excitation–emission matrix
- PARAFAC:
-
parallel factor analysis
- C1 and C3:
-
humic-like substance in WEOM
- C2:
-
tryptophan-like substance in WEOM
- SUVA254 :
-
specific ultraviolet visual absorption at 254 nm
- HIX:
-
humification index
- FI:
-
fluorescence index
- BIX:
-
freshness index
- F max :
-
maximum fluorescence intensity
References
Escobar M, Hue N, Pandalai S. Current developments in organic farming. Recent Res Dev Soil Sci. 2007;2:29–62.
Gattinger A, Muller A, Haeni M, Skinner C, Fliessbach A, Buchmann N, et al. Enhanced top soil carbon stocks under organic farming. Proc Natl Acad Sci USA. 2012;109(44):18226–31.
Sun H, Koal P, Liu D, Gerl G, Schroll R, Gattinger A, et al. Soil microbial community and microbial residues respond positively to minimum tillage under organic farming in Southern Germany. Appl Soil Ecol. 2016;108:16–24.
Kandeler E, Tscherko D, Spiegel H. Long-term monitoring of microbial biomass, N mineralisation and enzyme activities of a Chernozem under different tillage management. Biol Fertil Soils. 1999;28(4):343–51.
Ghimire R, Adhikari KR, Chen ZS, Shah SC, Dahal KR. Soil organic carbon sequestration as affected by tillage, crop residue, and nitrogen application in rice–wheat rotation system. Paddy Water Environ. 2012;10(2):95–102.
Kalbitz K, Solinger S, Park JH, Michalzik B, Matzner E. Controls on the dynamics of dissolved organic matter in soils: a review. Soil Sci. 2000;165(4):277–304.
Ohno T, Bro R. Dissolved organic matter characterization using multiway spectral decomposition of fluorescence landscapes. Soil Sci Soc Am J. 2006;70(6):2028–37.
Embacher A, Zsolnay A, Gattinger A, Munch JC. The dynamics of water extractable organic matter (WEOM) in common arable topsoils: I. Quantity, quality and function over a three year period. Geoderma. 2007;139(1–2):11–22.
Bausenwein U, Gattinger A, Langer U, Embacher A, Hartmann HP, Sommer M, et al. Exploring soil microbial communities and soil organic matter: variability and interactions in arable soils under minimum tillage practice. Appl Soil Ecol. 2008;40(1):67–77.
Grosso F, Temussi F, De Nicola F. Water-extractable organic matter and enzyme activity in three forest soils of the Mediterranean area. Eur J Soil Biol. 2014;64:15–22.
Dong WX, Hu CS, Chen SY, Zhang YM. Tillage and residue management effects on soil carbon and CO2 emission in a wheat–corn double-cropping system. Nutr Cycl Agroecosyst. 2009;83(1):27–37.
Sun BH, Hallett PD, Caul S, Daniell TJ, Hopkins DW. Distribution of soil carbon and microbial biomass in arable soils under different tillage regimes. Plant Soil. 2011;338(1–2):17–25.
Chantigny MH. Dissolved and water-extractable organic matter in soils: a review on the influence of land use and management practices. Geoderma. 2003;113(3–4):357–80.
Melero S, Lopez-Garrido R, Madejon E, Murillo JM, Vanderlinden K, Ordonez R, et al. Long-term effects of conservation tillage on organic fractions in two soils in southwest of Spain. Agric Ecosyst Environ. 2009;133(1–2):68–74.
Cookson WR, Murphy DV, Roper MM. Characterizing the relationships between soil organic matter components and microbial function and composition along a tillage disturbance gradient. Soil Biol Biochem. 2008;40(3):763–77.
Zhang M, He Z, Zhao A, Zhang H, Endale DM, Schomberg HH. Water-extractable soil organic carbon and nitrogen affected by tillage and manure application. Soil Sci. 2011;176(6):307–12.
Marinari S, Liburdi K, Fliessbach A, Kalbitz K. Effects of organic management on water-extractable organic matter and C mineralization in European arable soils. Soil Tillage Res. 2010;106(2):211–7.
Xu N, Wilson HF, Saiers JE, Entz M. Effects of crop rotation and management system on water-extractable organic matter concentration, structure, and bioavailability in a chernozemic agricultural soil. J Environ Qual. 2013;42(1):179–90.
Borisover M, Lordian A, Levy GJ. Water-extractable soil organic matter characterization by chromophoric indicators: effects of soil type and irrigation water quality. Geoderma. 2012;179–180:28–37.
Fellman J, D’Amore D, Hood E, Boone R. Fluorescence characteristics and biodegradability of dissolved organic matter in forest and wetland soils from coastal temperate watersheds in southeast Alaska. Biogeochemistry. 2008;88(2):169–84.
Zsolnay A, Baigar E, Jimenez M, Steinweg B, Saccomandi F. Differentiating with fluorescence spectroscopy the sources of dissolved organic matter in soils subjected to drying. Chemosphere. 1999;38(1):45–50.
Corvasce M, Zsolnay A, D’Orazio V, Lopez R, Miano TM. Characterization of water extractable organic matter in a deep soil profile. Chemosphere. 2006;62(10):1583–90.
McKnight DM, Boyer EW, Westerhoff PK, Doran PT, Kulbe T, Andersen DT. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol Oceanogr. 2001;46(1):38–48.
Huguet A, Vacher L, Relexans S, Saubusse S, Froidefond JM, Parlanti E. Properties of fluorescent dissolved organic matter in the Gironde Estuary. Org Geochem. 2009;40(6):706–19.
Wilson HF, Xenopoulos MA. Effects of agricultural land use on the composition of fluvial dissolved organic matter. Nat Geosci. 2009;2(1):37–41.
Schröder P, Huber B, Olazabal U, Kammerer A, Munch JC. Land use and sustainability: FAM research network on agroecosystems. Geoderma. 2002;105(3–4):155–66.
Kölbl A, Kögel-Knabner I. Content and composition of free and occluded particulate organic matter in a differently textured arable Cambisol as revealed by solid-state (13)C NMR spectroscopy. J Plant Nutr Soil Sci. 2004;167(1):45–53.
Flessa H, Ruser R, Schilling R, Loftfield N, Munch JC, Kaiser EA, et al. N2O and CH4 fluxes in potato fields: automated measurement, management effects and temporal variation. Geoderma. 2002;105(3–4):307–25.
Gee G, Bauder J. Particle-size analysis. Methods Soil Anal Part I. 1986;9:383–411.
Zsolnay A. Dissolved Humus in Soil Waters. In: Piccolo A, editor. Humic substances in terrestrial ecosystems. Amsterdam: Elsevier Science B.V; 1996. p. 171–223.
Zsolnay A. Dissolved organic matter: artefacts, definitions, and functions. Geoderma. 2003;113(3–4):187–209.
Embacher A, Zsolnay A, Gattinger A, Munch JC. The dynamics of water extractable organic matter (WEOM) in common arable topsoils: II. Influence of mineral and combined mineral and manure fertilization in a Haplic Chernozem. Geoderma. 2008;148(1):63–9.
Yang L, Hur J. Critical evaluation of spectroscopic indices for organic matter source tracing via end member mixing analysis based on two contrasting sources. Water Res. 2014;59:80–9.
Cuss CW, Shi YX, McConnell SM, Guéguen C. Changes in the fluorescence composition of multiple DOM sources over pH gradients assessed by combining parallel factor analysis and self-organizing maps. J Geophys Res Biogeosci. 2014;119(9):1850–60.
Chen H, Kenny JE. A study of pH effects on humic substances using chemometric analysis of excitation–emission matrices. AES. 2007;1:1–9.
Gauthier TD, Shane EC, Guerin WF, Seitz WR, Grant CL. Fluorescence quenching method for determining equilibrium constants for polycyclic aromatic hydrocarbons binding to dissolved humic materials. Environ Sci Technol. 1986;20(11):1162–6.
Akagi J, Zsoinay A. Effects of long-term de-vegetation on the quantity and quality of water extractable organic matter (WEOM): biogeochemical implications. Chemosphere. 2008;72(10):1462–6.
Lakowicz JR. Principles of fluorescence spectroscopy. Berlin: Springer, US; 2006.
Bro R. PARAFAC. Tutorial and applications. Chemom Intell Lab Syst. 1997;38(2):149–71.
Andersen CM, Bro R. Practical aspects of PARAFAC modeling of fluorescence excitation–emission data. J Chemom. 2003;17(4):200–15.
Singh S, D’Sa EJ, Swenson EM. Chromophoric dissolved organic matter (CDOM) variability in Barataria Basin using excitation–emission matrix (EEM) fluorescence and parallel factor analysis (PARAFAC). Sci Total Environ. 2010;408(16):3211–22.
Murphy KR, Stedmon CA, Graeber D, Bro R. Fluorescence spectroscopy and multi-way techniques. PARAFAC. Anal Methods. 2013;5(23):6557–66.
Baghoth SA, Sharma SK, Amy GL. Tracking natural organic matter (NOM) in a drinking water treatment plant using fluorescence excitation–emission matrices and PARAFAC. Water Res. 2011;45(2):797–809.
Lawaetz AJ, Stedmon CA. Fluorescence intensity calibration using the Raman scatter peak of water. Appl Spectrosc. 2009;63(8):936–40.
Stedmon CA, Bro R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: a tutorial. Limnol Oceanogr Methods. 2008;6:572–9.
R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2015. http://www.R-project.org/. Accessed 18 Oct 2015.
Murphy KR, Stedmon CA, Waite TD, Ruiz GM. Distinguishing between terrestrial and autochthonous organic matter sources in marine environments using fluorescence spectroscopy. Mar Chem. 2008;108(1–2):40–58.
Yamashita Y, Scinto LJ, Maie N, Jaffe R. Dissolved organic matter characteristics across a subtropical wetland’s landscape: application of optical properties in the assessment of environmental dynamics. Ecosystems. 2010;13(7):1006–19.
Huang W, McDowell WH, Zou XM, Ruan HH, Wang JS, Ma ZL. Qualitative differences in headwater stream dissolved organic matter and riparian water-extractable soil organic matter under four different vegetation types along an altitudinal gradient in the Wuyi Mountains of China. Appl Geochem. 2015;52:67–75.
Stedmon CA, Markager S, Bro R. Tracing dissolved organic matter in aquatic environments using a new approach to fluorescence spectroscopy. Mar Chem. 2003;82(3–4):239–54.
Williams CJ, Yamashita Y, Wilson HF, Jaffe R, Xenopoulos MA. Unraveling the role of land use and microbial activity in shaping dissolved organic matter characteristics in stream ecosystems. Limnol Oceanogr. 2010;55(3):1159–71.
Hong HS, Yang LY, Guo WD, Wang FL, Yu XX. Characterization of dissolved organic matter under contrasting hydrologic regimes in a subtropical watershed using PARAFAC model. Biogeochemistry. 2012;109(1–3):163–74.
Coble PG. Characterization of marine and terrestrial DOM in seawater using excitation–emission matrix spectroscopy. Mar Chem. 1996;51(4):325–46.
Stedmon CA, Markager S. Resolving the variability in dissolved organic matter fluorescence in a temperate estuary and its catchment using PARAFAC analysis. Limnol Oceanogr. 2005;50(2):686–97.
Sun HY, Wang CX, Wang XD, Rees RM. Changes in soil organic carbon and its chemical fractions under different tillage practices on loess soils of the Guanzhong Plain in north-west China. Soil Use Manag. 2013;29(3):344–53.
Six J, Elliott ET, Paustian K. Soil macroaggregate turnover and microaggregate formation: a mechanism for C sequestration under no-tillage agriculture. Soil Biol Biochem. 2000;32(14):2099–103.
Chivenge PP, Murwira HK, Giller KE, Mapfumo P, Six J. Long-term impact of reduced tillage and residue management on soil carbon stabilization: implications for conservation agriculture on contrasting soils. Soil Tillage Res. 2007;94(2):328–37.
Kaiser M, Piegholdt C, Andruschkewitsch R, Linsler D, Koch HJ, Ludwig B. Impact of tillage intensity on carbon and nitrogen pools in surface and sub-surface soils of three long-term field experiments. Eur J Soil Sci. 2014;65(4):499–509.
Luo Z, Wang E, Sun OJ. Can no-tillage stimulate carbon sequestration in agricultural soils? A meta-analysis of paired experiments. Agric Ecosyst Environ. 2010;139(1–2):224–31.
Marriott EE, Wander MM. Total and labile soil organic matter in organic and conventional farming systems. Soil Sci Soc Am J. 2006;70(3):950–9.
Parras-Alcantara L, Lozano-Garcia B. Conventional tillage versus organic farming in relation to soil organic carbon stock in olive groves in Mediterranean rangelands (southern Spain). Solid Earth. 2014;5(1):299–311.
Parras-Alcantara L, Diaz-Jaimes L, Lozano-Garcia B, Rebollo PF, Elcure FM, Munoz MDC. Organic farming has little effect on carbon stock in a Mediterranean dehesa (southern Spain). Catena. 2014;113:9–17.
Chatterjee A, Lal R. On farm assessment of tillage impact on soil carbon and associated soil quality parameters. Soil Tillage Res. 2009;104(2):270–7.
Mäder P, Fliessbach A, Dubois D, Gunst L, Fried P, Niggli U. Soil fertility and biodiversity in organic farming. Science. 2002;296(5573):1694–7.
Blanco-Canqui H, Lal R. Extent of soil water repellency under long-term no-till soils. Geoderma. 2009;149(1–2):171–80.
Sun L, Chang SX, Feng YS, Dyck MF, Puurveen D. Nitrogen fertilization and tillage reversal affected water-extractable organic carbon and nitrogen differentially in a Black Chernozem and a Gray Luvisol. Soil Tillage Res. 2015;146:253–60.
Kuntz M, Berner A, Gattinger A, Scholberg JM, Mader P, Pfiffner L. Influence of reduced tillage on earthworm and microbial communities under organic arable farming. Pedobiologia. 2013;56(4–6):251–60.
Ohno T, He Z, Tazisong IA, Senwo ZN. Influence of tillage, cropping, and nitrogen source on the chemical characteristics of humic acid, fulvic acid, and water-soluble soil organic matter fractions of a long-term cropping system study. Soil Sci. 2009;174(12):652–60.
Esperschütz J, Gattinger A, Mader P, Schloter M, Fliessbach A. Response of soil microbial biomass and community structures to conventional and organic farming systems under identical crop rotations. FEMS Microbiol Ecol. 2007;61(1):26–37.
Küstermann B, Munch JC, Hülsbergen K-J. Effects of soil tillage and fertilization on resource efficiency and greenhouse gas emissions in a long-term field experiment in Southern Germany. Eur J Agron. 2013;49:61–73.
Authors’ contributions
HYS: through writing—original draft preparation, conceptualization, project management, and realization. PK: through co-field work, advice and help on lab work, and draft preparation; GR: through field-trial management; RS: through advice on lab work and original draft preparation, and supervision; RGJ: through writing—review and editing, and supervision; JCM: through writing—conceptualization, review and editing, and supervision. All the authors read and approved the final manuscript.
Acknowledgements
The authors thank Franz Buegger and Gudrun Hufnagel for excellent sample analysis. Han Yin Sun was funded by Chinese Scholarship Council (CSC) and Helmholtz Zentrum München.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The data and materials were presented in the main manuscript.
Consent for publication
The authors all agreed for the publication of the manuscript in this journal.
Ethics approval and consent to participate
This manuscript is an original research paper and has not been published in other journals. The authors agreed to maintain the copyright rule.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Sun, H.Y., Koal, P., Gerl, G. et al. Water-extractable organic matter and its fluorescence fractions in response to minimum tillage and organic farming in a Cambisol. Chem. Biol. Technol. Agric. 4, 15 (2017). https://doi.org/10.1186/s40538-017-0097-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40538-017-0097-5