Abstract
Background
This study comprises the assessment of sorption of the agricultural antibiotic oxytetracycline to Brazilian soils, to prove the sorption to soil and the influence of the organic matter on the sorption capability, noting that tetracyclines are one of the major classes of antibiotics used in the global livestock industry, either for the treatment of diseases or as growth promoter, besides to be applied in agriculture to control bacteria and fungi. The high-performance liquid chromatography with UV detector was used to obtain quantitative data allowing the construction of soil-oxytetracycline sorption isotherms at pH 4.8, in order to confirm the sorption and to identify the influence of the organic matter on the sorption capacity.
Results
The Freundlich isotherm presented himself as a mathematical model suitable for the verification of the sorption by means of the application of chromatographic method, proving the sorption and a homogeneous molecular process of interaction, as well as the effect of organic matter content on the sorption capacity (K d values up to 9290 kg L−1).
Conclusions
The sorption capacity (K d) was quantified as high, denoting an achievement of the groundwater by the antibiotic OTC.

The sorption of the antibiotic oxytetracycline to soil organic matter can produce environmental risks for humans and animals.
Similar content being viewed by others
Background
Considering the many sources of pollution to which the environment is exposed due to human activities related to production and usage of several chemicals, studies for fate assessment have become a fundamental tool for determination of the polluting sources, exposure routes, and the potential risk to the environment and human health [1].
As a global trend, the Brazilian environmental legislation is advancing in order to monitor and restrict the presence of several organic and inorganic polluting species in the environment [2–4]. However, there is still a wide variety of chemicals that are not included in such legislation, being able to highlight care products, pharmaceuticals, and veterinary, a broad family of emerging pollutants, including antibiotics [5, 6]. Antibiotics are compounds that effectively inhibit the bacteria growth, and are commonly produced by microorganisms present in the environment, such as fungi [7]. The estimated global production of antibiotics is on the order of 200,000 tons per year, and half that amount used for use in animals, and the tetracyclines (Fig. 1) ranks among the most used classes of antibiotic [8]. Furthermore, the global consumption of antibiotics in food animal production was estimated at 63,151 tons in 2010 and is projected to rise by 67 %, to 105,596 tons, by 2030 [9].
The main representatives of the tetracyclines class are classified in the following descending order of veterinary use: oxytetracycline (OTC) > tetracycline > chlortetracycline [10]. The use of tetracycline antibiotics as growth promoter (GP) and to treat bacteria from the genus Salmonella in poultry is widespread in Brazil [11], and it is one of the major classes of antibiotics marketed and applied to the Brazilian livestock [12].
One concern in the use of veterinary tetracyclic antibiotics is that a large fraction of these, between 20 and 90 %, is not absorbed by the animal organism and is excreted unchanged in the urine and feces [13]. It can promote resistance to these antibiotics and, depending on its transport in the soil, reached the groundwater and confer resistance to the microorganisms in this water. If humans and animals will consume this water, microorganisms present inside them may transmit genes of resistance to the antibiotic to humans and animals [14, 15]. The application of animal manure in farmland stands out as a source of soil pollution by antibiotics [16], with the evidence of the presence of residues in soil resulting from this type of agricultural application [17, 18]. Besides this issue of environmental and public health concerning, the fact that the new generation of GP is not absorbed from the intestinal tract to avoid residues in meat [19] may increase the presence of these compounds in environmental matrices, as soil and water.
The soil organic matter (SOM) originates from decomposition of vegetable and animal biomass by means of chemical, biological and physical processes, with structural modifications giving rise to a number of organic compounds whose main representatives are humic substances (HS)—humic and fulvic acids and humin [20]. HS interact with various organic pollutants such as pesticides and oil, and inorganic such as heavy metals [21]. A recent study on the interaction between humic acid extracted from Brazilian soil and the OTC pointed to the existence of different physicochemical mechanisms of interaction that depends on pH values [22], which most probably should be repeated for organic matter (OM) present in the soil.
The study reported here aimed to verify, through the application of HPLC-UV analytical technique, the occurrence of sorption between the OTC and SOM molecules, followed by the construction of sorption isotherms—Freundlich isotherm. It was also of particular interest to evaluate the effect of OM content on the sorption. It is important to highlight the inexistence of studies on this theme for Brazil and tropical soils.
Materials
M1 soil, a red-yellow dystrophic latosol, was collected in a tropical forest in the São Paulo State (21040′4″ S, 47050′33″ W); T1 soil, an organosol (peat), was collected in a farm field, also in the São Paulo State (21033′20″ S, 47055′08″ W). Both samples were collected at depth from 0 to 15 cm and used in soil-OTC sorption experiment. Soil samples were dried at room temperature for 7 days. After this period, samples were homogenized in 2 mm sieve and sent to the laboratory for analyses and sorption experiments. Such soils were analyzed according to Embrapa routine procedures for soil analysis [23].
Methods
The soil-OTC sorption experiment was carried out in duplicate using the M1 and T1 soils as adaptation of the procedure developed by Jones et al. [24]. As the charge and speciation of OTC and soil are dependent on pH values, it chose a medium with a pH value of 4.8, close to the maximum mass fraction of the OTC zwitterion [25] and to minimize possible interference of H+ and other electronic charges on the sorption process.
A solution of OTC (oxytetracycline hydrochloride, HPLC grade ≥ 95 % purity, Sigma-Aldrich) was previously prepared at 240 mg L−1 (stock-solution) in a buffer solution of sodium acetate (Synth)/acetic acid (Synth) at 0.1 mol L−1 in ultrapure water (Milli-Q). The prepared working concentrations from the stock-solution were 120, 60, 30, 20, 10, and 5 mg L−1, with a control solution of 0 mg L−1 of OTC (buffer solution). A 10 mL aliquot of each solution was transferred to amber glass flask and, immediately, added to the soil mass to a final concentration of 5 g L−1 of soil at OTC solution. The mixtures were shaken for 48 h, protected from light and at room temperature (25 °C), and after this period followed for chromatographic analysis, the results were used in the construction of the sorption isotherms.
High-performance liquid chromatography analyses were carried out in a chromatograph Prostar Varian with a UV detector, with a column for reverse mode of polystyrene-divinylbenzene with 15 cm length, 4.6 mm internal diameter, and 5 µm particle size (Phenomenex), according to the conditions adapted from Loke et al. [26]: isocratic mobile phase (v/v) of 26 % of acetonitrile (JT Backer)/74 % of aqueous solution of trifluoroacetic acid (Mallinckrodt) at 0.05 % (v/v), with previous ultrasound treatment by 15 min; column temperature of 30 °C; flow of mobile phase of 1 mL min−1; and wavelength of UV detector at 355 nm. Acetonitrile of HPLC grade (JT Baker) and trifluoroacetic acid (Mallinckrodt) were used for the mobile phase, with a minimum purity grade of 99 %. An external calibration curve was constructed for OTC whose points were 5, 10, 20, 30, 60, 120, and 240 mg L−1. From this curve was determined the limit of quantification (LOQ) of the method, according to the IUPAC recommendations [27].
The determination of the partition coefficient (K d) was done by means of Eq. 1 [28], followed by Eqs. 1 and 3, which enables an estimative of the sorption intensity of xenobiotics on soil:
where C s is the OTC concentration adsorbed to the SOM (mmol g−1) and C e is the OTC concentration in the aqueous phase at equilibrium condition (mmol L−1). K d was chosen expressed as L kg−1, instead of L g−1.
The Freundlich isotherm applied to heterogeneous surfaces is defined by
where S is the concentration of solute sorbed (mg g−1), K f is the sorption coefficient (mg1−n L gn), C is the concentration of solute in equilibrium in aqueous solution (mg L−1), and N is the linearity parameter. In a simple case, N = 1 and K f is equivalent to K d for linear isotherms. The logarithmic form of the equation is used for the linear fit of the Freundlich isotherm:
Results and discussion
Table 1 shows the results of the compositional analysis and physicochemical characterization of the soil samples. The data presented demonstrated that the T1 sample had a larger content of OM and metal, which resulted in higher cationic exchange capacity (CEC) at high pH values, respectively, when compared to the sample M1.
Isotherms were built to verify the influence of soil type on the sorption of OTC. However, it is worth mentioning that the external calibration curve of the analytical method showed a satisfactory value for linear correlation coefficient (R = 0.999) [29]. Then, was obtained a LOQ of 4.0 mg L−1. Nevertheless, were obtained negative values for C e for the initial concentrations of OTC of 10.0 and 5.0 mg L−1, which demonstrated that the LOQ was inefficient for two concentrations, most likely due to the decreasing in the method sensitivity [29].
Freundlich, Langmuir, and partition isotherms were tested (not shown), with the Freundlich isotherm being the more suitable mathematical model, as shown in Fig. 2.
It should be noted that the N value of the Freundlich isotherm allows infer the shape of the isotherm and the sorption mechanism, where N = 1 represents an linear isotherm C, or partition, N < 1 the isotherm L, and N > 1 the isotherm S [30]. The higher the value of N, more heterogeneous is the sorption process [31].
From the linear fit—Eq. 3—of the curves obtained, it was found that the Freundlich isotherm, with the logarithmic linear fit done by the use of the software Origin®, was the best model. This isotherm is commonly used for the experimental representation of the sorption of organic compounds, as veterinary drugs, on heterogeneous surfaces (e.g., soil and OM) [31, 32]. Table 2 lists the fit parameters.
The values obtained for the K f from Eq. 2 showed that soil with higher content of OM (T1) showed the highest sorption capacity—a L-type isotherm—which increases with increasing concentration of OTC in the aqueous medium [33]. K f values obtained were in accordance with those from literature for OTC sorption to temperate soils [24], demonstrating that this sorption was weak, probably due to a lower OM content. However, Kong et al. [34] also observed the need to calculate the K d partition coefficient for sorption and desorption of OTC to soils. Thus, K d was calculated for both soils according to Eq. 1 described above, with values shown in Table 3.
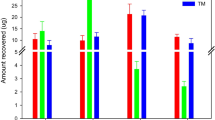
Figure 3 shows the graphic profile with the Kd for soil sample.
The behavior observed in Fig. 3 can be interpreted from the physicochemical characteristics of each soil. Soil texture (particle size), CEC, and OM content are the main factors influencing the OTC sorption to soil [24, 34]. Regarding the OM, probably its influence is limited to its content allied to the physicochemical particularities from its molecular composition, as considered by Schwarzenbach et al. [35].
To correlate the sorption profiles presented in Fig. 2 with the compositional analysis and physicochemical characterization in Table 1, it can be observed that the soil with higher content of OM and higher CEC (T1) showed the higher K d value (9290 kg L−1) in the presence of the higher initial concentration of OTC (120 mg L−1), probably due to the fact that the higher OTC concentration had a larger amount of sites available for intermolecular interactions. Another factor also considered for the largest value of K d for T1 is the greater presence of metallic ions, that leads to an increasing in the interaction between OTC and OM, since OTC is a strong chelating agent for metallic cations at pH about 5 [26, 36].
However, according to the values established by the Brazilian environmental legislation to evaluate the mobility capacity of chemicals in soils [37], K d values ranging from 0 to 24 kg L−1 feature a low sorption capacity to transport the OTC molecule from soil to groundwater by means of leaching process. The K d values in Table 3 were different from those observed in the literature for soils in other regions of the world [38], especially for the soil T1. However, these values expressed the behavior of OTC sorption to Brazilian soils.
Obeying the Freundlich isotherm, the sorption of OTC to soils occurred by means of the formation of multilayers [39], from the sorbed species (OTC) on the sorbent surface (soils).
Conclusions
There was a weak sorption of OTC to Brazilian soils, where it was dependent on the OM content, presenting a potential risk to the environment arising from their weak sorption capacity to such soils, making it available to transport the antibiotic to the groundwater. OTC adsorption to soil obeyed Freundlich isotherm (L-type), for a multilayer OTC deposition on the soil surface. From such findings, it is reasonable to assume that environmental agencies and legislative bodies should provide more attention to the presence of antibiotics in environmental matrices, which leads to the need for further studies related to this subject to produce data enabling stipulate the maximum values allowed for the presence in soil.
Abbreviations
- CEC:
-
cationic exchange capacity
- GP:
-
growth promoter
- HPLC:
-
high-performance liquid chromatography
- HS:
-
humic substances
- IUPAC:
-
International Union of Pure and Applied Chemistry
- LOQ:
-
limit of quantification
- OM:
-
organic matter
- OTC:
-
oxytetracycline
- K d :
-
partition coefficient
- K f :
-
sorption coefficient
- SOM:
-
soil organic matter
- UV:
-
ultraviolet
References
Environmental risk assessment for veterinary medical products other than GMO-containing and immunological products. http://ec.europa.eu/health/files/eudralex/vol-7/a/7ar1a_en.pdf.
Health Ministry of Brazil: Portaria n. 518, de 25 de março de. http://www.aeap.org.br/doc/portaria_518_de_25_de_marco_2004.pdf.
Guiding values for soil and groundwater in São Paulo State. http://solo.cetesb.sp.gov.br/solo/valores-orientadores-para-solo-e-agua-subterranea/.
Procedure for contaminated sites management in São Paulo State. http://cetesb.sp.gov.br/areas-contaminadas/wp-content/uploads/sites/45/2015/07/DD-103-07-C-E-Procedimento-para-Gerenciamento-de-%C3%81reas-Contaminadas.pdf.
Gavrilescu M, Demnerova K, Aamand J, Agathos S, Fava F. Emerging pollutants in the environment: present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnol. 2015;32:147–56.
Leblonde T, Cossu-Leguille C, Harteman P. Emerging pollutants in wastewater: a review of the literature. Int J Hyg Environm Health. 2011;214:442–8.
Martinez JL. Environmental pollution by antibiotics and by antibiotic resistance determinants. Environ Pollut. 2009;157:2893–902.
Kϋmmer K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere. 2009;75:417–34.
Van Boeckel TP, Brower C, Gilbert M, Grenfell BT, Levin AS, Robinson TP, Teillant A, Laxmirayan R. Global trends in antimicrobial use in food animals. Proc Natl Acad Sci USA. 2015;112:1–6.
Martínez-Caballo E, Gonzáles-Barreiro C, Scharf S, Gans O. Environmental monitoring study of selected veterinary antibiotics in animal manure and soils in Austria. Environ Pollut. 2007;148:570–9.
Santos DMS, Berchiery-Jr A, Fernandes AS, Tavechio AT, Amaral LA. Salmonella in frozen poultry carcasses. Pesquisa Veterinária Brasileira. 2000;20:39–42.
Rutz F, Lima GJMM. The use of antimicrobials as growth promoters in Brazil. In: Congress of the Brazilian association of poultry breeders. Porto Alegre; 2001.
Chee-Sandford JC, Aminov RI, Krapac IJ, Garrigues-Jeanjean N, Mackie RI. Occurrence and diversity of tetracycline resistance genes in lagoons and groundwater underlying two swine production facilities. Appl Environ Microbiol. 2001;67:1494–502.
Liou F, Ying G-G, Tao R, Zhao J-L, Yang J-F, Zhao L-F. Effects of six selected antibiotics on plant growth and soil microbial and enzymatic activities. Environ Pollut. 2009;157:1636–42.
Boxall ABA, Johnson P, Smith EJ, Sinclair CJ, Stutt E, Levy LS. Uptake of veterinary medicines from soils into plants. J Agric Food Chem. 2006;54:2288–97.
Venglovsky J, Sasakova N, Placha I. Pathogens and antibiotic residues in animal manures and hygienic and ecological risks related to subsequent land application. Bioresour Technol. 2009;100:5386–91.
Wang Q, Yates SR. Laboratory study of oxytetracycline degradation kinetics in animal manure and soil. J Agric Food Chem. 2008;56:1683–8.
Andrade NA. Myths and truths about the use of antibiotics in animal feed. Jornal do Conselho Regional de Medicina Veterinária—RJ. 2007;186:4–5.
Blanco G, Lemus JA, Grande J. Microbial pollution in wildlife: linking agricultural manuring and bacterial antibiotic resistance in red-billed choughs. Environ Res. 2009;109:405–12.
Stevenson FJ. Humus chemistry: genesis, composition, reaction. 2nd ed. New York: Willey; 1994.
Clapp CE, Hayes MHB, Senesi N, Bloom PR, Jardine PM, editors. Humic substances and chemical contaminants. Madison: Soil Society of America; 2001.
Vaz S Jr, Lopes WT, Martin-Neto L. Study of molecular interactions between humic acid from Brazilian soil and the antibiotic oxytetracycline. Environ Technol Innov. 2015;4:260–7.
Chemical analysis for assessment of soil fertility. http://hotsites.cnps.embrapa.br/blogs/paqlf/wp-content/uploads/2008/08/analises_quimicas_fertilidade.pdf.
Jones AD, Bruland GL, Agrawal SG, Vasudevan D. Factors influencing the sorption of oxytetracycline to soils. Environ Toxicol Chem. 2005;24:761–70.
Mitscher LA. The chemistry of the tetracycline antibiotics, vol. 9. New York: Marcel Dekker; 1978.
Loke M-L, Tjørnelund J, Halling-Sørensen B. Determination of the distribution coefficient (logK d) of oxytetracycline, tylosin A, olaquindox and metronidazole in manure. Chemosphere. 2002;48:351–61.
Gold book. http://goldbook.iupac.org/.
Giles CH, Smith D, Huitson A. A general treatment and classification of the solute adsorption isotherm. I. Theoretical. J. Colloid Interface Sci. 1974;47:755–65.
Ribani M, Bottoli CB, Collins CH, Jardim ICSF, Melo LF. Validation in chromatographic and electrophoretic methods. Quim Nova. 2004;27:771–80.
Hinz C. Description of sorption data with isotherm equations. Geoderma. 2001;99:225–43.
Ferreira JA, Martin-Neto L, Vaz CMP, Reginato JB. Sorption interactions between imazaquin and a humic acid extracted from a typical Brazilian oxisol. J Environ Qual. 2002;31:1665–70.
Yu L, Fink G, Wintgens T, Melin T, Ternes TA. Sorption behavior of potential organic wastewater indicators with soils. Water Res. 2009;43:951–60.
Perzynski GM, Sims JT, Vance GF. Soil and environmental quality. 3rd ed. Boca Ranton: CRC Taylor and Francis; 2005.
Kong W, Li C, Dolphi JM, Li S, He J, Qiao M. Characteristics of oxytetracycline sorption and potential bioavailability in soils with various physical–chemical properties. Chemosphere. 2012;87:542–8.
Schwarzenbach RP, Gschwend PM, Imboden DM. Environmental organic chemistry. 2nd ed. Hoboken: Wiley; 2003. p. 55–458.
Couto CMCM, Montenegro CBSM, Reis S. Tetracycline, oxytetracycline and chlortetracycline complexation with the cation coper (II). Química Nova. 2000;23:457–60.
Guideline for evaluation of ecotoxicity of chemical agents. http://www.ibama.gov.br/areas-tematicas/qualidade-ambiental.
Atkins P, de Paula J. Atkins’s physical chemistry. 8th ed. Oxford: Oxford University Press; 2006. p. 916–22.
Tolls J. Sorption of veterinary pharmaceuticals in soils: a review. Environ Sci Technol. 2001;35:3397–406.
Acknowledgements
The author thanks the Institute of Chemistry of São Carlos—University of São Paulo, Embrapa Instrumentation, FAPESP and CNPq for the facilities and funds. The author thanks also Dr. Tony Jarbas Ferreira Cunha (Embrapa Semi-Arid) for the scientific review.
Competing interests
The author declares that he has no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Vaz, S. Sorption behavior of the oxytetracycline antibiotic to two Brazilian soils. Chem. Biol. Technol. Agric. 3, 6 (2016). https://doi.org/10.1186/s40538-016-0056-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40538-016-0056-6