Abstract
The binary-flashing cycle (BFC) is supposed to be a promising technology for geothermal recovery due to the full use of geofluid. For further performance improvement, the potential of using mixtures of a hydrocarbon and a retardant in the BFC system is investigated. R245fa is selected as a retardant and blended with R600 to form zeotropic mixtures. With the thermal efficiency (ηth), exergy efficiency (ηex), net power output per ton geofluid (PRW), and exergy destruction rate (Ed) as evaluation indexes, the flowsheet modeling and optimization are conducted to explore the optimal compositions and operating parameters. It is revealed the optimal mass fraction of R600/R245fa is 0.44/0.56, at which the flammability of R600 is suppressed, the global warming potential (GWP) of R245fa is reduced. The maximum ηex and PRW are higher than those of component fluids. The maximum Ed occurs in the heat exchanger, which should be optimized. The recommended generation pressures are 1200–1430, 1240–1480, 1220–1460, and 1170–1420 kPa, respectively, for R600 mass fraction of 0.2, 0.4, 0.6, and 0.8. In addition, the flashing temperature is also optimized. Although the mixtures do not always yield superior performance, it is still beneficial to apply the mixtures to the BFC system through systematic consideration of safety and environmental friendliness.
Similar content being viewed by others
Introduction
The accelerated energy demand causes growing consumption of fossil fuels and massive discharge of pollutants, which promotes the development of renewable energy utilization. Among other renewable energy sources, the geothermal energy has the advantages of reliability, sustainability, high capacity factor and less ecological effect (Liu et al. 2017). Over 70% of the geothermal sources available in the world are of low-enthalpy with the temperature lower than 150 °C (Franco and Vaccaro 2014). As an efficient technology in the exploitation of the low–medium temperature geothermal energy, Organic Rankine Cycle (ORC) systems have drawn great attention (Pollet et al. 2018; Sadeghi et al. 2016).
Nevertheless, the thermal efficiency of the ORC system is generally less than 12% (Basaran and Ozgerer 2013), which is a serious obstacle for further application. It is not well-optimized from the view of thermodynamics. One major disadvantage of the ORC is the complete evaporation of working fluid accounting for large amount of the total input heat. As a consequence, the evaporation temperature and pressure are relatively low, leading to lower power output. As a modification, the binary-flashing cycle (BFC) is proposed to be an efficient system to overcome such a problem (Edrisi and Michaelides 2013; Liu et al. 2018; Michaelides and Scott 1984; Michaelides 2016; Shi and Michaelides 1989; Wang et al. 2016, 2018; Yuan and Michaelides 1993). The working fluid is not evaporated completely in the BFC system. And lesser heat rate is demanded in the evaporation process. As a consequence, the evaporation temperature and pressure are higher than those in the ORC system. The liquid-state working fluid from the evaporator is flashed in the flashing tank to produce more steam at a lower temperature. And an additional amount of power is obtained.
Up to date, the research focusing on the BFC is very limited. Michaelides and Scott (1984) analyzed the geothermal BFC system employing freon, ammonia, and isobutene as working fluids. It is indicated the BFC system would produce 20% more power than the ORC system. The superior thermodynamic performance of the BFC system is also found in Shi and Michaelides (1989). Yuan and Michaelides (1993) found that BFC system may provide up to 25% more work than the conventional ORC system under the optimum operation conditions. Edrisi and Michaelides (2013) compared several pure working fluids, including normal butane, isobutane, hexane, pentane, refrigerant-114, and ammonia, employed in the BFC system. It is suggested that among the six working fluids, hexane and pentane appear to be a better choice. Michaelides (2016) found that the overall entropy production is reduced in the BFC system, which is proposed to be the future cycle for geothermal power generation system. Wang et al. (2018) proposed a BFC system with a regenerator to recover the waste heat of the working fluid at the turbine exit. And the working fluid selection was conducted. Wang et al. (2016) carried out the comparison among the ORC, double-evaporator ORC and BFC. The thermal efficiency, power output per ton geothermal brine, and reinjection temperature of geothermal brine were selected as the evaluation indicators. Liu et al. (2018) investigated the performance characteristics and conducted the working selection for the BFC. It is indicated that there exists an optimum generation temperature and flashing temperature at which the system achieves the optimal performance.
A brief literature survey reveals that all the investigations on the BFC system focused solely on pure working fluids. The pure working fluids in BFC have a large entropy generation during evaporation and condensation process, due to the isothermal behavior. Fortunately, the issue can be partially solved by zeotropic mixtures, which exhibits non-isothermal characteristics during heat transfer process. Great efforts have been devoted to the using of the zeotropic mixtures in the ORC systems. The superiority of the zeotropic mixtures applying in the ORC system has been generally recognized (Dong et al. 2018; Harby 2017; Zhai et al. 2018).
However, none of the previous work investigated the BFC system using the zeotropic mixtures as a working fluid. To fill up this research gap, the objective of the present study is to verify the potential benefits of the zeotropic mixtures in the BFC system. Due to the excellent thermal performance and zero ozone depletion potential (ODP), R245fa is recommended as the working fluid in the ORC system (Feng et al. 2017; Imran et al. 2014; Liu et al. 2013). And it is frequently used in the experimental study (Dong et al. 2017; Galloni et al. 2015; Guillaume et al. 2017). However, it has relatively high global warming potential (GWP), which has certain unfavorable effects on the environment. The hydrocarbons are attractive due to the highest thermal performance and cheapness (Li et al. 2017; Song and Gu 2015). However, the flammability and chemical instability of the hydrocarbons limit their practical applications (Budisulistyo and Krumdieck 2015). In the present study, a blending of hydrocarbons and R245fa is proposed. Furthermore, the mixtures have the potential to suppress the flammability of the hydrocarbon and reduce the GWP of R245fa. The thermophysical properties of R245fa and R600 are listed in Table 1. The first law efficiency, the second law efficiency, and power capability per unit ton geofluid are chosen as the evaluation criteria.
BFC system description
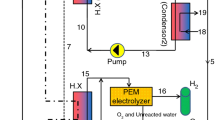
The BFC system mainly consists of two preheaters, a vapor generator, a separator, a high-pressure expander, a flash drum, a low-pressure expander, a condenser, and two working fluid pumps. A schematic diagram of the BFC system is depicted in Fig. 1. For simplicity, only one expander is depicted. The geothermal brine flows into the vapor generator and heats up the working fluid. The working fluid is partially vaporized at a predetermined dryness fraction. The gas–liquid mixture is sent to the separator, where the gas is separated from the mixture and flows into the high-pressure expander to produce work. The remaining liquid is sent to the flash drum, where it is flashed at a lower temperature and pressure. An additional quantity of vapor is produced and fed to the low-pressure expand to produce more work. The remaining liquid working fluid released from the flash drum is pumped to the preheater 2 and is heated by the geothermal brine from the vapor generator. The working fluid from the high-pressure and low-pressure expand is condensed to liquid state in the condenser by the cooling water. Subsequently, the working fluid is pumped to preheater 1, where the working fluid is heated by the geothermal brine from preheater 2. Then the left geothermal brine is re-injected to the reservoir. Finally, the working fluid from preheater 2 is sent to the vapor generator to continue cycle operation.
Thermodynamic model
The model is established on the typical simplifying assumptions as follows (Dai et al. 2016):
- 1.
All processes are operated under steady-state conditions;
- 2.
Pressure loss and heat dissipation in all components and pipelines are negligible except for the expander and pump;
- 3.
Friction losses, kinetic energy, and potential energy are neglected;
- 4.
Saturated liquid is assumed at the condenser exit;
- 5.
The irreversibility related to the separator is ignored;
- 6.
The geothermal brine has the properties of pure water.
Based on the assumptions above, a set of basic equations and the specific thermodynamic processes are described as follows.
The evaporator contains three components, i.e., preheater 1, preheater 2, and vapor generator.
For the preheater 1
For the preheater 2
For the vapor generator
The total heat transfer rate absorbed by the evaporator can be written by:
where Q is the heat transfer rate, kW; m is the mass flow rate, kg/s; cp is the specific heat capacity, kJ/(kg K); T is the temperature, °C; h is the specific enthalpy, kJ/kg; X is the dryness fraction; the subscripts “hf”, “wf”, “Pre1”, “Pre2”, “in”, “gen” and “fsh” denote geothermal brine, working fluid, preheater 1, preheater 2, vapor generator and flash drum, respectively; the subscripts “in” and “out” represent the inlet and exit of the components, respectively. The subscript numbers of the equations presented in this section represent the working state points in the cycle as shown in Fig. 1.
For the separator
For the flash drum
For the high-pressure expander
The power produced by the expander is given by:
For the condenser
where the subscripts “con” and “cf” denote the condenser and cooling water, respectively.
For the pump 1
The required power of the pump is given by:
where the subscript “pup” denotes the working fluid pump.
For the pump 2
Evaluation indicators
The net power output is expressed by:
where the subscript “net” represents net output.
The thermal efficiency is written by:
PRW is defined as the ratio of net power output to unit ton geothermal brine, which reflects the power capability per ton geothermal brine.
where mwat is the mass flow rate of geothermal brine, t/h.
The exergy efficiency is defined as the ratio of the net output power to input exergy:
The input exergy is expressed by:
The exergy destruction rate of the components can be expressed as follows:
The total exergy destruction rate is expressed by:
where ED is the exergy destruction rate, kW; T0 is the ambient temperature, °C; the subscript “m” represents the thermodynamic mean value.
It should be noted that the typical flashing temperature is approximately equal to the average of the generation and condensation temperature (Edrisi and Michaelides 2013).
There exists a practical flashing temperature in the BFC system. To investigate the influence of flashing temperature on the system performance conveniently, a symbol is defined as temperature difference between the practical flashing temperature and the typical flashing temperature:
where Tfsh_rea is the practical flashing temperature, °C.
Based on the above assumptions and equations, simulations and optimizations of zeotropic fluid composition are carried out by MATLAB software. All of the properties of the working fluids are acquired from REFPROP 9.1 (Lemmon et al. 2013). The operating conditions and parameters are listed in Table 2. Note that the analysis is performed by varying one parameter at a time while keeping the others as the typical values given in Table 2.
Model validation
Due to the lack of studies on BFC system running with the zeotropic mixtures, the validation of the present calculating program is conducted based on the results of pure working fluid. The ηth and PRW against Tgen are calculated by the present model and compared with the research investigated by Liu et al. (2018) under the same working condition using R245fa. The comparison result is depicted in Fig. 2. It can be observed that the maximum deviations of the ηth and PRW between the present solutions and results from Liu et al. (2018) are less than 3.3% and 1.5%, respectively. It exhibits a good agreement between the developed model and published reference values. The present model can be applied to predict the BFC system performance using the zeotropic mixtures within the acceptable error range.
Results and discussion
The Ωbue represents the mass fraction of R600 in the mixtures. The effect of Ωbue on the ηth and ηex is illustrated in Fig. 3. It is obvious that the ηth and ηex firstly decrease and then increase with the increment of Ωbue. And the ηth and ηex present remarkable similar variation tendency. The ηth of the mixtures remains higher than that of R245fa until Ωbue exceeds 0.36. The ηex reaches the maximum of 43.62% at 0.44 for Ωbue. When the Ωbue is larger than 0.24, the mixtures always yield higher ηex than their component fluids. Specific attention should be paid to the rebound and fluctuation in ηth as Ωbue approaches to 1. The mixtures play a more important role in raising ηex than ηth. Zabetakis (1965) studied the flammability envelopes of the mixtures of hydrocarbons and nonflammable working fluids. It is indicated that 0.3 volume fraction of retardants is adequate to make mixture nonflammable. The molecular weight of R245fa (134.05 g/mol) is much larger than that of R600 (58.12 g/mol). When the mass fraction of R245fa is 0.49, it is enough to suppress the flammability of the mixtures. Shu et al. (2014) proposed the mass fraction of retardants ranging from 0.3 to 0.7 in the ORC system, with the hydrocarbons and refrigerant retardants mixtures as working fluid. According to Ding (1997), the GWP of the mixtures is weighted by the mass fraction of each component. The blending with a certain amount of R600 is beneficial to reduce the GWP relative to R245fa employed alone. That is to say, when the ηth and ηex obtain the optimal values, the R245fa and R600 mixtures bestow with merits of both fluids sans their flammability and large GWP demerits.
Figure 4 shows the variations of Ed and PRW with respect to Ωbue. The PRW firstly decreases, then increases, and at last decreases slowly. The mixtures always present higher PRW in the range of 0.24–0.94 for Ωbue. The PRW achieve the maximum (7.14) at 0.44 for Ωbue. It is quite clear that the ηex and PRW get the maximal value simultaneously at 0.44 for Ωbue4. The maximal PRW of the mixtures is 9.2% and 3.5% higher than that of R245fa and R600, respectively. While an opposite trend is exhibited for Ed. The Ed increases rapidly, then decreases drastically, and at last tends to stable and fluctuates. The Ωbue should be not less than 0.32 to avoid larger Ed.
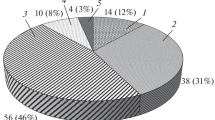
The Ed distribution in the BFC system with Ωbue of 0.44 is illustrated in Fig. 5. Note that the Ed of the separator is ignored. From the distribution pie chart, it is observed the maximum Ed occurs in evaporator, followed by the condenser, flash drum, expander, pump 1 and pump 2 in sequence. The Ed of the heat exchangers, i.e., evaporator and condenser, accounts for 57.37% of the total Ed. Therefore, more attention should be paid to cut down the Ed of the heat exchangers. The study on heat transfer mechanism and heat exchanger design should be enhanced. The Ed of the two working fluid pumps is both very low, accounting for only 1.34% and 0.91% of the Ed, respectively. That is to say the working fluid pump has little impact on the system performance.
The variation of ηth with Pgen under different Ωbue is presented in Fig. 6. For all the Ωbue examined, ηth firstly rises dramatically, reaches the maximum, and then declines slowly with the increased Pgen. Under a specific working condition, i.e., a certain flashing temperature and condensation temperature, the rise in Pgen causes the specific enthalpy drop across the turbine increasing, while it will raise the mass flow rate of the mixtures. With the two conflicting influences, an optimum Pgen exists and makes the ηth obtain the maximum value, which is well in conformity with Edrisi and Michaelides (2013). The optimum Pgen is 1430, 1480, 1460, and 1420 kPa for Ωbue of 0.2, 0.4, 0.6 and 0.8, respectively. The optimum Pgen is in a narrow range for different Ωbue.
The variation of PRW with Pgen under different Ωbue is depicted in Fig. 7. As can be seen, PRW emerges a curve first going up slowly then down remarkably. The optimum Pgen is 1200, 1240, 1220 and 1170 kPa for Ωbue of 0.2, 0.4, 0.6, and 0.8, respectively. It is obvious that the corresponding optimum Pgen of the maximum PRW is much lower than that of maximum ηth under the same Ωbue. The discharge temperature of the geothermal brine increases with increase in Pgen, leading to the drop of the input heat flux in the evaporator. Initially, the growth rate of ηth is larger than the decrease rate of input heat flux in the evaporator, PRW is increased. It can be discovered from Fig. 6 that when Pgen is less than the corresponding optimum Pgen, the growth rate of ηth is decreased. When the growth rate of ηth is less than the decrease rate of input heat flux in the evaporator, PRW is decreased. In Fig. 7, when Pgen is larger than the corresponding optimal Pgen of maximum ηth, the PRW decreases remarkably. The ηth and PRW cannot reach the maximum simultaneously. It is imperative to conduct multi-objective optimization to acquire the most suitable Pgen. The recommended Pgen are 1200–1430, 1240–1480, 1220–1460, and 1170–1420 kPa, respectively, for Ωbue of 0.2, 0.4, 0.6, and 0.8.
The variation of ηex with Pgen under different Ωbue is presented in Fig. 8. The ηex presents a similar variation tendency with the PRW when Pgen increases. Although there both exist optimum Pgen for the ηth and ηex, the variation trend has significant discrepancies. The optimum Pgen of the ηex is the same with that of PRW with the same Ωbue. The variation of Ed with Pgen under different Ωbue is presented in Fig. 9. As expected, as shown in Fig. 9, the variation tendency of the Ed runs opposite with that of PRW. The optimum Pgen are 1410, 1460, 1440, and 1390 kPa at 0.2, 0.4, 0.6, and 0.8 for Ωbue, respectively, which are slightly lower than those of maximum ηth.
Due to the adding of the separator and flashing tanking, the BFC system is equivalent to the coupling of the ORC system and organic working fluid flashing system. An additional Tfsh is required to be optimized. Figure 10 exhibits the variation of ηth with δT under different Ωbue. It can be observed that under various Ωbue, the ηth keeps going up almost linearly with the increasing of δT. The increase in δT means a raise of Tfsh. It corresponds to enlarge the Tfsh of the organic working fluid flashing system with the ORC system unchanged. It will be easy to understand the increase in the ηth. It is conceivable that when the Tfsh is equal to Tgen, the ηth reach the maximum, and the BFC system becomes to the ORC system. Take Ωbue = 0.4 as example, the ηth is improved by 28.3% with δT of 10 °C than that of − 10 °C.
Figure 11 illustrates the effect of δT on PRW with different Ωbue. It can be seen that the PRW initially increases and attains maximum value then decreases with the rising Ωbue. The increase in Tfsh causes the decline of the mass flow rate of working fluid vapor, and rise of specific enthalpy drop across the turbine, under a specific working condition, i.e., a certain Pgen and Tcon. With the interaction between the two effects, an optimum Tfsh exists and makes the PRW obtain the maximum value, which is well in conformity with Edrisi and Michaelides (2013). For all the Ωbue investigated, the PRW has a peak value with δT of 3 °C.
Figure 12 presents the influence of δT on the ηex with different Ωbue. The ηex firstly increases and then decreases for all Ωbue. There exists a δT at which the ηex achieve the maximum. The optimum δTs at 0.2, 0.4, 0.6 and 0.8 for Ωbue are 4, 3, 3, and 3 °C, respectively. As sated above, Wnet first rises and then declines. It is known that the raising Tfsh leads to the increase in geothermal brine reinjection temperature, which results in the decrease in input exergy. So the ηex increases significantly firstly. Afterward, because the decreased range of Wnet is larger than that of input exergy, ηex is decreased. By comprehensive analysis of Figs. 6 and 7, there exists an optimum δT of 3 °C at which the PRW and ηex achieve the maximum simultaneously.
The influence of δT on Ed is displayed in Fig. 13, and it can be seen that the Ed almost decreases linearly with the increase in δT. The increase in δT leads to the decrease in input heat of evaporator, and the degree of irreversibility in the heat transfer process is decreased. The Ed drops by 22.5% on average when δT increases from − 10 to 10 °C.
Conclusions
In the present study, a geothermal BFC system employing the zeotropic working fluid is explored theoretically. One of the contributions is the potential of applying R245fa and R600 mixtures in the BFC system. The following conclusions can be drawn:
- 1.
The ηth of the mixtures remains higher than that of R245fa until Ωbue exceeds 0.36. And the maximum ηth is slightly smaller than that of R600. The maximum ηex and PRW are higher than the component fluids, and reach the maximum simultaneously at 0.44 for Ωbue. In order to avoid larger Ed, the Xbue should be not less than 0.32. The optimal Ωbue is 0.44, at which the flammability of R600 is suppressed, and the GWP of R245fa is reduced.
- 2.
The maximum exergy destruction rate of the BFC system occurs in evaporator, followed by the condenser, flash drum, expander, pump 1 and pump 2 in sequence. The exergy destruction rate in the heat exchangers accounts for 57.37% of the total exergy destruction rate.
- 3.
For all the Ωbue examined, ηth, PRW and ηex firstly increase, then decline with the rising Pgen. And the variation of the Ed presents the opposite trend. The ηex and PRW attain superior under the same Pgen. The ηth and Ed gain the optimal values simultaneously on the whole. The recommended Pgen are 1200–1430, 1240–1480, 1220–1460, and 1170–1420 kPa at 0.2, 0.4, 0.6 and 0.8 for Ωbue.
- 4.
The ηth almost increases linearly with the increasing δT under various Ωbue. The ηex and PRW firstly increase and then decrease and reach the maximum under the same δT (3 °C). The Ed almost decreases linearly with the raising of δT.
Generally speaking, the mixtures may not always show outstanding thermal performance. Nevertheless, the mixtures can overcome the shortcomings of the component and extend the selection range of working fluid from the viewpoint of environmental impacts and safety aspects. The application of R245fa/R600 mixtures applying the BFC system is beneficial.
Finally and as future work, with the maximum ηth, maximum PRW and minimum capitalized cost as objective function, Ωbue, Pgen, δT, and perhaps other operating parameters as decision variables, multi-objective optimization will be conducted. What is more, the economic feasibility of the zeotropic mixtures employing in BFC system will be carried out.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- c p :
-
specific heat capacity (kJ/kg K−1)
- E D :
-
exergy destruction rate (kW)
- E in :
-
input exergy (kW)
- h :
-
specific enthalpy (kJ/kg)
- m :
-
mass flow rate (kg/s)
- P :
-
pressure (kPa)
- Q :
-
heat transfer rate (kW)
- T :
-
temperature (oC)
- PRW:
-
power output per unit geothermal brine mass flow rate (kW/t)
- qu:
-
quality
- W:
-
work (kW)
- η :
-
efficiency
- δ :
-
difference value
- 0:
-
ambient condition
- 1, 2, …, 12:
-
state points
- cf:
-
cooling water
- con:
-
condenser
- evp:
-
evaporator
- exp:
-
expander
- fsh:
-
flash tank
- gen:
-
vapor generator
- hf:
-
heat source fluid
- in:
-
inlet
- m:
-
thermodynamic mean value
- net:
-
net output
- out:
-
outlet
- Pre1:
-
preheater 1
- Pre2:
-
preheater 2
- pup1:
-
pump 1
- pup2:
-
pump 2
- s:
-
isentropic process
- tot:
-
total
- wat:
-
water
- wf:
-
working fluid
- BFC:
-
binary flashing cycle
- ODP:
-
ozone depletion potential
- ORC:
-
Organic Rankine Cycle
- GWP:
-
global warming potential
References
Basaran A, Ozgerer L. Investigation of the effect of different refrigerants on performances of binary geothermal power plants. Energy Convers Manag. 2013;76:483–98.
Budisulistyo D, Krumdieck S. Thermodynamic and economic analysis for the pre-feasibility study of a binary geothermal power plant. Energy Convers Manag. 2015;103:639–49.
Dai XY, Shi L, An QS, Qian WZ. Screening of hydrocarbons as supercritical ORCs working fluids by thermal stability. Energy Convers Manag. 2016;126:632–7.
Ding GL. Alternatives to CFCs for refrigeration appliances in Germany and TEWI. Shanghai: Annual conference of Shanghai Refrigeration Society; 1997. p. 153–7.
Dong JQ, Zhang XH, Wang JZ. Experimental investigation on heat transfer characteristics of plate heat exchanger applied in organic Rankine cycle (ORC). Appl Therm Eng. 2017;112:1137–52.
Dong BS, Xu GQ, Li TT, Quan YK, Wen J. Thermodynamic and economic analysis of zeotropic mixtures as working fluids in low temperature organic Rankine cycles. Appl Therm Eng. 2018;132:545–53.
Edrisi BH, Michaelides EE. Effect of the working fluid on the optimum work of binary-flashing geothermal power plants. Energy. 2013;50:389–94.
Feng YQ, Hung TC, He YL, Wang Q, Wang S, Li BX, Lin JR, Zhang WP. Operation characteristic and performance comparison of organic Rankine cycle (ORC) for low-grade waste heat using R245fa, R123 and their mixtures. Energy Convers Manag. 2017;144:163.
Franco A, Vaccaro M. Numerical simulation of geothermal reservoirs for the sustainable design of energy plants: a review. Renew Sustain Energy Rev. 2014;30:987–1002.
Galloni E, Fontana G, Staccone S. Design and experimental analysis of a mini ORC (organic Rankine cycle) power plant based on R245fa working fluid. Energy. 2015;90:768–75.
Guillaume L, Legros A, Desideri A, Lemort V. Performance of a radial-inflow turbine integrated in an ORC system and designed for a WHR on truck application: an experimental comparison between R245fa and R1233zd. Appl Energy. 2017;186:408–22.
Harby K. Hydrocarbons and their mixtures as alternatives to environmental unfriendly halogenated refrigerants: an updated overview. Renew Sustain Energy Rev. 2017;73:1247–64.
Imran M, Park BS, Kim HJ, Dong HL, Usman M, Heo M. Thermo-economic optimization of regenerative organic Rankine cycle for waste heat recovery applications. Energy Convers Manag. 2014;87:107–18.
Li L, Ge YT, Luo X, Tassou SA. Experimental investigations into power generation with low grade waste heat and R245fa Organic Rankine Cycles (ORCs). Appl Therm Eng. 2017;115:815–24.
Liu Q, Duan Y, Yang Z. Performance analyses of geothermal organic Rankine cycles with selected hydrocarbon working fluids. Energy. 2013;63:123–32.
Liu XM, Wei M, Yang LN, Wang X. Thermo-economic analysis and optimization selection of ORC system configurations for low temperature binary-cycle geothermal plant. Appl Therm Eng. 2017;125:153–64.
Liu X, Li HS, Bu XB, Wang LB, Xie N, Zeng J. Performance characteristics and working fluid selection for low-temperature binary flashing cycle. Appl Therm Eng. 2018;141:51–60.
Michaelides EE. Future directions and cycles for electricity production from geothermal resources. Energy Convers Manag. 2016;107:3–9.
Michaelides EE, Scott GJ. A binary-flashing geothermal power plant. Energy. 1984;9:323–31.
Lemmon EW, Huber MH, McLinden MO. REFPROP, NIST Standard Reference Database 23. Version 9.1, USA. 2013.
Pollet M, Gosselin L, Dallaire J, Mathieu Potvin F. Optimization of geothermal power plant design for evolving operating conditions. Appl Therm Eng. 2018;134:118–29.
Sadeghi M, Nemati A, Ghavimi A, Yari M. Thermodynamic analysis and multi-objective optimization of various ORC (organic Rankine cycle) configurations using zeotropic mixtures. Energy. 2016;109:791–802.
Shi H, Michaelides EE. Binary dual-flashing geothermal power plants. Int J Energy Res. 1989;13:127–35.
Shu GQ, Gao YY, Tian H, Wei HQ, Liang XY. Study of mixtures based on hydrocarbons used in ORC (Organic Rankine Cycle) for engine waste heat recovery. Energy. 2014;74:428–38.
Song G, Gu CW. Analysis of ORC (Organic Rankine Cycle) systems with pure hydrocarbons and mixtures of hydrocarbon and retardant for engine waste heat recovery. Appl Therm Eng. 2015;89:693–702.
Wang YX, Wang LB, Li HS, Bu XB. Thermodynamic calculation and optimization of geothermal power generation in Ganzi. J Harbin Eng Univ. 2016;37:873–7.
Wang LB, Bu XB, Li HS, Wang HZ, Ma WB. Working fluids selection for flashing organic rankine regeneration cycle driven by low-medium heat source. Environ Prog Sustain Energy. 2018;37:1201–9.
Yuan Z, Michaelides EE. Binary-flashing geothermal power plants. J Energy Resour Technol. 1993;115:232–6.
Zabetakis MG. Flammability characteristics of combustible gases and vapor. Washington DC: Bureau of Mines; 1965.
Zhai HX, An QS, Shi L. Zeotropic mixture active design method for organic Rankine cycle. Appl Therm Eng. 2018;129:1171–80.
Acknowledgements
We express our gratitude to W.B. Ma for technical support while writing the MATLAB program.
Funding
The authors gratefully acknowledge the financial supports provided by the National Key R&D Program of China (No. 2018YFB1501805), and Natural Science Foundation of Guangdong Province (No. 2016A030313174), and National Natural Science Foundation of China (No. 41972314).
Author information
Authors and Affiliations
Contributions
LW built the workflow of the BFC, designed the model, run all simulations on MATLAB, and drafted the manuscript. XB provided guidance and insight into evaluating the results and his experience in the use of MATLAB. HL provided guidance and insight into evaluating the results and helped drafting the manuscript. All authors contributed to the preparation of the manuscript and the interpretation of the data and model results. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, L., Bu, X. & Li, H. Investigation on geothermal binary-flashing cycle employing zeotropic mixtures as working fluids. Geotherm Energy 7, 36 (2019). https://doi.org/10.1186/s40517-019-0153-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40517-019-0153-4