Abstract
NF2 alteration is the most commonly–found genetic abnormality in meningiomas and is known to initiate events for aggressive-type meningiomas. Whereas the prognosis of meningiomas differs depending on their epigenomic/transcriptomic profile, the effect of NF2 alteration on the prognosis of benign meningiomas is not fully elucidated. This study aimed to probe the importance of NF2 alteration in prognosis of WHO grade I meningiomas. A long-term retrospective follow-up (5.3 ± 4.5 years) study involving 281 consecutive WHO grade I meningioma patients was performed. We assessed tumour recurrence in correlation with extent of resection (EOR), histopathological findings, tumour location, and NF2 alteration. “NF2 meningioma” was defined as meningiomas with presence of NF2 mutation and/or 22q loss. Overall, NF2 meningioma per se was not a predictor of prognosis in the whole cohort; however, it was a predictor of recurrence in supratentorial meningiomas, together with EOR and Ki-67. In a striking contrast, NF2 meningioma showed a better prognosis than non-NF2 meningioma in infratentorial lesion. Supratentorial NF2 meningiomas had higher Ki-67 and forkhead box protein M1 expression than those of others, possibly explaining the worse prognosis in this subtype. The combination of NF2 alteration, high Ki-67 and supratentorial location defines subgroup with the worst prognosis among WHO grade I meningiomas. Clinical connotation of NF2 alteration in terms of prognosis of WHO grade I meningioma differs in an opposite way between supratentorial and infratentorial tumors. Integrated anatomical, histopathological, and genomic classifications will provide the best follow-up schedule and proactive measures.
Similar content being viewed by others
Introduction
Meningiomas are the most common primary central nervous system tumours in the adult population, accounting for approximately 30% of intracranial tumours [1, 2]. Meningiomas originate at the meningeal arachnoid leaf, and are histologically benign in the majority of cases (75% are grade I per WHO classification) [3]. The remaining 25% of meningiomas, categorized as grade II or III per WHO classification, show a rapid growth and high recurrence rate (in WHO grade II, roughly 50% recurrence at 10 years, in WHO grade III, almost 100% recurrence at 10 years) [3]. In general, a 2nd surgery or radiotherapy are common complementary treatment practices for recurrent meningiomas in addition to surgical resection, especially in case of WHO grade II or III meningiomas [3]. However, WHO grade I meningiomas are also known to recur, with a sobering report of around 24–60% recurrence rate with long-term follow-up [1, 3,4,5,6,7,8]. Despite these reports, observation with or without imaging follow-ups remains commonplace following gross total resection (GTR) and subtotal resection (STR) in WHO grade I meningioma [1]. Thus, for the therapeutic management of WHO grade I meningiomas, identification of cases that are at a higher risk of and of the indicators of recurrence is crucial for making a right decision at the right timing in performing the complementary treatment.
According to previous reports, the extent of resection (EOR), Ki-67 index, and WHO grades based on histological findings are known as predictors of meningioma recurrence [4,5,6,7,8,9,10,11,12,13]. Many of these studies have focused on the clinical characteristics of atypical or malignant meningiomas, while recurrence of WHO grade I meningiomas has not yet been fully elucidated [13, 14].
Recent molecular analyses have shown that meningiomas are not genomically homogenous but have various genetic, epigenetic, and transcriptomic profiles [16,17,18,19,20,21,22,23,24,25]. For example, driver gene mutations are known to be highly dependent on the anatomical location of meningiomas [16,17,18,19,20,21]. Neurofibromatosis 2 (NF2) alteration is the most common genetic driver in sporadic meningiomas and is known to initiate events for aggressive-type meningiomas. However, the effect of driver gene mutations, especially NF2 alteration, on the prognosis of meningiomas has not been clearly established, except a few reports stating that their prognosis in all WHO grade meningiomas differs depending on the epigenomic/transcriptomic profiles [26,27,28,29,30,31]. Furthermore, tumour location has to be taken into account in considering recurrence of WHO grade I meningiomas because the location of the tumour is also related to its epigenetic profile [24, 32].
In this study, we aimed to investigate the clinical significance of NF2 alteration in the prognosis of WHO grade I meningiomas. We performed a long-term follow-up study to validate the effect of EOR, the significance of NF2 alteration, and tumour anatomical location on meningioma recurrence.
Materials and methods
Patient population
Patients who underwent surgical resection of sporadic meningioma between 2000 and 2019 were retrospectively queried using an institutional database. The study protocol was approved by our Institutional Review Board (G10028), and informed consent was obtained from all the patients. Patients with incomplete clinical or genetic data, any previous history of meningioma treatment, or any history of radiation therapy for the remaining tumour immediately after the first surgery, were excluded. Patients with WHO grade I meningioma were included in this study (Additional file 1: Figure S1).
Clinical data
All clinical data were collected through a retrospective chart review. The collected clinical endpoints included patient age, sex, and radiological follow-up. Preoperative and postoperative radiological data including tumour size, anatomical location, EOR, and the presence/absence and timing of recurrence were noted. Patients were followed-up with contrast-enhanced MRI (CE-MRI) within 2 days, around 6 months, and 1 year after surgery. If there was no tumour recurrence, follow-up was continued annually by CE-MRI. We defined tumour recurrence as apparent enlargement of the residual tumour on CE-MRI, by blind inter-observer agreement between the neuro-radiologists and neurosurgeons in charge.
The EOR was categorized as either GTR, which included Simpson grades I, II, and III, or STR, which included Simpson grades IV. The EOR was determined based on the postoperative imaging and operation records.
Histopathological data
The pathological diagnosis was established by the expert neuropathologist at our institution based on the 2016 WHO classification of tumours of the central nervous system. Formalin-fixed paraffin-embedded tissue was used for immunohistochemistry (IHC) in all cases. IHC was performed using whole-slide sections for forkhead box protein M1 (FOXM1) and Ki-67. The following antibodies were used: anti-Ki67 rabbit polyclonal (30–9; Ventana Medical Systems, Tucson, AZ) and FOXM1 rabbit monoclonal (EPR17379). Ki-67 index was calculated as the highest value in areas of maximum cellular density, identified by visual inspection. We define 4% as the cutoff of Ki-67 in reference to previous publications about Ki-67 and the recurrence of meningioma [33, 34]. FOXM1 IHC was quantified as the highest number of positive nuclei / high power field (HPF) within each meningioma using ImageJ (U.S. National Institutes of Health). “High” FOXM1 expression was defined as more than 3 nuclei/HPF, and “low” as otherwise.
Sanger sequencing and microsatellite analysis
Tumour samples were stored at −80 °C after tumour resection until genomic analysis. Tumour DNA was obtained from frozen samples using a DNA Extraction Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Mutation analysis was performed as previously described, including direct Sanger sequencing and microsatellite analysis [35].
Anatomical groups
Tumour location was defined as the location of the dural attachment of the tumour based on intraoperative observation as well as preoperative imaging. All meningiomas were classified into two groups, namely supratentorial or infratentorial lesions. Supratentorial lesions included meningiomas at the convexity, falx, parasagittal, sphenoid ridge, anterior fossa, middle fossa, clinoid, tuberculum sellae, and cavernous sinus. Infratentorial lesions included meningiomas at the clivus, petro-clivus, petrous, cerebellopontine angle, cerebellar convexity, jugular foramen, and foramen magnum. Tentorial meningiomas were divided into two groups depending on the dominant tumour protrusion direction, as evaluated using sagittal MRI images.
Subgroups encompassing driver gene mutation and tumour location
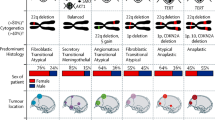
“NF2 meningioma” was defined as meningiomas with the presence of NF2 mutation and/or 22q loss [14]. Based on the driver gene mutation profile and the tumour location, meningiomas of all enrolled patients were categorized into the following four subgroups: “Supratentorial NF2”, “Infratentorial NF2”, “Supratentorial non–NF2”, and “Infratentorial non–NF2”. For each group, we evaluated clinical, radiological, and histopathological data, and progression-free survival (PFS).
Statistical analysis
Statistical analyses were performed using R version 3.1.2 (R Core Team, http://www.R-project.org). Numerical variables were expressed as the mean and standard deviation. Categorical data were compared between the subgroups using Fisher’s exact test. PFS was evaluated using the Kaplan–Meier method, followed by the log-rank test for each variable.
The Mann–Whitney U test was used to compare two non-parametric continuous variables.
The hazard ratio (HR) of all variables for the presence of recurrence as endpoints was analyzed using the univariate Cox proportional hazards model. Variables associated with endpoints in univariate analyses (p < 0.05) were included in a backward, stepwise manner using p-value multivariate analysis.
All reported p-values were two-sided, and in all comparisons, p-values of less than 0.05, were considered significant. Post-hoc pairwise comparisons between subgroups and prognostic grades were adjusted using the Bonferroni method.
Data availability
The authors confirmed that the data supporting the findings of this study will be shared upon request from any qualified investigator.
Results
Clinical characteristics
A total of 343 patients who underwent surgical treatment for sporadic meningioma were enrolled in this study at the University of Tokyo Hospital between 2000 and 2019 (Additional file 1: Fig. S1). The remaining 281 patients with WHO grade 1 meningioma were eligible for subsequent analyses. The average follow-up period after surgery was 5.3 ± 4.5 years (Table 1). Supratentorial and infratentorial meningiomas accounted for 188 (66.9%) and 93 (33.1%) patients, respectively (Table 1, Table 2). The detailed tumour locations are shown in Additional file 1: Table S1.
Surgical outcome
The overall recurrence rate of WHO grade I meningiomas was 16.0% in the follow-up period (Table 1 & Table 2), and the average time for recurrence was 4.1 ± 3.7 years (Additional file 1: Table S2). 5-year PFS was 83.4% (CI 77.3–88.1), and GTR was achieved in 84.0% of patients (Tables 1, 2).
Driver gene mutation and subgroups
Molecular analysis revealed that there were 152 NF2 meningiomas (54.1%) and non-NF2 meningiomas: 45.9% (129 cases) including “AKT1”: 11.4% (32 cases), “KLF4”:5.7% (16 cases), “POLR2A”:16 cases (5.7%), “SMO”: 0.7% (2 cases), and others:22.1% (62 cases) (Table 1, Additional file 1: Table S1). Based on the mutation and anatomical location, the cases were classified into the following subgroups: “Supratentorial NF2”, 109 cases (38.8%); “Infratentorial NF2”, 43 cases (15.3%); “Supratentorial non–NF2”, 79 cases (28.1%); and “Infratentorial non–NF2”, 50 cases (17.8%) (Table 1).
Association of the prognosis with EOR, tumour location, driver gene mutation, and subgroups.
Consistent with previous reports, the cases with GTR had a better prognosis than those with STR (5-year PFS: 86.9% in GTR vs. 65.4% in STR, p = 8.4 × 10–8) (Table 2, Fig. 1A) [4,5,6,7,8,9,10,11,12,13]. We found that the prognosis of the meningiomas was not different location-wise (5-year PFS: 82.1% in “Supratentorial” vs 86.7% in “Infratentorial”, p = 0.18) (Table 2, Fig. 1B) but was different mutation-wise (5-year PFS: 77.9% in NF2 vs. 90.3% in non-NF2, p = 0.04 (Table 2, Fig. 1C). By subgrouping with mutation and location, they became more distinctively different (5-year PFS: 72.8% in “Supratentorial NF2” vs. 94.1% in “Infratentorial NF2” vs 95.3% in “Supratentorial non–NF2” vs 80.2% in “Infratentorial non–NF2”, p = 6.2 × 10–4) (Table 2, Fig. 1D). In supratentorial lesion, the prognosis of NF2 meningioma was worse than that of non-NF2 meningioma. In infratentorial lesion, however, the prognosis of NF2 meningioma was better than that of non-NF2 meningioma (Table 2, Fig. 1D).
Meningiomas with GTR or STR
To avoid the effect of EOR on the recurrence, we further compared the prognosis in the meningioma with the same EOR (GTR or STR). Among tumors with GTR, the prognosis was better in non-NF2 meningioma (5-year PFS; 81.5% in NF2 meningioma vs. 94.1% in non-NF2 meningioma; p = 0.02) and also better in infratentorial meningioma (5-year PFS; 84.4% in “Supratentorial” vs. 93.1% in “Infratentorial”; p = 0.01) (Table 2, Fig. 2A, B). By subgrouping, the prognosis in the “Supratentorial NF2” subgroup was worst (5-year PFS: 77.3% in “Supratentorial NF2” vs. 93.8% in “Infratentorial NF2” vs. 94.4% in “Supratentorial non–NF2” vs. 92.3% in “Infratentorial non–NF2”, p = 6.2 × 10–3) (Table 2, Fig. 2C).
In contrast, in tumors with STR, the prognosis in “Supratentorial NF2” remained worse, but 5-year PFS in “Infratentorial non–NF2” was also low (5-year PFS: 50.0% in “Supratentorial NF2”, 100% in “Infratentorial NF2”, 100% in “Supratentorial non–NF2”, 51.3% in “Infratentorial non–NF2”; p = 0.05) (Table 2).
Histopathological findings and their correlation to prognosis
The histopathological findings of meningiomas are shown in Fig. 3 and Additional file 1: Table S1, and they are consistent with previous reports [1]. The prognosis was found to be different depending on Ki-67 index (Ki-67 ≥ 4 vs. Ki-67 < 4, p = 4.5 × 10–9). Among meningiomas with GTR, PFS was also different depending on Ki-67 index (Ki-67 ≥ 4 vs. Ki-67 < 4, p = 4.9 × 10–10), FOXM1 protein expression in 111 cases (high vs. low, p = 0.00052), and FOXM1 protein expression in 40 cases of supratentorial NF2 meningioma (high vs. low, p = 0.013) (Additional file 1 Fig. S2A, 2B, 2C).
Ki-67 index and FOXM1 protein expression in WHO grade I meningioma with GTR. Comparison of Ki-67 index based on each variable (A: driver gene mutation, B: tumour location, C: subgroups). Comparison of FOXM1 protein expression based on each variable (D: driver gene mutation, E: tumour location, F: subgroups). G: hematoxylin eosin (WHO grade I meningioma in supratentorial lesion with NF2 mutation, scale bar: 50 µm). H: Ki-67 index (WHO grade I meningioma in supratentorial lesion with NF2 mutation, scale bar: 50 µm). I: FOXM1 protein expression (WHO grade I meningioma in supratentorial lesion with NF2 mutation, scale bar: 50 µm). J: FOXM1 protein expression (WHO grade III anaplastic meningioma, scale bar: 100 µm). K: FOXM1 protein expression (brain metastasis from lung cancer, scale bar: 50 µm). FOXM1: forkhead box protein M1; WHO: World Health Organization. GTR: gross total resection; PFS: progression-free survival
HR for PFS in WHO grade I meningiomas
In univariate analyses, statistical associations were identified between worse prognosis and NF2 meningioma (HR:1.9, confidence interval [CI] 1.01–3.58, p = 0.04), STR (HR: 4.51, CI 2.46–8.25, p = 1.0 × 10–6), and Ki-67 index ≥ 4 (HR: 4.91, CI 2.7–8.95, p = 1.8 × 10–8) (Fig. 4). In subgroups (reference to “Supratentorial NF2), “Infratentorial NF2” (HR: 0.09, CI: 0.01–0.7, p = 0.02), “Supratentorial non–NF2” (HR: 0.25, CI 0.1–0.61, p = 0.002) showed longer PFS.
Multivariate analysis showed that NF2 meningioma per se was not proven to be a predictor of prognosis. However, STR (HR: 3.84, CI 2.07–7.12, p = 0.00001), Ki-67 index ≥ 4 (HR: 3.29, CI 1.76–6.27, p = 0.0002), and “Supratentorial NF2” (HR: 2.3, CI 1.19–4.44 p = 0.012) were found to be independent significant predictors of recurrence on multivariate analysis (Fig. 4).
In univariate analyses for the meningiomas with GTR, statistical associations were found between worse prognosis and identified with Ki-67 index ≥ 4 (HR: 8.91, CI 4.0–19.84, p = 8.4 × 10–8) (Fig. 4). In subgroups (reference to “Supratentorial NF2), “Supratentorial non–NF2” (HR: 0.33, CI: 0.12–0.90, p = 0.03) were positively associated with PFS.
In WHO grade I meningiomas with GTR, tumour location (supratentorial or infratentorial), NF2 status (presence or absence) and Ki-67 index (≥ 4 or not) were used as covariates in the multivariate analysis (Fig. 4). FOXM1 expression was omitted, as it is involved in the cell cycle and is assumingly closely associated with Ki-67 index [31]. Ki-67 index ≥ 4 (HR: 7.45, CI: 3.3–16.85, p = 1.3 × 10–6), and “Supratentorial NF2” (HR: 3.03, CI: 1.26–7.26 p = 0.01) were found to be significant predictors of prognosis.
Classifications of WHO grade I meningioma with GTR based on Ki-67 index, driver gene mutation, and tumour location.
According to the results of the multivariate analysis, we settled classes of WHO grade I meningioma that achieved GTR using three clinical/histopathological/genomic factors as follows: “Good”: Ki-67 index < 4 and non-NF2 meningioma, Ki-67 index < 4 and “Infratentorial NF2”; Intermediate: MIB-1 index ≥ 4 or “Supratentorial NF2”; Poor: Ki-67 index ≥ 4 and “Supratentorial NF2” (Fig. 5). The results in terms of 5-year and 10-year PFS were, Good, 96.1%/ 96.1%; Intermediate, 89.7% /83.9%; and Poor, 43.0%/21.5%. PFS was also different depending on each class (p = 3.8 × 10–13) (Fig. 5).
Discussion
Recent genetic and clinical studies have evaluated clinical/molecular characteristics of meningiomas including all WHO grades [22,23,24,25,26,27,28,29,30,31]; however, recurrence of WHO grade I meningiomas, especially after GTR has not been well-studied based on the assumption that benign meningioma with perfect resection should be of paramount reassurance [3, 11]. In the present study, we performed a long-term follow-up study (5.3 ± 4.5 years) for 281 WHO grade I meningiomas. The study revealed the significance of NF2 mutation and/or 22q loss in WHO grade I meningiomas in predicting recurrence, which was intriguingly reinforced by tumour anatomical locations. By integrating driver gene mutation, tumour location, and Ki-67 index, we now present a novel prognostic three-class category to predict tumour recurrence for WHO grade I meningiomas following GTR.
Association between driver gene mutation and recurrence in WHO grade I meningiomas
According to the recent study about the association between subgrouping by driver gene mutation and recurrence of meningiomas [14], only the Hedgehog group remained a significant genetic predictor of tumour recurrence by multivariate analysis in all WHO grade meningiomas. Furthermore, regarding WHO grade I meningiomas, the PFS of the group with the Hedgehog and the tumour necrosis factor-receptor associated factor 7 (TRAF 7) were shorter than that of the NF2 group [14]. In our results, the prognosis of the meningiomas was different mutation-wise (5-year PFS: 77.9% in NF2 vs 90.3% in non-NF2, p = 0.04 (Table 2, Fig. 1C) and univariate analysis identified between worse prognosis and NF2 meningioma (HR:1.9, confidence interval [CI] 1.01–3.58, p = 0.04)”, however, the statistical significance was marginal. Corroborating their results, NF2 meningioma per se was not a significant predictor by multivariate analysis for PFS in WHO grade I meningiomas in the current study. This finding is counterintuitive, considering that NF2 alteration are known to initiate events for aggressive-type meningiomas [36]. Actually, the latest trends of the molecular subgroups for meningioma focus on whether NF2 meningioma or not according to the data of single-cell RNA sequencing in meningiomas [25, 37, 38].
Addressing this pathophysiological question, the present study probed the connotation of driver gene mutation status in association with tumour location and revealed that the clinical significance of the NF2 mutation or 22q loss differed remarkably or inversely with tumour location. The presence of NF2 alteration was found to have a significantly worse prognostic impact in supratentorial meningiomas, while it has a tendency to have a better prognostic effect in infratentorial meningiomas. More specifically, “Supratentorial” and “NF2” was the worst combination as a predictor of PFS in subgroups. And this was echoed by a significantly higher Ki-67 index and FOXM1 expression in this subgroup compared with others.
Recently, meningioma development was reviewed from an embryological perspective by Fountain et al. and Boetto et al. [39, 40]. Collaborating these reports and our result, we speculate that the difference in the prognostic implication of NF2 alteration between supra- and infratentorial space may be attributed to the spatial distribution of the tumour cell of origin (neural crest vs mesoderm). Correspondingly, an integrated study surrounding location, phenotype, genotype, and meningeal embryology might be meaningful study in the future.
Association between other variates and recurrence in WHO grade I meningiomas
The EOR has been known as a prognostic factor for meningiomas [4,5,6,7,8,9], and in clinical practice, the Simpson grading system has been commonly utilized as a landmark to predict recurrence of meningiomas [41]. In our study, the EOR was a strong predictor of tumour recurrence. However, from a neurosurgical viewpoint, the EOR is highly and inevitably dependent on tumour location [24, 32]. Furthermore, the genomic profile is strongly correlated with tumour location [16,17,18,19,20,21]. Thus, the effect of each variable, including EOR, tumour location, and molecular subgroups, should be equally considered when evaluating meningioma recurrence.
In our results, the GTR/STR rate was not statistically different between supratentorial and infratentorial tumours (Table 2). However, most of the infratentorial tumours in which GTR was not achieved were non-NF2 meningiomas (4.7% in “Infratentorial NF2” vs. 26.0% in “Infratentorial non–NF2”, p = 0.04) (Table 2). In addition, they were mainly located surrounding brainstem such as at clivus, and petro-clival region (Additional file 1: Fig. S3). Previous studies reported that one of the prognostic factors for meningioma recurrence was posterior fossa [15] and that STR was an independent predictor of recurrence in skull base meningiomas [42]. Our results showed short PFS in infratentorial non–NF2 meningiomas (Fig. 1, Table 2). This result needs careful interpretation; one explanation is that only STR was achieved in many of infratentorial non–NF2 meningiomas due to the surgical difficulty related to their proximity to the cranial nerves, vessels, and brainstem. This contributed to a higher rate of recurrence compared with infratentorial NF2 meningiomas, which are often located at cerebellar convexity.
In contrast, even if GTR was achieved, the 5-year PFS in patient with NF2 meningioma remained short in the supratentorial space (Table 2). We speculated that despite being WHO grade I, the short PFS of these meningiomas reflected the aggressive histological features such as high Ki-67 index and high FOXM1 expression. This result should weigh in our decision-making regarding the appropriate management of WHO grade I supratentorial meningioma after GTR. Actually, the prognosis of “Poor” class WHO grade I supratentorial meningioma was quite similar to that of WHO grade II or III meningiomas (Fig. 5). Facing this sobering reality, we here recommend integrated histopathological and molecular diagnosis, close follow-up, and possibly proactive treatment protocol to be implemented in cases of WHO grade I meningioma with GTR when characterized by NF2 alteration, supratentorial location, and high Ki-67 index.
Our study had several limitations that should be addressed in future investigations. First, our study suffers from retrospective, single-institution design, which restricted variables for the assessment to those included in the database. Charts were reviewed retrospectively, and thus, not all clinical/genomic data could be collected. The duration of follow-up was not ideally sufficient to evaluate the prognosis of WHO grade I meningiomas, especially considering that the average time to recurrence was only a little shorter than the median follow-up time. As NF2 mutation was predominantly the commonest mutation in meningioma, we simplified the analyses by categorizing the meningioma as NF2 or non-NF2. In the future, we hope to analyze the effects of several other driver gene mutation types in association with anatomical location in meningioma recurrence. Further external studies on a large number of cases are warranted to validate our results to a better understanding of the clinical behaviour of this disease.
Previously, NF2 alteration, multiple copy number variations (CNV; e.g. 22q loss, 1p loss, etc.), high FOXM1 expression, low immune cell infiltration, and loss of H3K27me3 were reported as characteristics of aggressive behaviour of meningiomas [28, 43]. Recent reports have speculated structure variants such as multiple CNVs, especially 1p loss, as an epigenetic “second-hit” following NF2 alteration for aggressive-type meningiomas [22, 24, 38, 45]. Although we posit that supratentorial NF2 meningiomas might have such an aggressive feature as copy number alteration in addition to Ki-67 and FOXM1, it was beyond the scope of this study. Thus, evaluation of CNV status in NF2 mutated meningiomas depending on anatomical location is also needed in the future.
In terms of evaluating tumour anatomical location, while there are many ways of categorization, here we divided the cases into supra- or infra-tentorium, which possibly reflected the biological differences of the brain tumours, as evidenced by ependymomas [46]. However, more granular anatomical classification should be utilized ideally for clinical utilization. We will focus on the recurrence and the molecular status of meningiomas in each granular anatomical location.
Conclusion
In conclusion, by conducting this long-term follow-up study of a large number of patients with WHO grade I meningiomas, we demonstrated that the clinical significance of NF2 alteration status in WHO grade I meningiomas was different depending on tumour anatomical location, i.e., either supratentorial or infratentorial. By integrating driver gene mutation and tumour location, the “Supratentorial NF2” subgroup as well as the EOR and Ki-67 index, were identified as a significant predictor of recurrence of WHO grade I meningioma, clinically similar to the poor prognosis of WHO grade II/III. Correspondingly, even if GTR is achieved in WHO grade I meningiomas, this study suggested that close follow-up and proactive measures should be considered in cases characterized by NF2 alteration, supratentorial location, and high Ki-67 index. We anticipate that this integrated anatomical, histopathological, and genomic classification carries significant clinical implications and will provide the best follow-up schedule and proactive measures, as well as improve the daily clinical practice for patients with this most common brain tumour.
Competing interests
The authors report no competing interests.
References
Louis DN, Perry A, Reifenberger G, Deimling AV, Figarella-Branger D, Cavenee WK et al (2016) The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131(6):803–820
Claus EB, Bondy ML, Schildkraut JM, Wiemels JL, Wrensch M, Black PM (2005) Epidemiology of intracranial meningioma. Neurosurgery 57(6):1088–1095
Rogers L, Barani I, Chamberlain M, Kaley TJ, McDermott M, Raizer J et al (2015) Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg 122(1):4–23
Mirimanoff RO, Dosoretz DE, Linggood RM, Ojemann RG, Martuza RL (1985) Meningioma: analysis of recurrence and progress-Sion following neurosurgical resection. J Neurosurg 62(1):18–24
Condra KS, Buatti JM, Mendenhall WM, Friedman WA, Marcus RB Jr, Rhoton AL (1997) Benign meningiomas: primary treatment selection affects survival. Int J Radiat Oncol Biol Phys 39(2):427–436
Stafford SL, Perry A, Suman VJ, Meyer FB, Scheithauer BW, Lohse CM et al (1998) Primarily resected meningiomas: outcome and prognostic factors in 581 Mayo Clinic patients, 1978 through 1988. Mayo Clin Proc 73(10):936–942
Soyuer S, Chang EL, Selek U, Shi W, Maor MH, DeMonte F et al (2004) Radiotherapy after surgery for benign cerebral meningioma. Radiother Oncol 71(1):85–90
Taylor BW Jr, Marcus RB Jr, Friedman WA, Ballinger WE Jr, Million RR (1988) The meningioma controversy: postoperative radiation therapy. Int J Radiat Oncol Biol Phys 15(2):299–304
Pollock BE, Stafford SL, Utter A, Giannini C, Schreiner SA (2003) Stereotactic radiosurgery provides equivalent tumor control to Simpson Grade 1 resection for patients with small- to medium-size meningiomas. Int J Radiat Oncol Biol Phys 55(4):1000–1005
Roggendorf W, Schuster T, Peiffer J (1987) Proliferative potential of meningiomas determined with the monoclonal antibody Ki-67. Acta Neuropathol 73(4):361–364
Abry E, Thomassen IØ, Salvesen ØO, Torp SH (2010) The significance of Ki-67/MIB-1 labeling index in human meningiomas: a literature study. Pathol Res Pract 206(12):810–815
Oya S, Kawai K, Nakatomi H, Saito N (2012) Significance of Simpson grading system in modern meningioma surgery: integration of the grade with MIB-1 labeling index as a key to predict the recurrence of WHO Grade I meningiomas. J Neuorsurg 117:121–128
Fukushima Y, Oya S, Nakatomi H, Shibahara J, Hanakita S, Tanaka S (2013) Effect of dural detachment on long-term tumor control for meningiomas treated using Simpson Grade IV resection. J Neuorsurg 119:1373–1379
Youngblood MW, Miyagishima DF, Jin L, Gupte T, Li C, Duran D et al (2020) Associations of meningioma molecular subgroup and tumor recurrence. Neuro Oncol. https://doi.org/10.1093/neuonc/noaa226
Haddad AF, Young JS, Kanungo I, Sudhir S, Chen JS, Raleigh DR et al (2020) WHO Grade I meningioma recurrence: identifying high risk patients using histopathological features and the MIB-1 index. Front Oncol 10:1522
Clark VE, Erson-Omay EZ, Serin A, Yin J, Cotney J, Ozduman K et al (2013) Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science 339(6123):1077–1080
Clark VE, Harmancı AS, Bai H, Youngblood MW, Lee TI, Baranoski JF et al (2016) Recurrent somatic mutations in POLR2A define a distinct subset of meningiomas. Nat Genet 48(10):1253–1259
Brastianos PK, Horowitz PM, Santagata S, Jones RT, McKenna A, Getz G et al (2013) Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat Genet 45(3):285–289
Abedalthagafi M, Bi WL, Aizer AA, Merrill PH, Brewster R, Agarwalla PK et al (2016) Oncogenic PI3K mutations are as common as AKT1 and SMO mutations in meningioma. Neuro Oncol 18(5):649–655
Youngblood MW, Duran D, Montejo JD, Li C, Omay SB, Ozduman K et al (2019) Correlations between genomic subgroup and clinical features in a cohort of more than 3000 meningiomas. J Neurosurg 1–10
Boetto J, Bielle F, Sanson M, Peyre M, Kalamarides M (2017) SMO mutation status defines a distinct and frequent molecular subgroup in olfactory groove meningiomas. Neuro Oncol 19(3):345–351
Harmancı AS, Youngblood MW, Clark VE, Coskun S, Henegriu O, Duran D et al (2017) Integrated genomic analyses of de novo pathways underlying atypical meningiomas. Nat Commun 8(1):14433
Patel AJ, Wan YW, Al-Ouran R, Revelli JP, Cardenas MF, Oneissi M et al (2019) Molecular profiling predicts meningioma recurrence and reveals loss of DREAM complex repression in aggressive tumors. Proc Natl Acad Sci U S A 116(43):21715–21726
Sahm F, Schrimpf D, Stichel D, Jones DTW, Hielscher T, Schefzyk S et al (2017) DNA methylation–based classification and grading system for meningioma: a multicentre, retrospective analysis. Lancet Oncol 18(5):682–694
Nassiri F, Liu J, Patil V, Mamatjan Y, Wang JZ, Hugh-White R et al (2021) A clinically applicable integrative molecular classification of meningiomas. Nature 597(7874):119–125
Berghoff AS, Hielscher T, Ricken G, Furtner J, Scchrimpf D, Widhalm G et al (2022) Prognostic impact of genetic alterations and methylation classes in meningioma. Brain Pahtol 32(2):e12970
Maas SLN, Stichel D, Hielscher T, Sievers P, Berghoff AS, Schrimpf D, Sill M et al (2021) Integrated molecular-morphologic meningioma classification: a multicenter retrospective analysis, retrospectively and prospectively validated. J Clin Oncol 39(34):3839–3852
Nassiri F, Wang JZ, Singh O, Karimi S, Dalcourt T, Ijad N et al (2021) Loss of H3K27me3 in meningiomas. Neuro Oncol 23(8):1282–1291
Meta R, Boldt H, Kristensen BW, Sahm F, Sjursen W, Torp SH (2021) The prognostic value of methylation signatures and NF2 mutations in atypical meningiomas. Cancers (Basel) 13(6):1262
Driver J, Hoffman S, Tavakol S, Woodward E, Maury EA, Bhave V et al (2021) A molecularly integrated grade for meningioma. Neuro Oncol (online ahead of print).
Nassiri F, Mamatjan Y, Suppiah S, Badhiwala JH, Mansouri S, Karimi S et al (2019) DNA methylation profiling to predict recurrence risk in meningioma: development and validation of a nomogram to optimize clinical management. Neuro Oncol 21(7):901–910
Sanson M, Kalamarides M (2017) Epigenetics: a new tool for meningioma management? Lancet Oncol 18(5):569–570
Abry E, Ingrid T, Salvesen O, Torp SH (2010) The significance of Ki-67/MIB-1 labelling index in human meningiomas: a literature study. Pathol Res Pract 206(12):810–815
Miran C, Skyrman S, Bartek J, Jensen LR, Kihlstrom L, Forander P et al (2020) The Ki-67 proliferation index as a marker of time to recurrence in intracranial meningioma. Neurosurgery 87(6):1289–1298
Okano A. Miyawaki1 S, Hongo H, Dofuku S, Teranishi Y, Mitsui J et al (2021) Tumor locations of meningiomas based on embryologic origin of meninges determine the diversity of pathological diagnosis and genetic abnormality. Sci Rep. (in press)
Prager BC, Vasudevan HN, Dixit D, Bernatchez JA, Wu Q, Wallace LC et al (2020) The meningioma enhancer landscape delineates novel subgroups and drives druggable dependencies. Cancer Discov 10(11):1722–1741
Blume C, Dogan D, Schweizer L, Peyre M, Doll S, Picard D et al (2021) Integrated phospho-proteogenomic and single-cell transcriptomic analysis of meningiomas establishes robust subtyping and reveals subtype-specific immune invasion. BioRxiv 05(11):443369. https://doi.org/10.1101/2021.05.11.443369
Choudhury A, Magill S, Eaton CD, Prager BC, Chen WC, Seo K et al (2020) Meningioma epigenetic grouping reveals biologic drivers and therapeutic vulnerabilities. MedRxiv. https://doi.org/10.1101/2020.11.23.20237495
Fountain DM, Smith MJ, O’Leary C, Pathmanaban ON, Roncarioli F, Bobola N et al (2021) The spatial phenotype of phenotypically distinct meningiomas demonstrate potential implication of the embryology of the meninges. Oncogene 40(5):875–884
Boetto J, Peyre M, Kalamarides M (2021) Meningiomas from a developmental perspective: exploring the crossroads between meningeal embryology and tumorigenesis. Acta Neurochir (Wien) 163(1):57–66
Simpson D (1957) The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry 20(1):22–39
Mansouri A, Klironomos G, Taslimi S, Kilian A, Gentili F, Khan OH et al (2016) Surgically resected skull base meningiomas demonstrate a divergent postoperative recurrence pattern compared with non–skull base meningiomas. J Neurosurg 125:431–440
Katz LM, Hielscher T, Liechty B, Silverman J, Zagzag D, Sen R et al (2018) Loss of histone H3K27me3 identifies a subset of meningiomas with increased risk of recurrence. Acta Neuropathol 135(6):955–963
Vasudevan HN, Braunstein SE, Phillips JJ, Pekmezci M, Tomlin B, Wu A et al (2018) Comprehensive molecular profiling identifies FOXM1 as a key transcription factor for meningioma proliferation. Cell Rep 22(13):3672–3683
Williams EA, Santagata S, Wakimoto H, Shanker Gm, Barker FG 2nd, Sharaf R et al (2020) Distinct genomic subclasses of high-grade/progressive meningiomas NF2-associated2-exclusive, and NF2-agnostic. Acta Neuropathol Commun. 8(1):171
Pajtler KW, Witt H, Sill M, Jones DTW, Hovestadt V, Kratochwil F et al (2015) Molecular classification of ependymal tumors across all CNS compartments, histopathological grades, and age groups. Cancer Cell 27(5):728–743
Acknowledgements
We are grateful to Dr. Matsuura for helping with examinations.
Funding
This project was supported by a Grant-in-Aid for Scientific Research (B) (No. 21H03041) from the Japan Society for the Promotion of Science to Dr. Saito, a Grant-in-Aid for Scientific Research (C) (No. 19K09473) from the Japan Society for the Promotion of Science to Dr. Miyawaki, a Grant-in-Aid for Research Activity Start-up (No. 19K24023) from the Japan Society for the Promotion of Science to Dr. Teranishi, a Grant-in-Aid for Young Scientists (20K17954) from the Japan Society for the Promotion of Science to Dr. Teranishi, and a research grant from the Takeda Science Foundation to Dr. Miyawaki.
Author information
Authors and Affiliations
Contributions
Experimental design: Dr. Yu Teranishi, Dr. Atsushi Okano, Dr. Satoru Miyawaki, Dr. Masahiro Shin, Dr. Hirofumi Nakatomi, Dr. Nobuhito Saito. Implementation of the experiments: Yu Teranishi1, Atsushi Okano1, Satoru Miyawaki1, Kenta Ohara1 Daiichiro Ishigami1, Hiroki Hongo1, Shogo Dofuku1, Dr. Jun Mitsui. Analysis and interpretation of the data: Dr. Yu Teranishi, Dr. Satoru Miyawaki, Dr. Hirokazu Takami, Dr. Masako Ikemura, Daisuke Komura, Hiroto Katoh, Dr. Ushiku, Dr. Shumpei Ishikawa, Dr. Masahiro Shin, Dr. Hirofumi Nakatomi, Dr. Nobuhito Saito. All authors read and approved the final manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Figure S1. Flowchart in this study, Figure S2. PFS of patients with WHO grade I meningioma with GTR evaluated using the Kaplan-Meier method followed by the log-rank test for each variable, A: Ki-67 index, B: FOXM1 protein expression, C: FOXM1 protein expression in supratentorial NF2 meningiomas. GTR: gross total resection; PFS: progression-free survival; FOXM1: forkhead box protein M1, Figure S3. Detailed tumor location WHO grade I meningiomas considering recurrence, and EOR, Table S1. Detailed patient characteristics, Table S2. Comparing variables depending on driver gene mutation, tumor location, and subgroups
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Teranishi, Y., Okano, A., Miyawaki, S. et al. Clinical significance of NF2 alteration in grade I meningiomas revisited; prognostic impact integrated with extent of resection, tumour location, and Ki-67 index. acta neuropathol commun 10, 76 (2022). https://doi.org/10.1186/s40478-022-01377-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40478-022-01377-w