Abstract
The discovery of fibroblast growth factor receptor (FGFR) gene family alterations as drivers of primary brain tumors has generated significant excitement, both as potential therapeutic targets as well as defining hallmarks of histologic entities. However, FGFR alterations among neuroepithelial lesions are not restricted to high or low grade, nor to adult vs. pediatric-type tumors. While it may be tempting to consider FGFR-altered tumors as a unified group, this underlying heterogeneity poses diagnostic and interpretive challenges. Therefore, understanding the underlying biology of tumors harboring specific FGFR alterations is critical. In this review, recent evidence for recurrent FGFR alterations in histologically and biologically low-grade neuroepithelial tumors (LGNTs) is examined (namely FGFR1 tyrosine kinase domain duplication in low grade glioma, FGFR1-TACC1 fusions in extraventricular neurocytoma [EVN], and FGFR2-CTNNA3 fusions in polymorphous low-grade neuroepithelial tumor of the young [PLNTY]). Additionally, FGFR alterations with less well-defined prognostic implications are considered (FGFR3-TACC3 fusions, FGFR1 hotspot mutations). Finally, a framework for practical interpretation of FGFR alterations in low grade glial/glioneuronal tumors is proposed.
Similar content being viewed by others
Introduction
The search for disease-defining genetic alterations in brain tumors has characterized the last several decades in neuropathology: one particularly exciting arena has been the discovery of a host of fibroblast growth factor receptor (FGFR) gene family alterations as apparent drivers of primary brain tumors. However, this particular group of lesions has proven especially challenging as they are not confined to either high or low grade, nor to adult vs. pediatric lesions. In fact, FGFR alterations are implicated across a host of human cancers, promoting oncogenesis as a result of overexpression, amplification, mutations, and structural variations [28, 35, 51, 73].
The FGFR family consists of four highly conserved transmembrane tyrosine kinase receptors (FGFR1–4) and represents a fundamental receptor tyrosine kinase (RTK) signaling pathway. FGFRs dimerize in the presence of any of 22 known ligands, triggering downstream signaling pathways well-implicated in tumorigenesis; these include the mitogen activated protein kinase (MAPK) and phosphoinositide-3-kinase (PI3K)/Akt pathways among others [14, 20, 34, 45]. Beyond playing an important role in CNS embryonal development, FGFR signaling influences angiogenesis and tumor cell migration, differentiation, proliferation, and survival. Not surprisingly, FGFRs have emerged as a major target for cancer therapeutics across tumor types and multiple targeting strategies are under investigation [5, 13, 16, 19, 24, 30, 47, 48].
The optimal use of targeted therapy in brain tumors remains under investigation, and its efficacy in low-grade tumors, which would conceivably be slow growing, has been difficult to assess [72]. Although detection of these possible therapeutic targets is of great clinical interest, high quality clinical data remains limited. Ahead of this, understanding the biological implications of specific FGFR alterations, and how this relates to tumor subclassification, is paramount; this is particularly true among histologically low-grade tumors.
Recently the Consortium to Inform Molecular and Practical Approaches to CNS tumor Taxonomy-Not Official WHO (cIMPACT-NOW) released update 4, which specifically addressed so-called “pediatric-type diffuse gliomas” [22]. In contrast to the IDH- wild type, diffuse gliomas encountered in adults, diffuse gliomas in children and adolescents most commonly harbor a different constellation of mutations and fusions including alterations in FGFR1 [56, 77]. The guidelines recommend distinguishing these from adult-type tumors to provide more accurate prognostication, and in some instances guide therapy; delineating relevant diffuse gliomas as harboring either tyrosine kinase domain duplication (TKDD) or single nucleotide variants in FGFR1. This is an important step in brain tumor classification and more accurately reflects the relatively prolonged disease course and better overall survival of these pediatric lesions, certainly when compared to IDH-wild type, “adult” tumors. However, while it may be tempting to further consider FGFR-altered tumors as a unified group, there remains significant heterogeneity among them.
In this review, recent evidence for recurrent FGFR alterations in histologically and biologically low grade neuroepithelial tumors (LGNTs) is examined. These include FGFR1 tyrosine kinase domain duplication in low grade glioma, FGFR1-TACC1 fusions in extraventricular neurocytoma (EVN), and FGFR2-CTNNA3 fusions in polymorphous low grade neuroepithelial tumor of the young (PLNTY). Additionally, FGFR alterations with less well-defined prognostic implications are considered (FGR3-TACC3 fusions, FGFR1 hotspot mutations). The structure of these alterations is summarized in Fig. 1. Finally, a proposed framework for interpreting the implications of specific FGFR alterations regarding tumor subclassification and prognostication is presented.
Summary of common FGFR alterations in brain tumors. Some alterations are strongly associated with low grade neuroepithelial lesions: FGFR1-TKD, FGFR1-TACC1 fusion, FGFR2-CTNNA3 fusion. Others (including FGFR1 hotspot mutations and FGFR3-TACC3 fusions) are described in low-grade as well as high-grade tumors, requiring cautious interpretation when encountered in histologic LGNTs
Genotypic-phenotypic correlations in low grade lesions with FGFR alterations
Emerging evidence has demonstrated that certain low-grade histologic entities appear to be dominated by specific FGFR alterations. While these mutations have not yet been raised to the level of definitional characteristics by the WHO (and are therefore not required for rendering a diagnosis), there remains (with rare exceptions), a virtual absence in the reported literature of associated high-grade histology and/or aggressive clinical behavior in association with select FGFR alterations. As such, by and large, these alterations may be reasonably regarded as hallmarks of the following low grade neuroepithelial tumors.
FGFR1- tyrosine kinase domain duplication (FGFR1-TKDD) in low grade glioma (LGG)
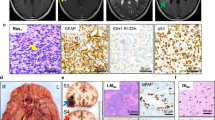
Among the most important insights gained from landmark sequencing studies examining the molecular landscape of pediatric low grade glial and glioneuronal tumors was the identification of an intragenic duplication of the entire FGFR1 region encoding the tyrosine kinase domain (TKD). This duplication includes exons 10–18 and produces an in-frame fusion separated by a linker element of variable length [56, 77]. Histologically, lesions harboring FGFR1-TKDD appear to be predominately diffuse gliomas located in the cerebral cortex. Duplication of the FGFR1 TKD has also been reported in low-grade astrocytomas more suggestive of other specific histologic entities including pilocytic astrocytoma (typically extracerebellar) and dysembryoplastic neuroepithelial tumor (DNET, Fig. 2a, b) [23, 37, 40, 60, 77].
Histologic features of FGFR-altered LGNTs. Three examples of LGNTs bearing characteristic FGFR-alteration are shown: DNET with FGFR1- TKD (a, b), EVN with FGFR1-TACC1 fusion (c, d), and PLNTY with FGFR2-CTNNA3 fusion (e, f). Note that while histologic features of each lesion met diagnostic criteria in keeping with a specific entity, LGNTs share many overlapping histologic features including bland neurocytic/ oligodedroglioma-like nuclear features and of lack of significant proliferative or mitotic activity
While encompassing a significant subset of LGNT (7.4–24%), this alteration appears. to be virtually absent in high-grade gliomas (HGG) [38, 77]. In the original report, a cohort of 33 HGG were screened for duplication of the FGFR1 region encoding the TKD, revealing only one tumor (diagnosed as anaplastic oligoastrocytoma, WHO grade III) that had progressed from a grade II tumor. No FGFR1-TKDD positive cases were detected in adult-type oligodendrogliomas, IDH-mutant and 1p/19q co-deleted [77]. Since then, the association of FGFR1-TKDD with anaplastic histologic features has proven to be an exceedingly rare phenomenon. One reported case of a rosette forming glioneuronal tumor (RGNT) having focal DNET-like features exhibited multiple local recurrences over a ten-year period, ultimately demonstrated elevated mitoses and high-grade histology, and was shown to harbor FGFR1-TKDD in addition to a frameshift mutation in ATRX [33]. Additionally, a glioneuronal tumor with features of pilocytic astrocytoma and pleomorphic xanthoastrocytoma also harboring FGFR1-TKDD was reported to demonstrate focally elevated mitotic activity; molecular characterization revealed multiple additional variants of unknown significance [3]. It is noteworthy in this instance that, while histologic criteria for anaplasia were met, without long term follow-up data, the biologic and prognostic significance of these findings are unclear. Excepting these rare instances, FGFR1-TKDD has been associated with tumors manifesting bland histology and benign clinical behavior.
FGFR1-TACC1 fusion in extra ventricular neurocytoma
Among the most highly recurrent chromosomal translocations across human cancers are those involving fusions of FGFR genes with members of the purportedly oncogenic TACC protein family (TACC1, TACC2, and TACC3 [21, 52, 75]). TACC proteins contain a coiled-coil domain at the C-terminus (TACC domain), which facilitates localization of the fusion protein to the centrosome and mitotic spindle [36, 53] in tum promoting aneuploidy and tumorigenesis [49, 69]. Constitutive FGFR activity and downstream MAPK/PI3K/mTOR pathway activation also results from the fusion [32, 43].
It is important to note that the highest frequency of FGFR-TACC chromosomal translocations is in HGG, namely IDH-wild type GBM where the fusion is between FGFR3 and TACC3, located 48 kb apart on chromosome 4p16 [18, 50, 69] see FGFR3 fusions). Among FGFR-fusion positive glioblastomas, much less frequently encountered are FGFR fusions other than FGFR3-TACC3, including FGFR1-TACC1 [18, 69, 70]. Homologous with regards to respective chromosomal locations, FGFR1 and TACC1 are located on chromosome 8p11; the molecular mechanisms with regards to downstream MAPK pathway activation as a result of FGFR1-TACC1 fusion are also thought to be similar to those of FGFR3-TACC3, though less extensively studied and modeled [44].
In sharp contrast to FGFR3-TACC3, FGFR1-TACC1 appears to more commonly associated with low-grade histology and biology, being especially prevalent in the context of extra ventricular neurocytoma (EVN). EVN is a rare primary brain tumor occurring within the parenchyma, outside the ventricular system. While a range of histopathological features may be encountered in EVN, these tumors generally resemble central neurocytoma (Fig. 2c, d). Not surprisingly, accurate diagnosis is confounded by overlapping morphological features with other LGNT entities. DNA methylation-based analysis of a cohort of EVN found that while a subset of histologically diagnosed EVN could be regrouped with other defined, established entities, a large fraction formed a clearly distinct, separate epigenetic group. Importantly, copy number analysis and RNA sequencing demonstrated FGFR1-TACC1 fusion as a recurrent feature within the EVN methylation group (60%), in addition to a small number of other FGFR rearrangements (FGFR3-TACC3, FGFR1-EVI5) [67].
Indeed, many of the earlier descriptions of EVN predate newer molecular classification of brain tumors and may have been confounded by histologic overlap with other entities. The relationship between rare cases described as FGFR1-TACC1 fusion-positive HGG/GBM and cases of so-called “atypical EVN” with necrosis, vascular proliferation, and/or elevated mitotic activity, is unclear [25, 29, 41, 44, 69]. The majority of EVNs are well-differentiated and generally benign [11]. In the absence of elevated proliferative rate/mitotic activity, and particularly after complete resection, the rate of recurrence is low [25, 41]. While definitive grading criteria have yet to be established and survival data studied in additional independent cohorts, EVN corresponds histologically to WHO grade II, which is in keeping with reported survival data in molecularly-defined EVN, including those bearing FGFR1-TACC1 fusions [67].
FGFR2- fusion (FGFR2-CTNNA3) in PLNTY
A recently described entity, “polymorphous low-grade neuroepithelial tumor of the young” or “PLNTY”, has been shown to harbor molecular abnormalities involving the MAPK pathway, including FGFR genes, and a unique fusion involving FGFR2 [39]. These tumors, while morphologically somewhat variable, are characterized by infiltrative growth, oligodendroglioma-like cytologic features, and frequent calcification (Fig. 2e, f). Strong cluster of differentiation 34 (CD34) immunohistochemical expression has also been described. Belonging to a group of epilepsy associated low-grade neuroepithelial tumors in children and young adults, PLNTYs appear to have a predilection for the superficial cerebral hemispheres (particularly the temporal lobes), in keeping with prior reports of “long-term epilepsy associated tumors (LEATs)” [10, 39]. Most importantly, all indications point to the indolent behavior of PLNTY [9, 31, 37, 71].
In the original description by Huse et al. (2017) a novel fusion transcript was identified among the series of PLNTY, wherein FGFR2 (including the kinase domain) joined with exons 14–18 of CTNNA3 (to include the entirety of its C-terminal dimerization domain) [37, 58]. The oncogenic fusion is thought to result in homodimerization and autophosphorylation of FGFR2 and downstream MAPK/PI3K/mTOR pathway activation, similar to other FGFR fusions as previously discussed [15, 69, 71]. Molecular profiling of PLNTYs has demonstrated that they carry a distinct DNA methylation signature, suggesting that they are in fact a distinct biologic entity among at least a subset of LGNTs, including previously described “pediatric oligodendrogliomas” [56, 77]. No reports of FGFR2-CTNNA3 fusion in association with a high-grade or aggressive tumor have been made to date. However, it is important to note that while FGFR2-CTNNA3 appears to be relatively specific signature of PLNTY, the molecular landscape of PLNTY includes genetic abnormalities involving either BRAF or even FGFR3. These other alterations are not unique to PLNTY, and inasmuch as they are frequently also encountered in higher grade entities, should not be regarded as diagnostic of this entity or as predictive of a benign clinical course.
Other FGFR alterations: unclear implications in LGNT
Several other alterations in FGFR genes have been reported in association with LGNTs, but their distribution is not limited to tumors with low grade histology or benign behavior. Therefore, the implications of these alterations in isolation are less clear. Cautious interpretation is advised, particularly in settings where infiltrating or undersampled tumor is a possibility.
FGFR3 fusions
The reality is that the implications of FGFR3 fusion are clear: as previously stated, FGFR3 fusions, most commonly FGFR3-TACC3, are by and large a feature of IDH-wild type glioblastoma, WHO grade IV [18]. Although FGFR-fusion positive GBM constitutes a small subset of GBM as a whole (~ 3%), the sheer preponderance of GBM relative to other types of glioma renders this the most common scenario in which FGFR3 fusions will be encountered in most neuropathology practice settings [7, 18, 69].
Difficulty arises when this genetic feature of GBM is encountered in lower grade histologic entities. Detection of FGFR3 fusions in histologically low-grade tumors is well-documented [18, 27, 37, 38, 77]. However, many of these cases were not been reported with sufficient long-term follow up to determine their clinical biology. This is not to say that FGFR3 fusions cannot be associated with benign histologic entities; the sole FGFR3-TACC3 fusion positive case in the original series of PLNTY for example was devoid of any high-grade features suggestive of GBM and demonstrated no evidence of disease or seizures after an extensive interval (89 months) [37]. Of note, FGFR3-TACC3 fusions in GBM characteristically arise in order individuals, with frequent co-mutation of TERT promoter and loss of CDKN2A/2B, features that should help distinguish these cases from true LGNT, including PLNTY.
FGFR3-TACC3 fusion gliomas, both low and high grade, exhibit characteristic histologic features, including monomorphous oligodendroglioma-like nuclei, “chicken-wire” capillary networks, and frequent microcalcifications [7]. While this may be reflective of the common end-result of FGFR fusions in all tumors (namely enhanced downstream signaling through MAP kinase pathway effectors), the histologic similarities suggest the possibility of FGFR3-fusion positive GBM arising from lower-grade precursor lesions. To date, however, there has been insufficient evidence to support this, and the relationship between high and low grade FGFR- fusion positive tumors, if any, remains unclear. Rather, FGFR3 fusions should prompt a careful evaluation of clinical and neuroradiologic features and call for close surveillance following surgery, when encountered in an ostensibly LGNT.
FGFR1 hotspot (N546 & K656) mutations
Another frequently reported FGFR alteration among LGNTs is mutation of two hotspot residues (N546 & K656) in the tyrosine kinase domain of FGFR1, well-known to be activating and oncogenic [6, 46, 57, 76]. These two residues are the most commonly mutated residues in FGFR1 in human cancers and interestingly are described predominately in CNS tumors, mostly histologic pilocytic astrocytomas [40, 78]. Somatic hotspot and germline mutations in FGFR1 have also been implicated in the pathogenesis of DNET [60]. Of note, encephalocraniocutaneous lipomatosis (ECCL) a sporadic neurocutaneous syndrome with features of disordered RAS-MAPK signaling, appears to be mediated in at least a subset of cases by these very FGFR1 mutations (in mosaic, somatic distribution) and also carries an increased risk of low-grade gliomas, again predominately of pilocytic astrocytoma histology [6, 8, 42, 54, 64]. It is emerging however, that while these FGFR1-mutant tumors certainly can be described histologically and biologically as low grade, they are distinct from typical pilocytic astrocytoma (WHO grade I), which are predominately driven by BRAF fusions. In fact, in some early studies, FGFR1 mutation in pilocytic astrocytoma was associated with a significantly poorer prognosis, although sample size was small [4]. While no specific differentiating histologic criteria have been reported, it has emerged that there are distinguishing clinicopathologic features of these tumors; subsequent larger studies have revealed that pilocytic astrocytoma with FGFR1 mutation are predominately extracerebellar and frequently midline in location, (in contrast to BRAF-fusion positive pilocytic astrocytomas, which predominate in the cerebellum) [40]. At the same time, hotspot FGFR1 mutations have also been observed in adult and pediatric HGG, at the level of GBM (WHO grade IV) [12, 40, 57]. Notably, FGFR1 hotspot mutations have been detected in up to 18% of adult midline glioma with high grade histology [55]. These FGFR1-mutant HGG frequently demonstrated a recurrent mutational profile in which H3 alterations (H3F3A K27M) and somatic mutations in NF1 [40] were detected. Although this profile can be seen in tumors histologically equivalent to pilocytic astrocytoma, the underlying molecular features are strongly suggestive of biologic overlap with diffuse midline glioma, H3 K27M-mutant (WHO grade IV) [40, 65].
FGFR1 hotspot mutations have also emerged as a molecular hallmark of rosette-forming glioneuronal tumor (RGNT) [26, 66]. RGNTs predominately affect young adults and are relatively rare neuroepithelial tumors with distinctive histologic features namely, the presence of neurocytes in rosettes or perivascular pseudo-rosettes in addition to an astrocytic component resembling pilocytic astrocytoma. It is on the basis of histology that the diagnosis is rendered. While in recent studies FGFR1 hotspot mutations were invariably detected among RGNTs [66], their presence is not currently required for the diagnosis, (and as previously discussed, is certainly not unique to RGNT). Moreover, while RGNT corresponds histologically to WHO grade I and is generally considered benign, dissemination and progression have been reported in rare instances [1, 2, 62, 68, 74]. Of note, frequent co-mutation with PIK3CA as well as NF1 have been reported in RGNT [66]. Mutation of PI3K pathway genes has been associated with aggressive clinical behavior in LGNTs, although further study is needed to determine their prognostic value in RGNT [26, 61]. On the whole, while there is clearly a role for FGFR1 hotspot mutations in the pathogenesis of LGNT, their specificity for low grade histology and clinical behavior is highly dependent on histologic features and broader molecular context.
Practical approaches to FGFR alterations in LGNT
Based on available evidence, it appears that some FGFR alterations are more tightly correlated with specific histologic entities among LGNTs, while others may be encountered among variable tumor types, spanning histologic grades and clinical behavior. This poses significant challenges for molecular pathologists, neuropathologists and clinicians: how to determine which amongst these lesions are truly low grade, versus those with increased biologic potential. A practical approach to consider when encountering and “triaging” FGFR alterations in LGNT should involve determining 1) the presence of any atypical features and 2) the presence of additional molecular alterations. Atypical features worth noting in LGNT include both histologic and clinical features. For example, elevated mitotic activity, proliferation indices, and other indicators of high-grade histology should always be noted, even if only focally present in tumors bearing the FGFR alterations described herein. While definitive grading criteria await establishment, in general, bona fide LGNTs are not expected to display significant mitoses, necrosis, or vascular proliferation; proliferative indices would not be expected to exceed 1–2%. Similarly, a multidisciplinary clinical view should be given due consideration in these instances; atypical neuroimaging, and unusual clinical setting (i.e. PLNTY in an older individual [9, 59]) could potentially serve as important indicators of the true nature of the lesion.
By and large, FGFR alterations in LGNTs appear to be a reassuring finding, particularly when they are present in an otherwise genomically quiet background. Most LGNTs appear to be driven by a single molecular pathway, and typically by a single driver genetic alteration [56, 77]. This can be a challenge to definitively determine when taking a minimalist molecular diagnostic approach. Although next-generation sequencing may not be possible to perform in every case, determining the absence of additional alterations (loss of CDKN2A/2B, TERT promoter mutation, H3- mutation etc.) may be critical to determining the nature of the FGFR-alteration bearing tumor and broader genomic testing should be strongly considered [22].
Conclusion
While for the purpose of this review, the role of FGFR alterations has been described in relation to specific histologic entities, the reality is that there is significant overlap of histologic features amongst LGNTs (Fig. 2). Although there is utility to the genotypic-phenotypic association between FGFR-alteration and tumor type, it may be more accurate to consider FGFR-altered neuroepithelial lesions as spanning a histologic spectrum. That this group also includes higher grade tumors implies that the spectrum is a biological one as well. Furthermore, it is important to bear in mind that FGFR- altered tumors are an important subset of a larger group of glial/glioneuronal tumors that are primarily driven by altered MAPK signaling [17, 37, 52, 71].
As previously noted, oncogenic FGFR signaling appears to play a role in a variety of cancer types, including extraneural tumors; FGFR pathway inhibition as a therapeutic strategy remains an area of active investigation. As clinical trials of FGFR inhibitors in brain tumors are ongoing or only recently completed (NCT01975701, NCT028224133, NCT02052778, NCT01948297), we have yet to fully explore the efficacy of this therapeutic approach. Recently, for example, a study found that FGFR inhibitors (AZ4547, dovatinib, PD173074, ponatinib) were more effective in reducing the growth of pediatric diffuse midline glioma, H3 K27M-mutant (diffuse intrinsic pontine glioma, DIPG) cells in vitro compared to Temozolomide [63]. However, much about the role of FGFR inhibitors in treatment of brain tumors, LGNTs in particular, remains to be understood. Optimal design of clinical trials and interpretation of data will be directly dependent on accurate classification of tumors bearing these FGFR alterations.
The complexity of FGFR signaling means that more research will also be necessary to better understand how FGFRs contribute to cancer biology beyond tumor initiation. The role of FGFRs in disease progression as well as associated mechanisms of treatment resistance remain largely unknown (but are certainly relevant issues in the treatment of low grade tumors). With advancing knowledge, we will continue to more accurately identify and stratify LGNTs based on their underlying molecular features, increasingly guiding therapeutic decisions now and in the imminent future.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
References
Allinson KS, O'Donovan DG, Jena R, Cross JJ, Santarius TS (2015) Rosette-forming glioneuronal tumor with dissemination throughout the ventricular system: a case report. Clin Neuropathol 34:64–69
Anan M, Inoue R, Ishii K, Abe T, Fujiki M, Kobayashi H, Goya T, Nakazato Y (2009) A rosette-forming glioneuronal tumor of the spinal cord: the first case of a rosette-forming glioneuronal tumor originating from the spinal cord. Hum Pathol 40:898–901
Ballester LY, Penas-Prado M, Leeds NE, Huse JT, Fuller GN (2018) FGFR1 tyrosine kinase domain duplication in pilocytic astrocytoma with anaplasia. Cold Spring Harb Mol Case Stud 4:a002378
Becker AP, Scapulatempo-Neto C, Carloni AC, Paulino A, Sheren J, Aisner DL, Musselwhite E, Clara C, Machado HR, Oliveira RS et al (2015) KIAA1549: BRAF gene fusion and FGFR1 hotspot mutations are prognostic factors in pilocytic astrocytomas. J Neuropathol Exp Neurol 74:743–754
Becker D, Lee PL, Rodeck U, Herlyn M (1992) Inhibition of the fibroblast growth factor receptor 1 (FGFR-1) gene in human melanocytes and malignant melanomas leads to inhibition of proliferation and signs indicative of differentiation. Oncogene 7:2303–2313
Bennett JT, Tan TY, Alcantara D, Tetrault M, Timms AE, Jensen D, Collins S, Nowaczyk MJM, Lindhurst MJ, Christensen KM et al (2016) Mosaic activating mutations in FGFR1 cause encephalocraniocutaneous lipomatosis. Am J Hum Genet 98:579–587
Bielle F, Di Stefano AL, Meyronet D, Picca A, Villa C, Bernier M, Schmitt Y, Giry M, Rousseau A, Figarella-Branger D et al (2018) Diffuse gliomas with FGFR3-TACC3 fusion have characteristic histopathological and molecular features. Brain Pathol 28:674–683
Bieser S, Reis M, Guzman M, Gauvain K, Elbabaa S, Braddock SR, Abdel-Baki MS (2015) Grade II pilocytic astrocytoma in a 3-month-old patient with encephalocraniocutaneous lipomatosis (ECCL): case report and literature review of low grade gliomas in ECCL. Am J Med Genet A 167A:878–881. https://doi.org/10.1002/ajmg.a.37017
Bitar M, Danish SF, Rosenblum MK (2018) A newly diagnosed case of polymorphous low-grade neuroepithelial tumor of the young. Clin Neuropathol 37:178–181
Blumcke I, Aronica E, Urbach H, Alexopoulos A, Gonzalez-Martinez JA (2014) A neuropathology-based approach to epilepsy surgery in brain tumors and proposal for a new terminology use for long-term epilepsy-associated brain tumors. Acta Neuropathol 128:39–54
Brat DJ, Scheithauer BW, Eberhart CG, Burger PC (2001) Extraventricular neurocytomas: pathologic features and clinical outcome. Am J Surg Pathol 25:1252–1260
Cancer Genome Atlas Research N (2008) Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455:1061–1068
Chae YK, Ranganath K, Hammerman PS, Vaklavas C, Mohindra N, Kalyan A, Matsangou M, Costa R, Carneiro B, Villaflor VM et al (2017) Inhibition of the fibroblast growth factor receptor (FGFR) pathway: the current landscape and barriers to clinical application. Oncotarget 8:16052–16074
Chen Y, Li X, Eswarakumar VP, Seger R, Lonai P (2000) Fibroblast growth factor (FGF) signaling through PI 3-kinase and Akt/PKB is required for embryoid body differentiation. Oncogene 19:3750–3756
Churi CR, Shroff R, Wang Y, Rashid A, Kang HC, Weatherly J, Zuo M, Zinner R, Hong D, Meric-Bernstam F et al (2014) Mutation profiling in cholangiocarcinoma: prognostic and therapeutic implications. PLoS One 9:e115383
Darwis NDM, Nachankar A, Sasaki Y, Matsui T, Noda SE, Murata K, Tamaki T, Ando K, Okonogi N, Shiba S et al (2019) FGFR signaling as a candidate therapeutic target for cancers resistant to carbon ion radiotherapy. Int J Mol Sci 20:4563
Deng MY, Sill M, Chiang J, Schittenhelm J, Ebinger M, Schuhmann MU, Monoranu CM, Milde T, Wittmann A, Hartmann C et al (2018) Molecularly defined diffuse leptomeningeal glioneuronal tumor (DLGNT) comprises two subgroups with distinct clinical and genetic features. Acta Neuropathol 136:239–253
Di Stefano AL, Fucci A, Frattini V, Labussiere M, Mokhtari K, Zoppoli P, Marie Y, Bruno A, Boisselier B, Giry M et al (2015) Detection, characterization, and inhibition of FGFR-TACC fusions in IDH wild-type glioma. Clin Cancer Res 21:3307–3317
Dieci MV, Arnedos M, Andre F, Soria JC (2013) Fibroblast growth factor receptor inhibitors as a cancer treatment: from a biologic rationale to medical perspectives. Cancer Discov 3:264–279
Doherty P, Walsh FS (1996) CAM-FGF receptor interactions: a model for axonal growth. Mol Cell Neurosci 8:99–111
Duncan CG, Killela PJ, Payne CA, Lampson B, Chen WC, Liu J, Solomon D, Waldman T, Towers AJ, Gregory SG et al (2010) Integrated genomic analyses identify ERRFI1 and TACC3 as glioblastoma-targeted genes. Oncotarget 1:265–277
Ellison DW, Hawkins C, Jones DTW, Onar-Thomas A, Pfister SM, Reifenberger G, Louis DN (2019) cIMPACT-NOW update 4: diffuse gliomas characterized by MYB, MYBL1, or FGFR1 alterations or BRAF(V600E) mutation. Acta Neuropathol 137:683–687
Fina F, Barets D, Colin C, Bouvier C, Padovani L, Nanni-Metellus I, Ouafik L, Scavarda D, Korshunov A, Jones DT et al (2017) Droplet digital PCR is a powerful technique to demonstrate frequent FGFR1 duplication in dysembryoplastic neuroepithelial tumors. Oncotarget 8:2104–2113
Frinchi M, Bonomo A, Trovato-Salinaro A, Condorelli DF, Fuxe K, Spampinato MG, Mudo G (2008) Fibroblast growth factor-2 and its receptor expression in proliferating precursor cells of the subventricular zone in the adult rat brain. Neurosci Lett 447:20–25
Furtado A, Arantes M, Silva R, Romao H, Resende M, Honavar M (2010) Comprehensive review of extraventricular neurocytoma with report of two cases, and comparison with central neurocytoma. Clin Neuropathol 29:134–140
Gessi M, Moneim YA, Hammes J, Goschzik T, Scholz M, Denkhaus D, Waha A, Pietsch T (2014) FGFR1 mutations in rosette-forming glioneuronal tumors of the fourth ventricle. J Neuropathol Exp Neurol 73:580–584
Granberg KJ, Annala M, Lehtinen B, Kesseli J, Haapasalo J, Ruusuvuori P, Yli-Harja O, Visakorpi T, Haapasalo H, Nykter M et al (2017) Strong FGFR3 staining is a marker for FGFR3 fusions in diffuse gliomas. Neuro-Oncology 19:1206–1216
Greulich H, Pollock PM (2011) Targeting mutant fibroblast growth factor receptors in cancer. Trends Mol Med 17:283–292
Guerreiro Stucklin AS, Ryall S, Fukuoka K, Zapotocky M, Lassaletta A, Li C, Bridge T, Kim B, Arnoldo A, Kowalski PE et al (2019) Alterations in ALK/ROS1/NTRK/MET drive a group of infantile hemispheric gliomas. Nat Commun 10:4343
Guillemot F, Zimmer C (2011) From cradle to grave: the multiple roles of fibroblast growth factors in neural development. Neuron 71:574–588
Gupta VR, Giller C, Kolhe R, Forseen SE, Sharma S (2019) Polymorphous low-grade neuroepithelial tumor of the young: a case report with genomic findings. World Neurosurg 132:347–355
Hadari YR, Gotoh N, Kouhara H, Lax I, Schlessinger J (2001) Critical role for the docking-protein FRS2 alpha in FGF receptor-mediated signal transduction pathways. Proc Natl Acad Sci U S A 98:8578–8583
Halfpenny A, Ferris SP, Grafe M, Woltjer R, Selden N, Nazemi K, Perry A, Solomon DA, Gultekin SH, Moore S et al (2019) A case of recurrent epilepsy-associated rosette-forming glioneuronal tumor with anaplastic transformation in the absence of therapy. Neuropathology 39:389–393
Hart KC, Robertson SC, Kanemitsu MY, Meyer AN, Tynan JA, Donoghue DJ (2000) Transformation and stat activation by derivatives of FGFR1, FGFR3, and FGFR4. Oncogene 19:3309–3320
Helsten T, Elkin S, Arthur E, Tomson BN, Carter J, Kurzrock R (2016) The FGFR landscape in cancer: analysis of 4,853 tumors by next-generation sequencing. Clin Cancer Res 22:259–267
Hood FE, Royle SJ (2011) Pulling it together: the mitotic function of TACC3. Bioarchitecture 1:105–109
Huse JT, Snuderl M, Jones DT, Brathwaite CD, Altman N, Lavi E, Saffery R, Sexton-Oates A, Blumcke I, Capper D et al (2017) Polymorphous low-grade neuroepithelial tumor of the young (PLNTY): an epileptogenic neoplasm with oligodendroglioma-like components, aberrant CD34 expression, and genetic alterations involving the MAP kinase pathway. Acta Neuropathol 133:417–429
Johnson A, Severson E, Gay L, Vergilio JA, Elvin J, Suh J, Daniel S, Covert M, Frampton GM, Hsu S et al (2017) Comprehensive genomic profiling of 282 pediatric low- and high-grade gliomas reveals genomic drivers, tumor mutational burden, and hypermutation signatures. Oncologist 22:1478–1490
Johnson DR, Giannini C, Jenkins RB, Kim DK, Kaufmann TJ (2019) Plenty of calcification: imaging characterization of polymorphous low-grade neuroepithelial tumor of the young. Neuroradiology 61:1327–1332
Jones DT, Hutter B, Jager N, Korshunov A, Kool M, Warnatz HJ, Zichner T, Lambert SR, Ryzhova M, Quang DA et al (2013) Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat Genet 45:927–932
Kane AJ, Sughrue ME, Rutkowski MJ, Aranda D, Mills SA, Lehil M, Fang S, Parsa AT (2012) Atypia predicting prognosis for intracranial extraventricular neurocytomas. J Neurosurg 116:349–354
Kocak O, Yarar C, Carman KB (2016) Encephalocraniocutaneous lipomatosis, a rare neurocutaneous disorder: report of additional three cases. Childs Nerv Syst 32:559–562
Kouhara H, Hadari YR, Spivak-Kroizman T, Schilling J, Bar-Sagi D, Lax I, Schlessinger J (1997) A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell 89:693–702
Lasorella A, Sanson M, Iavarone A (2017) FGFR-TACC gene fusions in human glioma. Neuro-Oncology 19:475–483
LaVallee TM, Prudovsky IA, McMahon GA, Hu X, Maciag T (1998) Activation of the MAP kinase pathway by FGF-1 correlates with cell proliferation induction while activation of the Src pathway correlates with migration. J Cell Biol 141:1647–1658
Lew ED, Furdui CM, Anderson KS, Schlessinger J (2009) The precise sequence of FGF receptor autophosphorylation is kinetically driven and is disrupted by oncogenic mutations. Sci Signal 2:ra6
Li M, Bernard O (1992) FDC-P1 myeloid cells engineered to express fibroblast growth factor receptor 1 proliferate and differentiate in the presence of fibroblast growth factor and heparin. Proc Natl Acad Sci U S A 89:3315–3319
Marseglia G, Lodola A, Mor M, Castelli R (2019) Fibroblast growth factor receptor inhibitors: patent review (2015-2019). Expert Opin Ther Pat 29:965–977
Nelson KN, Meyer AN, Siari A, Campos AR, Motamedchaboki K, Donoghue DJ (2016) Oncogenic gene fusion FGFR3-TACC3 is regulated by tyrosine phosphorylation. Mol Cancer Res 14:458–469
Parker BC, Annala MJ, Cogdell DE, Granberg KJ, Sun Y, Ji P, Li X, Gumin J, Zheng H, Hu L et al (2013) The tumorigenic FGFR3-TACC3 gene fusion escapes miR-99a regulation in glioblastoma. J Clin Invest 123:855–865
Patani H, Bunney TD, Thiyagarajan N, Norman RA, Ogg D, Breed J, Ashford P, Potterton A, Edwards M, Williams SV et al (2016) Landscape of activating cancer mutations in FGFR kinases and their differential responses to inhibitors in clinical use. Oncotarget 7:24252–24268
Pekmezci M, Villanueva-Meyer JE, Goode B, Van Ziffle J, Onodera C, Grenert JP, Bastian BC, Chamyan G, Maher OM, Khatib Z et al (2018) The genetic landscape of ganglioglioma. Acta Neuropathol Commun 6:47
Peset I, Vernos I (2008) The TACC proteins: TACC-ling microtubule dynamics and centrosome function. Trends Cell Biol 18:379–388
Phi JH, Park SH, Chae JH, Wang KC, Cho BK, Kim SK (2010) Papillary glioneuronal tumor present in a patient with encephalocraniocutaneous lipomatosis: case report. Neurosurgery 67:E1165–E1169
Picca A, Berzero G, Bielle F, Touat M, Savatovsky J, Polivka M, Trisolini E, Meunier S, Schmitt Y, Idbaih A et al (2018) FGFR1 actionable mutations, molecular specificities, and outcome of adult midline gliomas. Neurology 90:e2086–e2094
Qaddoumi I, Orisme W, Wen J, Santiago T, Gupta K, Dalton JD, Tang B, Haupfear K, Punchihewa C, Easton J et al (2016) Genetic alterations in uncommon low-grade neuroepithelial tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and align with morphology. Acta Neuropathol 131:833–845
Rand V, Huang J, Stockwell T, Ferriera S, Buzko O, Levy S, Busam D, Li K, Edwards JB, Eberhart C et al (2005) Sequence survey of receptor tyrosine kinases reveals mutations in glioblastomas. Proc Natl Acad Sci U S A 102:14344–14349
Rangarajan ES, Izard T (2013) Dimer asymmetry defines alpha-catenin interactions. Nat Struct Mol Biol 20:188–193
Riva G, Cima L, Villanova M, Ghimenton C, Sina S, Riccioni L, Munari G, Fassan M, Giangaspero F, Eccher A (2018) Low-grade neuroepithelial tumor: unusual presentation in an adult without history of seizures. Neuropathology 38:557–560
Rivera B, Gayden T, Carrot-Zhang J, Nadaf J, Boshari T, Faury D, Zeinieh M, Blanc R, Burk DL, Fahiminiya S et al (2016) Germline and somatic FGFR1 abnormalities in dysembryoplastic neuroepithelial tumors. Acta Neuropathol 131:847–863
Rodriguez EF, Scheithauer BW, Giannini C, Rynearson A, Cen L, Hoesley B, Gilmer-Flynn H, Sarkaria JN, Jenkins S, Long J et al (2011) PI3K/AKT pathway alterations are associated with clinically aggressive and histologically anaplastic subsets of pilocytic astrocytoma. Acta Neuropathol 121:407–420
Schlamann A, von Bueren AO, Hagel C, Zwiener I, Seidel C, Kortmann RD, Muller K (2014) An individual patient data meta-analysis on characteristics and outcome of patients with papillary glioneuronal tumor, rosette glioneuronal tumor with neuropil-like islands and rosette forming glioneuronal tumor of the fourth ventricle. PLoS One 9:e101211
Schramm K, Iskar M, Statz B, Jager N, Haag D, Slabicki M, Pfister SM, Zapatka M, Gronych J, Jones DTW et al (2019) DECIPHER pooled shRNA library screen identifies PP2A and FGFR signaling as potential therapeutic targets for DIPGs. Neuro-Oncology 21:867
Schubbert S, Shannon K, Bollag G (2007) Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer 7:295–308
Schwartzentruber J, Korshunov A, Liu XY, Jones DT, Pfaff E, Jacob K, Sturm D, Fontebasso AM, Quang DA, Tonjes M et al (2012) Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482:226–231
Sievers P, Appay R, Schrimpf D, Stichel D, Reuss DE, Wefers AK, Reinhardt A, Coras R, Ruf VC, Schmid S et al (2019) Rosette-forming glioneuronal tumors share a distinct DNA methylation profile and mutations in FGFR1, with recurrent co-mutation of PIK3CA and NF1. Acta Neuropathol 138:497–504
Sievers P, Stichel D, Schrimpf D, Sahm F, Koelsche C, Reuss DE, Wefers AK, Reinhardt A, Huang K, Ebrahimi A et al (2018) FGFR1:TACC1 fusion is a frequent event in molecularly defined extraventricular neurocytoma. Acta Neuropathol 136:293–302
Silveira L, DeWitt J, Thomas A, Tranmer B (2019) Disseminated rosette-forming glioneuronal tumor with spinal drop metastasis, a uniquely aggressive presentation of rare tumor. World Neurosurg 132:7–11
Singh D, Chan JM, Zoppoli P, Niola F, Sullivan R, Castano A, Liu EM, Reichel J, Porrati P, Pellegatta S et al (2012) Transforming fusions of FGFR and TACC genes in human glioblastoma. Science 337:1231–1235
Stransky N, Cerami E, Schalm S, Kim JL, Lengauer C (2014) The landscape of kinase fusions in cancer. Nat Commun 5:4846
Surrey LF, Jain P, Zhang B, Straka J, Zhao X, Harding BN, Resnick AC, Storm PB, Buccoliero AM, Genitori L et al (2019) Genomic analysis of dysembryoplastic neuroepithelial tumor spectrum reveals a diversity of molecular alterations dysregulating the MAPK and PI3K/mTOR pathways. J Neuropathol Exp Neurol 78:1100–1111
Tom MC, Cahill DP, Buckner JC, Dietrich J, Parsons MW, Yu JS (2019) Management for different glioma subtypes: are all low-grade gliomas created equal? Am Soc Clin Oncol Educ Book 39:133–145
Wu YM, Su F, Kalyana-Sundaram S, Khazanov N, Ateeq B, Cao X, Lonigro RJ, Vats P, Wang R, Lin SF et al (2013) Identification of targetable FGFR gene fusions in diverse cancers. Cancer Discov 3:636–647
Xu J, Yang Y, Liu Y, Wei M, Ren J, Chang Y, Huan Y, Yin H, Xue Y (2012) Rosette-forming glioneuronal tumor in the pineal gland and the third ventricle: a case with radiological and clinical implications. Quant Imaging Med Surg 2:227–231
Yao R, Natsume Y, Saiki Y, Shioya H, Takeuchi K, Yamori T, Toki H, Aoki I, Saga T, Noda T (2012) Disruption of Tacc3 function leads to in vivo tumor regression. Oncogene 31:135–148
Yoon K, Nery S, Rutlin ML, Radtke F, Fishell G, Gaiano N (2004) Fibroblast growth factor receptor signaling promotes radial glial identity and interacts with Notch1 signaling in telencephalic progenitors. J Neurosci 24:9497–9506
Zhang J, Wu G, Miller CP, Tatevossian RG, Dalton JD, Tang B, Orisme W, Punchihewa C, Parker M, Qaddoumi I et al (2013) Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet 45:602–612
Cosmic (Catalogue of Somatic Mutations in Cancer) (2019) Https://cancer.sanger.ac.uk/cosmic. Accessed 5 Dec 2019
Acknowledgements
The author would like to thank Dr. Marc Rosenblum and Dr. Matthias Karajannis for their insights and expertise, as well as Ms. Shadia Carlo for administrative assistance in preparation of this manuscript.
Funding
The author has no relevant funding to disclose.
Author information
Authors and Affiliations
Contributions
The manuscript was generated in its entirety by the sole author. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The author has no competing interests to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Bale, T.A. FGFR- gene family alterations in low-grade neuroepithelial tumors. acta neuropathol commun 8, 21 (2020). https://doi.org/10.1186/s40478-020-00898-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40478-020-00898-6