Abstract
Background
Phospholipases A2 (PLA2s) are abundant components of snake venoms that have been extensively studied due to their pharmacological and pathophysiological effects on living organisms. This study aimed to assess the antitumor potential of BthTX-I, a basic myotoxic PLA2 isolated from Bothrops jararacussu venom, by evaluating in vitro processes of cytotoxicity, modulation of the cell cycle and induction of apoptosis in human (HL-60 and HepG2) and murine (PC-12 and B16F10) tumor cell lines.
Methods
The cytotoxic effects of BthTX-I were evaluated on the tumor cell lines HL-60 (promyelocytic leukemia), HepG2 (human hepatocellular carcinoma), PC-12 (murine pheochromocytoma) and B16F10 (murine melanoma) using the MTT method. Flow cytometry technique was used for the analysis of cell cycle alterations and death mechanisms (apoptosis and/or necrosis) induced in tumor cells after treatment with BthTX-I.
Results
It was observed that BthTX-I was cytotoxic to all evaluated tumor cell lines, reducing their viability in 40 to 50 %. The myotoxin showed modulating effects on the cell cycle of PC-12 and B16F10 cells, promoting delay in the G0/G1 phase. Additionally, flow cytometry analysis indicated cell death mainly by apoptosis. B16F10 was more susceptible to the effects of BthTX-I, with ~40 % of the cells analyzed in apoptosis, followed by HepG2 (~35 %), PC-12 (~25 %) and HL-60 (~4 %).
Conclusions
These results suggest that BthTX-I presents antitumor properties that may be useful for developing new therapeutic strategies against cancer.
Similar content being viewed by others
Background
According to the World Health Organization, cancer is one of the leading causes of morbidity and mortality worldwide. Cancer is a generic term for a large group of diseases that may affect any part of the body [1]. These diseases are characterized by uncontrolled growth and spread of abnormal cells, and may be caused by external factors (smoking, infectious organisms, radiation and chemical products) and internal factors (inherited or metabolic mutations, hormones and immune conditions) [2].
A correct diagnosis of cancer is essential for appropriate and effective treatment, because each cancer type requires specific treatments that may include surgery, radiotherapy and/or chemotherapy. Nonetheless, chemotherapy is flawed especially considering that no chemotherapeutic agent available acts exclusively on tumor cells, affecting also many types of normal body cells and leading to several undesirable effects [2, 3].
Currently there is great medical and scientific interest in the investigation of natural products for therapeutic purposes, in search of new molecules that could be used as antitumor agents with fewer side effects than the usual chemotherapy, or serve as molecular models for the development of more effective drugs against malignant tumors [4, 5].
In recent years, studies on snake venoms and their components have demonstrated the antitumor effects of different toxins [6–8]. The focus of these researches has been the understanding of the mechanisms of action of toxins on different types of tumors. It is known that the cytotoxicity induced by animal venoms is mainly related to changes in the cellular metabolism of cells, especially tumorous ones, however, the mechanisms of action of these toxins are still to be clarified, which makes them target of many studies with interest in their therapeutic potential [8].
Some phospholipases A2 (PLA2s) have been described to possess antitumor and antiangiogenic properties [9–11]. PLA2s are enzymes of great scientific interest due to their involvement in several human inflammatory diseases and in envenomations by snakes and bees. In addition, PLA2s present important effects on lipid membranes and are related to the formation of various substances involved in numerous biological activities, such as prostaglandins, prostacyclins, thromboxanes and leukotrienes [12, 13].
Myotoxins are PLA2 homologues that present a substitution of the aspartic acid residue at position 49 (Asp49) by a lysine (Lys49), with changes in their calcium binding site. These variations are related to the low or no catalytic activity presented by these molecules [14]. Nevertheless, myotoxic PLA2s have diverse biological effects, such as myotoxicity, edema, anticoagulation, neurotoxicity, indirect hemolysis, cytotoxicity, and also antibacterial, antiviral and antiparasitic activities [15].
Bothropstoxin-I (BthTX-I) is a single chain protein of 13.7 kDa and pI 8.2, with 121 amino acid residues and 7 disulfide bonds, isolated from Bothrops jararacussu snake venom [16]. This basic myotoxin showed action on the gastrocnemius muscle of mice and cytotoxicity in murine muscle cells (C2C12) and also had its cytotoxic effects evaluated both in vitro and in vivo on different tumor cell lines such as Jurkat, SKBR3, B16F10 and S180, showing promising antitumor properties [17, 18].
Considering these previous findings on BthTX-I and the wide variety of cytotoxic effects presented by snake toxins, additional studies using different tumor cell lines are necessary in order to increase the knowledge of the antitumor and biotechnological potential of this B. jararacussu myotoxin. Thereby, this study aimed to evaluate the in vitro effects of BthTX-I on human (HL-60 and HepG2) and murine (PC-12 and B16F10) tumor cell lines by assessing its induced cytotoxicity, cell cycle alterations and death mechanisms.
Methods
Materials
Toxin
BthTX-I was isolated from Bothrops jararacussu venom according to the methodology described by Cintra et al. [19]. The lyophilized protein was stored at −20 °C and solubilized in phosphate buffered saline (PBS) immediately before its use in the tests.
Cell lines
HL-60 (CCL-240, promyelocytic leukemia), HepG2 (HB-8065, human hepatocellular carcinoma), PC-12 (CRL-1721, murine pheochromocytoma) and B16F10 (CRL-6475, murine melanoma) tumor cell lines were obtained from ATCC (American Type Culture Collection, USA).
Methods
Cell culture
Cells were grown in monolayer in 25 cm2 flasks. HL-60 cells were cultured in 5 mL of RPMI culture medium (Gibco 31800–022, USA) supplemented with 10 % fetal bovine serum (FBS, Gibco 12657, USA) and 1 % antibiotic (streptomycin and penicillin, P4333 Sigma, USA). PC-12 cells were cultured in 5 mL of RPMI supplemented with 15 % fetal equine serum (Gibco 26050–088, New Zealand), 5 % FBS and 1 % antibiotic. B16F10 and HepG2 cells were grown in DMEM culture medium (Gibco 31600–034, USA) also supplemented with 10 % FBS and 1 % antibiotic. The vials containing the cells were incubated at 37 °C in a humidified incubator containing 5 % CO2, until reaching a state of confluence (~5 × 106 cells) when they require subculture. The tests with BthTX-I were performed with cells between the 3rd and 6th day of subculture. Cell viability tests using the Trypan blue dye were performed before any experimentation to ensure the accuracy of the results.
Cytotoxic assays using MTT
For the cytotoxicity assay, tumor cells (HL-60, PC-12, HepG2 or B16F10) were seeded into 96-well plates, followed by incubation for 24 h at 37 °C in a humidified incubator containing 5 % CO2. After this period, the cells were treated with 50 μL of PBS (negative control) or 50 μL of BthTX-I samples at different concentrations (5; 10; 25; 50 or 100 μg/mL). Experimental positive control received 50 μL of a cisplatin solution at 1 mg/mL (Incel, Darrow®), which is an antineoplastic agent that binds to DNA, inducing structural changes and, consequently, apoptosis. After treatment, the wells received 20 μL of MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (Sigma M2128, USA) (500 μg/mL, final concentration) and plates were incubated for 3 h at 37 °C and 5 % CO2. Then, plates were centrifuged at 900 g for 5 min and then inverted to discard the supernatant, followed by addition of 100 μL of DMSO (Sigma D2650, USA) to each well. The plates were kept under stirring until complete dissolution of crystals (~20 min) and then the absorbance at 570 nm was determined in a Powerwave XS2 microreader (Biotek) [20].
Cell cycle analysis
Tumor cells (HL-60, PC-12, HepG2 or B16F10) were plated into 24-well plates (1 × 106 cells/well), followed by incubation for 24 h at 37 °C in a humidified incubator containing 5 % CO2. After this period, the cells were treated with 50 μL of PBS (negative control) or 50 μL of BthTX-I samples at different concentrations (5; 10; 25; 50 or 100 μg/mL). After 24 h of treatment, cells were transferred to flow cytometry tubes and centrifuged at 900 g for 5 min, supernatant was discarded and pellet resuspended in 1 mL of PBS. Then, cells were centrifuged again, the supernatant was discarded, the pellet resuspended in 100 μL of cold PBS and 2 mL of cold 70 % ethanol and the samples were stored at −20 °C for 24 h. At the end of this period, the tubes were centrifuged, the supernatant discarded and the pellet was resuspended in 300 μL of cold PBS with 10 μL of RNase (10 mg/mL) and incubated for 30 min at 37 °C. Immediately after the incubation, samples were placed in an ice bath with 164 μL of HFS (hypotonic fluorescence solution, a reagent that measures the amount of DNA in the cell and defines its cell cycle position) and incubated on ice for 1 h, followed by analysis of 10,000 events in a FACSCanto flow cytometer (Becton Dickinson, USA). The files generated by the equipment were read in the FlowJo 7.6.1 software for the analysis of cell division phases: G0/G1, S and G2/M. The results were expressed as the percentage of cells in each phase of the cycle [6].
Analysis of apoptosis/necrosis by flow cytometry
The assessment of the apoptotic and necrotic effects induced by BthTX-I on different tumor cell lines (HL-60, PC-12, HepG2 or B16F10) was determined by flow cytometry, using a kit containing annexin V FITC and propidium iodide (PI) [21]. The population of necrotic cells is marked with PI (PI+), while the cells in apoptosis are marked with annexin V FITC (AV+) and the population of both necrotic and apoptotic cells is marked as PI+/AV+.
Tumor cells were plated into 24-well plates (5 × 105 cells/well), followed by incubation for 24 h at 37 °C in a humidified incubator containing 5 % CO2. After this period, the cells were treated with 50 μL of PBS (negative control) or 50 μL of BthTX-I samples at different concentrations (5; 10; 25; 50 or 100 μg/mL). Experimental positive control received 50 μL of a cisplatin solution at 1 mg/mL. After 24 h of treatment, cells were transferred to flow cytometry tubes, and centrifuged at 900 g for 5 min, supernatant was discarded and pellet resuspended in 1 mL of PBS. The same process was repeated, this time with addition of 300 μL of 1X annexin V to the resuspended pellet and 5 μL of FITC-annexin V to each tube. The samples were incubated for 15 min in ice, adding 1 μL of propidium iodide (PI) immediately after incubation, followed by analysis of 10,000 events in a FACSCanto flow cytometer (Becton Dickinson, USA) using Diva software.
Statistical analysis
Results were analyzed by the software Graph Pad Prism 5 using the method one-way ANOVA and Tukey’s post-test, considering values of p < 0.05 as significant. All treatments were compared to the negative control (PBS).
Results
Evaluation of the cytotoxicity induced by BthTX-I
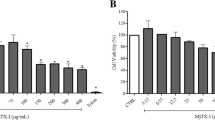
The antitumor activity of BthTX-I was assessed by treating tumor cell lines with different concentrations of the myotoxin. For HL-60, it was observed that the cell viability after treatment with BthTX-I was of approximately 80 % for the lowest concentration evaluated (5 μg/mL), while at the concentrations of 10, 25, 50 and 100 μg/mL, cell viabilities of 46; 36; 40 and 38 %, respectively, were observed (Fig. 1 – a). For HepG2, BthTX-I concentrations from 5 to 25 μg/mL led to cell viabilities of approximately 40 %, whereas at the concentrations of 50 and 100 μg/mL, the values of viability observed were of 35 % and 30 %, respectively (Fig. 1 – b). For PC-12, cell viability ranged from 40 to 60 %, with lowest values observed after treatment with BthTX-I at the concentration of 25 μg/mL (Fig. 1 – c). B16F10 cells showed around 40 % cell viability at all concentrations tested (Fig. 1 – d).
Cell viability of the tumor cell lines HL-60 (a), HepG2 (b), PC-12 (c) and B16F10 (d) after treatment with BthTX-I. The cytotoxicity was evaluated by the MTT method 24 h after treatment of tumor cells with BthTX-I (5–100 μg/mL). Results expressed as mean ± SD of three independent experiments (n = 3). Statistically significant differences (p < 0.05) were labeled with * (in comparison to PBS). PBS (negative control); Cisp (cisplatin, positive control)
Cell cycle kinetics analysis
The cell cycle kinetics analysis was performed in order to detect the role of BthTX-I in the cell division process of tumor cells. HL-60 and HepG2 cells treated with different concentrations of the toxin showed distribution in all three phases of the cell cycle (G0/G1, S, G2/M), with no significant differences (p < 0.05) in comparison with the negative control (PBS). Around 70 % of the HL-60 cells remained in G0/G1, 15 % in S and 15 % in G2/M phases (Fig. 2 – a), while 70 % of HepG2 cells remained in G0/G1, 20 % in S and 10 % in G2/M phases (Fig. 2 – b).
Assessment of cell cycle progression of the tumor cell lines HL-60 (a), HepG2 (b), PC-12 (c) and B16F10 (d) treated with BthTX-I using flow cytometry. Tumor cells were treated with BthTX-I at different concentrations (5 to 100 μg/mL). Cells treated with PBS were used as negative control (PBS). The cells were fixed with ethanol and stained with propidium iodide present in the HFS solution. G0/G1: resting/preparation for synthesis; S: DNA synthesis; G2/M: preparation for mitosis. Results expressed as mean ± SD of three independent experiments (n = 3). Statistically significant differences (p < 0.05) are labeled with * (in comparison with G0/G1 phase of PBS)
PC-12 and B16F10 cells treated with different concentrations of BthTX-I showed delayed cell cycles, with an increased number of cells in the G0/G1 phase, followed by a reduction in the percentage of cells in the S phase and an absence of cells in the G2/M phase. The increase in the percentage of PC-12 cells in the G0/G1 phase, compared with the negative control, was of ~16 % for the treatments with 10; 25 and 50 μg/mL of BtTX-I, and of 26 % with the toxin at 100 μg/mL (Fig. 2 – c). This increase for B16F10 cells was of approximately 24 % after treatment with BthTX-I at all the concentrations evaluated (Fig. 2 – d).
Analysis of the apoptotic/necrotic effects of BthTX-I
All concentrations of BthTX-I induced apoptosis/necrosis (PI+/AV+) in leukemic cells (HL-60), with the highest percentage of PI+/AV+ cells (~20 %) observed at the concentrations of 50 and 100 μg/mL. There was also a small percentage of cells that were only in necrotic process (1-5 %) at all the concentrations evaluated (Fig. 3 – a).
Assessment of apoptotic/necrotic effects of BthTX-I on the tumor cell lines HL-60 (a), HepG2 (b), PC-12 (c) and B16F10 (d) by flow cytometry. Tumor cells were treated with BthTX-I at different concentrations (25, 50 or 100 μg/mL). Cisplatin (Cisp, positive control) was used as a reference for the induction of apoptosis and cells treated only with PBS were used as negative control. The population of necrotic cells was labeled with propidium iodide (PI+), apoptosis was labeled with FITC-annexin V (AV+) and population of necrotic and apoptotic cells was labeled with PI+/AV+. Results expressed as mean ± SD of three independent experiments (n = 3). Statistically significant differences (p < 0.05) were labeled with * (values of PI+/AV+ compared with PBS); # (values of AV+ compared with PBS) or + (values of PI+ compared with PBS)
The treatment of HepG2 cells with BthTX-I at the concentrations of 25 and 50 μg/mL induced apoptosis/necrosis (PI+/AV+) in 24 % of the cell population, and apoptosis (AV+) in 44 and 34 % of cells, respectively. At the concentration of 100 μg/mL, 16 % of cells were in the process of necrosis (PI+), 40 % in apoptosis/necrosis (PI+/AV+) and 28 % in apoptosis (AV+) (Fig. 3 – b).
BthTX-I at the concentrations of 25 and 50 μg/mL induced about 10 % of PC-12 cell death by apoptosis/necrosis and 15 % by apoptosis. The concentration of 100 μg/mL promoted apoptosis in approximately 20 % of cells (Fig. 3 – c).
The treatment of B16F10 cells with different concentrations of BthTX-I (25, 50 and 100 μg/mL) promoted a high percentage of cell death by apoptosis (AV+) (30 to 40 %), with lower percentages of cells exclusively in the processes of necrosis (3, 8 and 4 %, respectively) or apoptosis (~5 %) (Fig. 3 – d).
Discussion
Many toxins isolated from snake venoms have been used as tools for understanding different pathophysiological events, since these proteins present several biological effects such as antiparasitic, antimicrobial and antitumor activities [22–24]. In that context, snake venom PLA2s of both acidic and basic character, and also synthetic peptides derived from homologous PLA2s, have been extensively studied due to their wide variety of pharmacological effects, including their antitumor and antiangiogenic properties [25–27].
In recent years, PLA2s have been investigated as tools for better understanding the mechanisms related to cancer. This class of toxins seems to act directly on the metabolism of phospholipid membranes, promoting changes in the lipid biosynthesis and deregulation of lipogenesis in different cell lines, including tumorous ones [28].
In the present study, BthTX-I induced significant cytotoxicity in all tested tumor cell lines. In general, all concentrations of the myotoxin inhibited the viability of HL-60, HepG2, PC-12 and B16F10 cells in 50 to 60 %. These results are consistent with many others which have shown that these toxins have the ability to induce death of various tumor and non-tumor cell lines [11, 29, 30].
Cisplatin (Incel, Darrow®) was used in the present study as a positive control of cytotoxicity, being able to promote death of all tumor cell lines evaluated, with lower effects on B16F10 cells. It is an inorganic platinum compound that binds to DNA and provokes structural changes and inhibition of transcription and replication, thus inducing apoptosis. Considering its cytotoxic effects, cisplatin is widely used as an antineoplastic agent in the treatment of several types of cancer [31].
Gebrim et al. [18] evaluated the in vitro antitumor activity of BthTX-I using tumor cell lines such as Jurkat, SKBR3 and B16F10, and showed that the native protein presented 75 to 90 % cytotoxicity on these tumor cell lines. Regarding in vivo experiments, injection of BthTX-I modified with p-bromophenacyl bromide (BPB, an agent that inhibits PLA2s by covalently binding to their active site) in mice transplanted with S180 tumor cells reduced 30 % of the tumor size on the 14th day and 76 % on the 60th day, when compared to the untreated control group. These findings are in accordance with other studies that suggested that the antitumor properties of PLA2s are independent of their catalytic activity [32, 33].
De Moura et al. [11] evaluated the cytotoxic effect of three PLA2s isolated from B. mattogrossensis venom, BmatTX-I (Lys49), BmatTX-II (Lys49) and BmatTX-III (Asp49) on Jurkat and SKBR-3 tumor cells. These authors observed that the Lys49 myotoxins were more cytotoxic than the Asp49 PLA2 to both tumor cells. Similar to that observed for BthTX-I, the toxins BmatTX-I and BmatTX-II at a concentration of 100 μg/mL inhibited the cell viability of Jurkat cells in approximately 50 %. Equivalent effects were observed when Jurkat cells were treated with 100 μg/mL of two acidic Asp49 PLA2s, BmooTX-I from B. moojeni venom and MTX-I from B. brazili venom [34, 35]. BthA-I-PLA2, an acidic PLA2 from B. jararacussu venom, also showed potential antitumor effects on Jurkat, SKBR-3 and Ehrlich ascites tumor (EAT) cells, with the enzyme at 100 μg/mL promoting death of 50 to 70 % of the tumor cells [36].
Studies suggest that several biological effects of the myotoxins are related to the interaction of their C-terminal region with cell membranes, which explains the synthesis of peptides derived from this region described in different studies [18, 35, 37]. The C-terminal region is usually described as capable of disrupting the hydrophilic matrix of membranes, opening pores and allowing the entrance of the toxins into the intracellular medium [38].
Investigations involving the mechanisms of action of snake venom toxins on tumor cells are still scarce in the literature. However, some suggestions of mechanisms have been proposed for components such as L-amino acid oxidases (LAAOs), which have been described as potent cytotoxic agents. Several studies attributed the cytotoxic effects of LAAOs mainly to the release of hydrogen peroxide to the medium, resulting in oxidative stress and consequently in cell death [7, 39].
The effect of cytotoxic agents can be evaluated by cell cycle analyses. The cell cycle is a series of continuous and repetitive processes that occur during a normal cell division [40]. The cycle is divided into interphase and mitosis. The interphase is further subdivided into the phases G0, G1, S and G2, in which the DNA duplication and preparation of cells for the next stage occurs. The mitosis is characterized by the cell division process itself [40, 41].
Flow cytometry experiments were performed for the cell cycle analyses, with the purpose of evaluating the effects of BthTX-I in the process of cell division. Nuclear DNA of cells was stained by a specific fluorochrome (PI), allowing its quantification and reflecting the position of cells in the cell cycle. BthTX-I was not able to induce any significant change in the cell cycle of HL-60 and HepG2 tumor cells. Conversely, the toxin caused a delay in the G0/G1 phase of the cell cycle of PC-12 and B16F10 tumor cells. The fact that the myotoxin promoted delay in the cell cycle indicates that it disrupts the mitotic progression and hence a smaller number of cells are doubled, which would implicate an important antitumor mechanism.
Although few studies have been conducted with snake venom toxins regarding their effects on the cell cycle progression, some other snake toxins were also described as able to promote delay of the cell cycle in the G0/G1 phase [6, 42]. Checkpoint proteins (CKI’s, CDK’s and CHK) are probably preventing these cells from leaving the G0/G1 phase. The CKI’s are able to suspend the cell cycle at any sight of DNA damage, directing the cells to repair systems. The cell cycle only continues when the damaged DNA is restored, and in case the repair systems are unable to correct the damage, cell death processes are induced [43].
Exposure to toxic agents can trigger cell death by processes of apoptosis or necrosis, and both processes occur depending on the intensity and duration of exposure. Necrosis is an accidental form of cell death in which a failure in the cell homeostasis occurs after the cell is damaged, leading to an inflammatory process [44–46]. Apoptosis is a controlled form of cell death, defined by a number of biochemical and morphological characteristics, such as the exposure of phosphatidylserine to the outside of the plasma membrane, the cell nucleus condensation and the cleavage of chromatin (DNA) in oligonucleosomal fragments [47].
The apoptosis-inducing ability of BthTX-I was assessed by flow cytometry using annexin V conjugated to fluorescein, which acts as a marker for the phosphatidylserine exposed on cells undergoing apoptosis stage. The necrotic effect was analyzed by staining with propidium iodide (PI), which marks the nucleic acid of dead cells.
Our results showed that different BthTX-I concentrations induced tumor cell death by processes of apoptosis and/or necrosis. The myotoxin mainly induced apoptosis (AV+) in PC-12 and B16F10 cells at all concentrations tested (25, 50 or 100 μg/mL), which suggests this process as the predominant cell death mechanism induced by BthTX-I on these murine tumor cells. Regarding human tumor cell lines (HL-60 and HepG2), apparently distinct death mechanisms could be observed: the percentage of HepG2 cells in apoptosis (AV+) was practically equal to those in apoptosis/necrosis (PI+/AV+), while for HL-60 cells, the major percentage of cells was marked as PI+/AV+, indicating death mechanisms related to both apoptosis and necrosis.
These findings corroborate the data obtained for other enzymes isolated from snake venoms, which promoted death in various cell lines by different mechanisms, such as apoptosis and necrosis [48, 49]. Several factors may determine whether cells go into apoptosis or necrosis, such as the toxin concentration, the ability of cells to manage local membrane perturbations and the metabolic status of cells [50]. Mora et al. [9] evaluated the induction of apoptosis and necrosis by a Lys49 PLA2 homologue from Bothrops asper snake venom in a lymphoblastoid cell line, and concluded that the enzyme was able to induce significantly different effects depending on the concentration of toxin used.
Conclusion
BthTX-I was shown to be cytotoxic for both human (HepG2 and HL-60) and murine (PC-12 and B16F10) tumor cell lines by inducing cell death mechanisms of apoptosis and/or necrosis. Additionally, BthTX-I was able to promote delay in the G0/G1 phase of the cell cycle of murine tumor cells. The results revealed important functional characteristics of this myotoxin isolated from B. jararacussu venom, demonstrating a remarkable antitumor potential still little explored. Studies on the mechanisms of action of BthTX-I are very important so that it may serve as a model for the development of new therapeutic agents to treat various human diseases such as cancer in the future.
References
World Health Organization. Cancer. http://www.who.int/mediacentre/factsheets/fs297/en/(2012). Accessed 20 Jan 2015.
American Cancer Society. Cancer facts and figures 2014. Atlanta: American Cancer Society, 2014. http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2014/. Accessed 20 Jan 2015.
Abdel-Rahman MA, Abdel-Nabi IM, El-Naggar MS, Abbas OA, Strong PN. Conus vexillum venom induces oxidative stress in Ehrlich’s ascites carcinoma cells: an insight into the mechanism of induction. J Venom Anim Toxins incl Trop Dis. 2013;19(1):10.
Wang L, Martins-Green M. Pomegranate and its components as alternative treatment for prostate cancer. Int J Mol Sci. 2014;15(9):14949–66.
Guo G, Yao G, Zhan G, Hu Y, Yue M, Cheng L, et al. N-methylhemeanthidine chloride, a novel Amaryllidaceae alkaloid, inhibits pancreatic cancer cell proliferation via down-regulating AKT activation. Toxicol Appl Pharmacol. 2014;280(3):475–83.
Alves-Paiva RM, de Freitas Figueiredo R, Antonucci GA, Paiva HH, de Lourdes Pires Bianchi M, Rodrigues KC, et al. Cell cycle arrest evidence, parasiticidal and bactericidal properties induced by L-amino acid oxidase from Bothrops atrox snake venom. Biochimie. 2011;93(5):941–47.
Costa TR, Burin SM, Menaldo DL, de Castro FA, Sampaio SV. Snake venom L-amino acid oxidases: an overview on their antitumor effects. J Venom Anim Toxins incl Trop Dis. 2014;20:23.
Calderon LA, Sobrinho JC, Zaqueo KD, de Moura AA, Grabner AN, Mazzi MV, et al. Antitumoral activity of snake venom proteins: new trends in cancer therapy. Biomed Res Int. 2014. doi:10.1155/2014/203639.
Mora R, Valverde B, Díaz C, Lomonte B, Gutiérrez JM. A Lys49 phospholipase A(2) homologue from Bothrops asper snake venom induces proliferation, apoptosis and necrosis in a lymphoblastoid cell line. Toxicon. 2005;45(5):651–60.
Villalobos JC, Mora R, Lomonte B, Gutiérrez JM, Angulo Y. Cytotoxicity induced in myotubes by a Lys49 phospholipase A2 homologue from the venom of the snake Bothrops asper: evidence of rapid plasma membrane damage and a dual role for extracellular calcium. Toxicol In Vitro. 2007;21(8):1382–9.
de Moura AA, Kayano AM, Oliveira GA, Setúbal SS, Ribeiro JG, Barros NB, et al. Purification and biochemical characterization of three myotoxins from Bothrops mattogrossensis snake venom with toxicity against Leishmania and tumor cells. Biomed Res Int. 2014. doi:10.1155/2014/195356.
Gutiérrez JM, Chaves F, Cerdas L. Inflammatory infiltrate in skeletal muscle injected with Bothrops asper venom. Rev Biol Trop. 1986;34(2):209–14.
Teixeira CF, Landucci EC, Antunes E, Chacur M, Cury Y. Inflammatory effects of snake venom myotoxic phospholipases A2. Toxicon. 2003;42(8):47–962.
Gutiérrez JM, Lomonte B. Phospholipase A2 myotoxins from Bothrops snake venoms. Toxicon. 1995;33(11):1405–24.
Soares AM, Giglio JR. Chemical modifications of phospholipases A2 from snake venoms: effects on catalytic and pharmacological properties. Toxicon. 2003;42(8):855–68.
Homsi-Brandeburgo MI, Queiroz LS, Santo-Neto H, Rodrigues-Simioni L, Giglio JR. Fractionation of Bothrops jararacussu snake venom: partial chemical characterization and biological activity of bothropstoxin. Toxicon. 1988;26(7):615–27.
Chioato L, Aragão EA, Lopes Ferreira T, Medeiros AI, Faccioli LH, Ward RJ. Mapping of the structural determinants of artificial and biological membrane damaging activities of a Lys49 phospholipase A2 by scanning alanine mutagenesis. Biochim Biophys Acta. 2007;1768(5):1247–57.
Gebrim LC, Marcussi S, Menaldo DL, de Menezes CS, Nomizo A, Hamaguchi A, et al. Antitumor effects of snake venom chemically modified Lys49 phospholipase A2-like BthTX-I and a synthetic peptide derived from its C-terminal region. Biologicals. 2009;37(4):222–9.
Cintra AC, Marangoni S, Oliveira B, Giglio JR. Bothropstoxin-I: amino acid sequence and function. J Protein Chem. 1993;12(1):57–64.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63.
Kommoju PR, Macheroux P, Ghisla S. Molecular cloning, expression and purification of L-amino acid oxidase from the Malayan pit viper Calloselasma rhodostoma. Protein Expr Purif. 2007;52(1):89–95.
Costa Torres AF, Dantas RT, Toyama MH, Diz Filho E, Zara FJ, de Queiroz MG R, et al. Antibacterial and antiparasitic effects of Bothrops marajoensis venom and its fractions: Phospholipase A2 and L-amino acid oxidase. Toxicon. 2010;55(4):795–804.
Vargas LJ, Quintana JC, Pereañez JA, Núñez V, Sanz L, Calvete J. Cloning and characterization of an antibacterial L-amino acid oxidase from Crotalus durissus cumanensis venom. Toxicon. 2013;64:1–11.
Doumanov J, Mladenova K, Topouzova-Hristova T, Stoitsova S, Petrova S. Effects of vipoxin and its components on HepG2 cells. Toxicon. 2015;94:36–44.
Soares AM, Guerra-Sá R, Borja-Oliveira CR, Rodrigues VM, Rodrigues-Simioni L, Rodrigues V, et al. Structural and functional characterization of BnSP-7, a Lys49 myotoxic phospholipase A(2) homologue from Bothrops neuwiedi pauloensis venom. Arch Biochem Biophys. 2000;378(2):201–9.
Cavalcante WL, Silva MD, Gallacci M. Influence of temperature upon paralyzing and myotoxic effects of bothropstoxin-I on mouse neuromuscular preparations. Chem Biol Interact. 2005;151(2):95–100.
Araya C, Lomonte B. Antitumor effects of cationic synthetic peptides derived from Lys49 phospholipase A2 homologues of snake venoms. Cell Biol Int. 2007;31(3):263–8.
Mashima T, Seimiya H, Tsuruo T. De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br J Cancer. 2009;100(9):1369–72.
Mora-Obando D, Díaz C, Angulo Y, Gutiérrez JM, Lomonte B. Role of enzymatic activity in muscle damage and cytotoxicity induced by Bothrops asper Asp49 phospholipase A2 myotoxins: are there additional effector mechanisms involved? PeerJ. 2014;2:e569.
Gasanov SE, Dagda RK, Rael ED. Snake venom cytotoxins, phospholipase A2s, and Zn2+-dependent metalloproteinases: mechanisms of action and pharmacological relevance. J Clin Toxicol. 2014;4(1):1000181.
Pabla N, Dong Z. Curtailing side effects in chemotherapy: a tale of PKCO in cisplatin treatment. Oncotarget. 2012;3(1):107–11.
Stábeli RG, Amui SF, Sant’Ana CD, Pires MG, Nomizo A, Monteiro MC, et al. Bothrops moojeni myotoxin-II, a Lys49-phospholipase A2 homologue: an example of function versatility of snake venom proteins. Comp Biochem Physiol C Toxicol Pharmacol. 2006;142(3–4):371–81.
Rodrigues RS, Izidoro LF, de Oliveira Jr RJ, Sampaio SV, Soares AM, Rodrigues VM. Snake venom phospholipases A2: a new class of antitumor agents. Protein Pept Lett. 2009;16(8):894–8.
Santos-Filho NA, Silveira LB, Oliveira CZ, Bernardes CP, Menaldo DL, Fuly AL, et al. A new acidic myotoxic, anti-platelet and prostaglandin I2 inductor phospholipase A2 isolated from Bothrops moojeni snake venom. Toxicon. 2008;52(8):908–17.
Costa TR, Menaldo DL, Oliveira CZ, Santos-Filho NA, Teixeira SS, Nomizo A, et al. Myotoxic phospholipases A(2) isolated from Bothrops brazili snake venom and synthetic peptides derived from their C-terminal region: cytotoxic effect on microorganism and tumor cells. Peptides. 2008;29(10):1645–56.
Roberto PG, Kashima S, Marcussi S, Pereira JO, Astolfi-Filho S, Nomizo A, et al. Cloning and identification of a complete cDNA coding for a bactericidal and antitumoral acidic phospholipase A2 from Bothrops jararacussu venom. Protein J. 2004;23(4):273–85.
Lomonte B, Angulo Y, Moreno E. Synthetic peptides derived from the C-terminal region of Lys49 phospholipase A2 homologues from viperidae snake venoms: biomimetic activities and potential applications. Curr Pharm Des. 2010;16(28):3224–30.
Montecucco C, Gutiérrez JM, Lomonte B. Cellular pathology induced by snake venom phospholipase A2 myotoxins and neurotoxins: common aspects of their mechanisms of action. Cell Mol Life Sci. 2008;65(18):2897–912.
Costa TR, Menaldo DL, Prinholato da Silva C, Sorrechia R, Albuquerque S, Pietro RC, et al. Evaluating the microbicidal, antiparasitic and antitumor effects of CR-LAAO from Calloselasma rhodostoma venom. Int J Biol Macromol. 2015;80:489–97.
Vermeulen K, Van Bockstaele DR, Berneman ZN. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003;36(3):131–49.
Schafer KA. The cell cycle: a review. Vet Pathol. 1998;35(6):461–78.
Zhang L, WU WT. Isolation and characterization of ACTX-6: a cytotoxic L-amino acid oxidase from Agkistrodon acutus snake venom. Nat Prod Res. 2008;22:554–63.
Douglas RM, Haddad GG. Genetic models in applied physiology: invited review: effect of oxygen deprivation on cell cycle activity: a profile of delay and arrest. J Appl Physiol. 2003;94(5):2086–3.
Dypbukt JM, Ankarcrona M, Burkitt M, Sjöholm A, Ström K, Orrenius S, et al. Different prooxidant levels stimulate growth, trigger apoptosis, or produce necrosis of insulin-secreting RINm5F cells. The role of intracellular polyamines. J Biol Chem. 1994;269(48):30553–60.
Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, Lipton SA. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-D-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci USA. 1995;92(16):7162–6.
Ande SR, Kommoju PR, Draxl S, Murkovic M, Macheroux P, Ghisla S, et al. Mechanisms of cell death induction by L-amino acid oxidase, a major component of ophidian venom. Apoptosis. 2006;11(8):1439–51.
Schultz DR, Harrington Jr WJ. Apoptosis: programmed cell death at a molecular level. Semin Arthritis Rheum. 2003;32(6):345–69.
Samel M, Tõnismägi K, Rönnholm G, Vija H, Siigur J, Kalkkinen N, et al. L-Amino acid oxidase from Naja naja oxiana venom. Comp Biochem Physiol B Biochem Mol Biol. 2008;149(4):572–80.
Ciscotto P, de Avila RA M, Coelho EA, Oliveira J, Diniz CG, Farías LM, et al. Antigenic, microbicidal and antiparasitic properties of an l-amino acid oxidase isolated from Bothrops jararaca snake venom. Toxicon. 2009;53(3):330–41.
Leist M, Single B, Castoldi A, Kuhnle S, Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med. 1997;185(8):1481–6.
Acknowledgments
The authors would like to thank the financial support provided by the State of São Paulo Research Foundation (FAPESP, grants n. 2010/03243-43 and 2011/23236-4), the Coordination for the Improvement of Higher Education Personnel (CAPES) and the National Council for Scientific and Technological Development (CNPq process n. 476932/2012-2). We are also grateful to Fabiana Rosseto Morais, from FCFRP-USP, for the technical assistance in the flow cytometry analyses. Thanks are also due to the Center for the Study of Venoms and Venomous Animals (CEVAP) of UNESP for enabling the publication of this special collection (CNPq process 469660/2014-7).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CPS, RMAP and ACOC conceived and performed the experiments of this study. CPS, TRC and DLM analyzed the results and drafted the manuscript. LMGA and SVS supervised and critically discussed the study, and contributed with infrastructure and materials. All authors read and approved the final version of the manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Prinholato da Silva, C., Costa, T.R., Paiva, R.M.A. et al. Antitumor potential of the myotoxin BthTX-I from Bothrops jararacussu snake venom: evaluation of cell cycle alterations and death mechanisms induced in tumor cell lines. J Venom Anim Toxins Incl Trop Dis 21, 44 (2015). https://doi.org/10.1186/s40409-015-0044-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40409-015-0044-5