Abstract
Immunotherapy has become the major treatment for tumors in clinical practice, but some intractable problems such as the low response rate and high rates of immune-related adverse events still hinder the progress of tumor immunotherapy. Hence, it is essential to explore additional immunotherapy treatment targets. In this review, we focus on the structure, expression and expression-related mechanisms, interactions, biological functions and the progress in preclinical/clinical research of IGSF11 and VISTA in tumors. We cover the progress in recent research with this pair of immune checkpoints in tumor immune regulation, proliferation, immune resistance and predictive prognosis. Both IGSF11 and VISTA are highly expressed in tumors and are modulated by various factors. They co-participate in the functional regulation of immune cells and the inhibition of cytokine production. Besides, in the downregulation of IGSF11 and VISTA, both inhibit the growth of some tumors. Preclinical and clinical trials all emphasize the predictive role of IGSF11 and VISTA in the prognosis of tumors, and that the predictive role of the same gene varies from tumor to tumor. At present, further research is proving the enormous potential of IGSF11 and VISTA in tumors, and especially the role of VISTA in tumor immune resistance. This may prove to be a breakthrough to solve the current clinical immune resistance, and most importantly, since research has focused on VISTA but less on IGSF11, IGSF11 may be the next candidate for tumor immunotherapy.
Similar content being viewed by others
Introduction
Immunotherapy has become the major therapeutic method for tumors with a favorable treatment effect. Various immune checkpoints are the mature targets in tumor immunotherapy, like PD1/PD-L1 (Programmed Cell Death 1/ Programmed Cell Death Ligand 1), CTLA4 (Cytotoxic T-Lymphocyte Associated Protein 4) and LAG3 (Lymphocyte Activating 3), are in ongoing clinical trials [1]. But there are still some intractable problems, since the response rate of immunotherapy is too low but the irAEs (immune-related adverse events) are relatively high. For example, the response rate of anti-PD1/PD-L1 monotherapy or combination therapy is only about 30% in non-small cell lung cancer (NSCLC) [2], but the irAEs’ incidence of anti-CTLA-4 inhibitors is nearly 70% in 1265 oncologic patients from 22 clinical trials [3]. Thus, it is urgent to explore novel immune checkpoints with a higher response rate and lower incidence of irAEs, and we focus on IGSF11 and VISTA. IGSF11 (immunoglobulin superfamily 11 gene, also known as BT-IgSF, BTIGSF, CT119, CXADRL1, VSIG3) belongs to the immunoglobulin superfamily, and is a 46 KDa protein containing 431 amino acids. It is located on chromosome 3q13.32, exerts in cell adhesion [4, 5], migration [6], proliferation, differentiation [4, 7, 8], synapses’ induction [9, 10], maintaining the integrity of blood-testis barrier [5, 11] and meiotic diplotene of somatic cells and germ cells [12]. Besides this, it serves as the ligand of VISTA, regulating the function of immune cells, especially for T cells [13]. VISTA (V-domain immunoglobulin suppressor of T cell activation, also known as VSIR, B7-H5, B7H5, C10orf54, Dies1, PD-1H, SISP1), belongs to the immunoglobulin family, but is limited with other B7 family members, VISTA is a 34 KDa protein containing 311 amino acids, located on chromosome 10q22.1. There are two proved ligands of VISTA, one is IGSF11, the other is PSGL1 (P-selectin glycoprotein ligand 1) [13, 14]. Besides these, VSIG8, NSC622608 and Galectin 9 may be potential ligands for VISTA [15, 16]. The interaction between ligands and receptors may be modulated by the pH in microenvironment [14], and exert innate and adaptive immune regulation [17, 18]. The overexpression of VISTA may induce the secretion of TNF-α, IL-10, IL-6 and IL-1B [19], whereas the deficiency of VISTA may affect the production of MIG, IP10, MCP-1, the number of CD4+ T cells in blood and myeloid cells in spleen [20, 21]. Besides which, VISTA can also function in microglia inflammation [22, 23], and chemokines’ responsiveness [24]. IGSF11 and VISTA are also a pair of immune checkpoints that exert in tumor proliferation and immune regulation [25], which has enormous potential to be used as a novel tumor immunotherapy target and biomarker [26]. In this review, we illuminate the structure, expression, biological effect and clinical application of IGSF11 and VISTA, aiming at summarizing the recent research progress for further exploration (Fig. 1).
[The structure, expression, binding site and the immune regulation of IGSF11 and VISTA]. (a) Pattern diagram of the interaction between IGSF11 and VISTA. IGSF11 binds with VISTA mainly by V-type and C-type immunoglobulin-like domain. (b) The membrane expression, binding site and domains related with immune regulation of IGSF11 and VISTA
IGSF11/VISTA structure and expression
IGSF11/VISTA structure
The structural research about IGSF11 and VISTA may provide more targets for specific antibody design. IGSF11 is a type I transmembrane protein containing an extracellular domain, transmembrane domain and cytoplasmic domain [13]. Three domains construct the extracellular domain, including the C-type domain, V-type domain (with signal peptide) and PDZ domain; the V-type and C-type immunoglobulin-like domain are responsible for binding with VISTA [13]. The crystal structure of the extracellular domain is identified at 2.64 angstrom resolution, which may provide the structural basis for IGSF11 antibody [27]. The structure of VISTA contains as follows: a large 130 aa IgV-like domain linked with a 33 aa stalk segment; a 20 aa transmembrane region linked with a 96 aa cytoplasmic tail [28, 29]. The concentration of histidine residues in the extracellular domain is striking, and is involved in the inhibition of T cell activation [30]; SH2 domain (Src homology domain 2) locates at the middle of the cytoplasmic tail, SH3 domain (Src homology domain 3) is also a part of VISTA, casein kinase 2 and phosphokinase C phosphorylation sites consisting of the cytoplasmic domain [15, 31]. The crystal structure of VISTA is unique because of its extended CC’ loop region (a target of the VISTA block) with an attached helix and two disulfide bonds, the binding epitope of VISTA overlaps with IGSF11, and research on the VISTA crystal structure may also provide a novel target for antibody design [29].
IGSF11/VISTA expression and expression-related regulation mechanisms
Both IGSF11 and VISTA are highly expressed in tumors, the expression and expression-related regulation mechanisms of IGSF11 and VISTA are listed in Table 1. Compared to the minimal expression of IGSF11 in the normal tissue, IGSF11 is highly expressed in gastrointestinal tumors, including colorectal cancer, hepatocellular carcinoma and gastric cancer [32], however, the role of IGSF11 in esophageal carcinoma (which is also a part of digestive tract), is still unknown. In the breast tumor model, the expression of IGSF11 is associated with TGF-β; TGF-β regulates the EMT triggers, which promote the expression of LincRNA Platr18, then induces the expression of IGSF11, and the whole process may be related to the metastasis of breast cancer [33].
VISTA is found highly expressed in normal cells and malignant cells, and exerts in tumor immune regulation. VISTA is raised in tumor infiltration related cells, including T cells, especially in Tregs naïve CD4+ and CD8+ TCRαβ T cells, and TCRγδ T cells. MDSCs (Myeloid-derived suppressor cells) and macrophages are also found with high expression of VISTA [34,35,36]. Similar to CTLA-4, VISTA is detected raised in intracellular compartment and cell surface in myeloid cells, which may exert in cell signals exchange [37]. Specifically, VISTA inhibits the activation of TLR-induced IKK/NF-kB and MAPK/AP-1 signal pathways, to regulate the immunosuppression and inflammation of myeloid cells [38, 39]. The high expression of VISTA in naive T cells may be related to immune tolerance [40]. Exclusion for immune cells, VISTA expresses higher in tumors and may interact with IGSF11 in immune regulation, including NSCLC [41], ovarian cancer [42], gastric cancer [43], colorectal cancer [44], soft tissue sarcoma [25] and oral squamous cell carcinoma [45]. High expression of VISTA is found in gastric cancer, especially in EBVa gastric cancer and cancer with liver metastasis, the expression of VISTA is associated with PD-L1, the co-expression of VISTA and PD-L1 may help gastric cancer patients benefit from combination therapy [43]. In melanoma, it has been found that the expression of VISTA is highly associated with the expression of PD-1 and CD33 (MDSCc marker), indicating that both of them may work together in tumor immune inhibition [46]. After the treatment with ipilimumab, the expression of VISTA is raised higher on CD68+ macrophages, CD4+ T cells and CD8+ T cells, given that VISTA is the inhibitory immune checkpoint in prostate cancer, no matter whether for metastatic or localized. This may be the target to explain the ipilimumab treatment resistance and may provide a novel therapy strategy for prostate cancer [47]. In various tumors like breast cancer, NSCLC and gynecological oncology [48,49,50], VISTA is proven to be expressed on the membrane and in the cytoplasm. Single cell sequencing proves the high expression of VISTA in breast cancer, and the immunohistochemistry indicates that VISTA is highly expressed in cytoplasm and membrane and is positively associated with the expression of PD-1 (P = 0.038), pathological grade (P = 0.001) and lymph node status (P = 0.045) [51]. In NSCLC, VISTA is highly accumulated in stromal cell cytoplasm and membrane, and the high level is positively related with the PD-1/PD-L1 axis, and negatively associated with tumor EGFR mutations [52]. Similar results are proven in 758 NSCLC samples, where high expression of VISTA are also positively related with CD68+ macrophages and CD8+T cells, as well as the low mutation burden [53]. In cervical cancer, VISTA is found expressed in the membrane and cytoplasm of tumor cells, VISTA may co-express with Foxp3, Foxp3 is expressed in CIN I-III, but VISTA only expresses in CIN II-III, the co-expression of which indicates the prognosis of cervical patients (see part 5.2) [54]. Similarly, high expression of VISTA is proven on the membrane and in the cytoplasm of endometrial cancers, especially in G1/G2/G3 histopathologic grades and serous subtypes, in endometrial cancers, high infiltration of CD8+T cells is associated with more VISTA expression. Most subtypes of ovarian cancers like mucinous, serous, clear cell, endometrioid, and undifferentiated carcinoma, all proved to have high VISTA expression, especially in stage I and II cancers [55, 56]. In soft tissue sarcoma, the results of IHC shows that VISTA is highly accumulated on the membrane and cytoplasm of tumor cells, and positively associated with the expression of PD1 and PD-L1 [25], higher expression of PD1 is proved within sarcoma cells [57] and higher PD-L1 membrane expression is mainly found on tumor-infiltrating myeloid cells [58], especially on macrophages, these three immune checkpoints are involved in the regulation of tumor immunosuppression. The expression of VISTA is highly associated with CD11b, in colon cancer, CD11b+ cells always have a high VISTA expression, and VISTA+ cells have high CD11b expression in lung cancer cells [59], which may be related to antigen presentation and T cell activation. VISTA is found to co-express with other immune checkpoints TIM3 and IDO but is not correlated with survival in pancreatic ductal adenocarcinoma. This is a kind of tumor featured with immune escape, and the immune checkpoints co-expression may be responsible for the immune escape [60]. Similar results are proven in colorectal cancer, the high expression of C10orf54 (encoding VISTA) is positively associated with other immune checkpoints like PD1/PD-L1, TIGIT, BTLA and HAVGR2, and positively related with the anti-inflammation factors like Foxp3 and TGFb1, but negatively associated with the Kras mutation, also meaning that VISTA may be involved in immune escape, thus further investigations are warranted [44].
The expression of VISTA may be modulated by multiple factors, but nearly no reports for IGSF11, the mechanisms to regulate the expression of IGSF11 and VISTA can be found in Table 1. VISTA has its own unique promoter region. There are three potential transcription factors, including JunD, NF-κB (nuclear factor kappa B) and Fos, which bind to the promoter of VISTA and regulate its expression [40, 61]. There are not any known enhancers regulating VISTA expression that have been reported [62]. Besides, miRNA-125a/miRNA-125b both exert in VISTA expression post-transcriptional regulation by binding VISTA mRNA and inducing its degradation [63, 64]. In endometrial cancer, the expression of VISTA is modulated by DNA methylation, and the VISTA promoter region 2 may be responsible for the methylation regulation [55]. The expression of VISTA can be modulated by multiple factors in gastric cancer, and an EMT/MET ( epithelial-mesenchymal transition/ mesenchymal-epithelial transition) model is induced by TGFβ1. In this model, the expression of VISTA is associated with promoter methylation, especially in the specific CpG sites, and VISTA is proven to regulate its downstream effectors, ID2/ID3; besides, the overexpression of miR-125a-5p can significantly inhibit VISTA expression [65]. In melanoma, the inhibition of BRAF promotes the upregulation of FOXD3 (factor Forkhead box D3) by blocking MEK/ERK. FOXD3 serves as the transcription factors of VISTA in melanoma, and the upregulation of FOXD3 can effectively inhibit the expression of VISTA; thus, BRAF and FOXD3 co-participate in the expression of VISTA [66]. Besides, in various cancer cells, keratinocytes and T cells (including MCF-7, Jurkat T, HaCaT keratinocytes, THP-1, K562 and WT-3ab), the expression of VISTA is modulated by TGF-β/Smad3 signal pathway [67], however, the downstream of both of the axes needs further research.
The interaction between IGSF11 and VISTA
IGSF11 is the specific ligand of VISTA [13]. Co-IP proves the interaction between IGSF11 and VISTA, both SPR (surface plasmon resonance) and FACS (fluorescence-activated cell sorting) assays also prove the specificity between IGSF11 and VISTA [68]. They also prove that after IGSF11 binding with VISTA in V-type and C-type immunoglobulin-like domain, T cell proliferation and related cytokine production can all be inhibited, including IL-17(interleukin-17), CCL3(chemokine ligand 3), CXCL11(C-X-C motif chemokine 11) and CCL5(chemokine ligand 5) [13], and, this provides a theoretical basis for the application of IGSF11 in oncology. IGSF11 antibody and VISTA antibody can effectively block their interaction [27]. SG7 is an antibody targeting VISTA, and there are four epitopes inhibiting VISTA: H122, K38, E125, F36, which overlap with the other two VISTA antibodies, BMS767 and VSTB112 (Bristol Myers Squibb). SG7 can simultaneously compete with the two antibodies and reactivate the function of T cells [69]. The interaction between IGSF11 and VISTA can be affected by the pH of TME (tumor microenvironment), the binding affinity between IGSF11 and VISTA at pH 7.4 is 20 nM, but 80 nM at pH 6.0 (often seen in TME) [69]. HMBD-002 is another kind of antibody targeting the CC’ loop of VISTA, which can effectively block the interaction between VISTA and IGSF11, and further inhibit the production of IFN-γ from IGSF11-mediated T cells. The affinity between HMBD-002 and VISTA can also be affected by the pH, which has the highest affinity at pH 5.5–7.5 [70].
The biological function of IGSF11 and VISTA in tumors
The role of IGSF11 and VISTA in immune regulation, mainly in tumors
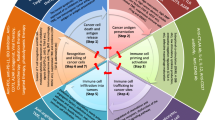
Both IGSF11 and VISTA are active in immune cell function and affect cytokine production (Fig. 2). In advanced human gliomas, high expression of IGSF11 is associated with deep immune infiltration, especially for CD4+ and CD8+ T cells, with high level of TGF-β (P < 0.0001), indicating that IGSF11 induces the infiltration of immune cells but weakens their function, finally creating an inhibitory immune microenvironment [71]. Similarly, VISTA is also raised high in glioma, and the expression of IL-10 and TGF-β increases with the increase in VISTA expression [72].
VISTA affects the function of a variety of immune cells, mainly for T cells. VISTA exerts negative immune regulation mainly by inhibiting the activation of T cell receptors, suppressing the proliferation and cytokine production of T cells, but less by affecting cell apoptosis [73, 74]. VISTA may serve with PD1/PD-L1 in immune regulation in tumors, especially for T cell function and activation [59, 75]. In soft tissue sarcomas, high expression of VISTA is found associated with higher level of TIL (tumour-infiltrating lymphocyte, P = 0.0033), PD1 (P = 0.0046), PD-L1 (P = 0.0031) and CD3 (P = 0.023). It has been proven that PD1/PD-L1 exert in tumor immune inhibition, which may form a balance with VISTA in soft tissue sarcoma immune regulation [25]. In pancreatic cancer, VISTA may induce the immune deficiency microenvironment, and compared to a melanoma, which is sensitive to immunotherapy, the expression of VISTA is higher on CD68+ macrophages in pancreatic cancer. VISTA may significantly inhibit the production of cytokines like TNF-α and IFN-γ; it can also cooperate with PD-L1 in CD8+ T-cell inhibition, and the co-inhibition of PD1/PD-L1 and VISTA may help restore the function of T cells in pancreatic cancer [76]. Contrarily, Hou et al. prove that VISTA is highly raised in both tumor cells and immune cells in pancreatic cancer, and especially, that a high level of VISTA is positively associated with CD19+ B cells, CD3+ T cells and CD68+ macrophages [77]. However, further cytological tests are needed to confirm whether the function of these cells is affected. Further, 13F3 (anti-VISTA monotherapy) can effectively improve the tumor microenvironment, reduce the number of tumor specific Treg cells and MDSCs, increase the number of TILs, and promote the function of T cells in melanoma and bladder carcinoma mouse models [34, 78]. In ovarian and endometrial cancer, VISTA can significantly inhibit T cell proliferation and cytokine IFN-γ production, especially for tumor infiltration CD8+ T cells, and it also proves that the downregulation of VISTA in endometrial cancer cells can restore T cell proliferation and cytokine production [42, 55]. Naïve mice generate the protective antitumor immunity after being vaccinated with irradiated MCA105 tumor cells, and MCA105 tumor cells are transfected by VISTA-RFP or Control-RFP to express higher VISTA. It proves that higher expression of VISTA can significantly interfere with the protective antitumor immunity, leading tumors to grow vigorously [79]. In NK/T cell lymphoma, the count of CD8+ TILs increases with the high expression of VISTA and higher with the co-expression of VISTA and PD-L1, and the single marker high expression of VISTA is correlated with the increase of Foxp3+ TILs, but the immune regulation mechanisms are still unknown, besides, high expression of VISTA predicts poor prognosis, thus, maybe the overexpression of VISTA promotes the accumulation of immune cells but inhibits their function? Further exploration is warranted [80]. In NSCLC, high expression of VISTA plays an immunomodulatory function and increases the count of tumor-infiltrating lymphocytes, tumor associated macrophages, effector T cells and PD-1 axis markers [52]. In AML, galectin-9 may be the novel ligand of VISTA and involved in the programmed death of T cells. After the binding between VISTA and galectin-9, caspase-3 and apoptotic death (granzyme B-dependent) are activated, the block of granzyme B can inhibit the apoptotic process, indicating that granzyme B is involved in the apoptosis of T lymphocytes and the interaction between VISTA and galectin-9, as well as the immune regulation of T cells [81]. It is reported that there is no any expression of VISTA in B cells, high expression in naïve CD4+ and Foxp3+ Regulatory T cells, highest in myeloid cells [17], thus, the immune regulatory role of VISTA for immune cells in tumors and the immune cells subsets in some other immune-related diseases are listed in Table 2.
The role of IGSF11 and VISTA in tumor growth, proliferation
Both IGSF11 and VISTA may be involved in tumor growth. In gastric cancer St-4 cells, the reduced expression of IGSF11 decreases the transfected St-4 cells number, the growth inhibitory effect can also be found in NIH3T3 cells and proved by colony formation assay in gastric cancer, but the intrinsic mechanisms are still unclear [32]. Besides, Katoh et al. indicate that IGSF11 is associated with some adhesion molecules’ encoding, like ESAM, FLJ22415 and CXADR, which may involve cell adhesion and drug delivery in gastric cancer [7]. In the fibrosarcoma mouse model, the overexpression of VISTA may induce the inhibition of T cells and boost the growth of tumor cells [79]. Similar results can be found in the glioma mouse model; VISTA-deficiency mice have a slower tumor growth rate, therefore, the block of VISTA may serve in tumor growth inhibition [82]. The melanoma animal model shows that the block of VISTA promotes the proliferation, infiltration and effector role of T cells, reducing the count and inhibiting the activation of MDSCs and Tregs, finally, boosting the growth of the melanoma [34]. In PDAC (pancreatic ductal adenocarcinoma), the expression of VISTA may be associated with TLR4; the downregulation of VISTA and TLR4 by siRNA and naloxone, respectively, can inhibit the growth of PDAC, indicating that VISTA and TLR4, with their downstream signal pathways, are all involved in PDAC growth [83]. Given that VISTA is also accumulated in cytoplasm of tumor cells, we consider that VISTA may bind with IGSF11 in cytoplasm and exert in tumor proliferation, but this hypothesis warrants more confirmation (Fig. 3).
The preclinical and clinical research progression of IGSF11 and VISTA
The research about targeted drugs
Both IGSF11 and VISTA have the potential to be the novel targets in tumor immunotherapy (Table 3). In gastric cancer, based on IGSF11, the polypeptide vaccine that is designed can effectively enhance the function of CTLs (cytotoxic T lymphocytes), which are identified for their favorable role in tumor inhibition and patients’ survival [32]. SG7 is such an antibody inhibiting the interaction between IGSF11 and VISTA. In the melanoma mouse model, the administration of SG7 for two weeks at 10 mg/kg can effectively inhibit the tumor growth; further, SG7 combined with anti-PD1 in the MC38 colon carcinoma model, also obtained similar results, which is a good sign for clinical application [69].
There are only 3 clinical trials targeting VISTA: JNJ-61610588, CA-170 and CI-8993 (NCT02671955, NCT02812875, NCT04475523). JNJ-61610588 is the first anti-VISTA antibody in clinical trial for solid tumors, but is terminated at present [84], but the studies about CA-170 get progress rapidly. The combination therapy of CA170 and KRAS peptide vaccine can effectively inhibit lung tumorigenesis in the mouse model. The administration of CA170 helps in boosting the infiltration of CD8+ T cells, decreasing the infiltration of MDSCs and Tregs, and exerting its potent anti-tumor effect, especially combined with the KRAS vaccine [85]. Clinical trial NCT02812875 has been completed and proved the potent anti-tumor effect of CA-170 [86], besides, in this clinical trial which enrolled 59 patients (13 NSCLC, 10 colorectal cancer, 8 SCCHN, 5 ovarian cancer, 4 melanoma, 3 renal cell carcinoma, 2 breast cancer, 2 esophageal cancer, 2 Hodgkin's Lymphoma, 2 Non-Hodgkin's Lymphoma, 8 other tumors), CA170 was administered 1200 mg twice daily in 21 days cycles. Of the patients, 33 patients had the best response based on RECIST (response evaluation criteria in solid tumors), but some reported grade 1–2 irAEs like nausea, constipation and fever, and some experienced grade 3–4 irAEs such as increased blood bilirubin, and amylase increase, which all occurred during the process of treatment [84].
CI-8993 is another antibody targeting VISTA, which is in a phase I clinical trial recruiting 50 relapsed and/or refractory solid tumor patients to evaluate its safety [87]. In the B16OVA melanoma model and MB49 bladder tumor model, VISTA mAbs show significant therapeutic effect. For the B16OVA melanoma model, the performance of 13F3 (VISTA mAb) can effectively inhibit the proliferation of tumor and induce its apoptosis, increase the count of IFN-γ–producing cells and promote the response of tumor specific T cells. Besides, the administration of αVISTA (VISTA mAb) shows similar anti-tumor effect in the B16OVA melanoma model, where the count of CD4+ T cells and CD8+ T cells increased (6.38% to 11.74%, 9.25% to 17.74%, respectively), and the percentage of MDSCs significantly decreased (37.74% to 25.64). For the MB49 bladder tumor model, the performance of VISTA mAb can effectively inhibit tumor growth by activating tumor-associated CD11c+DCs and inducing the production of IL-12 and TNF-α. Furthermore, VISTA expresses higher in CD62L− and ICOS− Tregs, and the block of VISTA decreases the count of Foxp3GFP+ iTregs and directly inhibit Tregs activation, enhancing the proliferation and cytotoxic function of CD8+ T cells [34].
The compound 6809‑0223 is a small molecule and a hit ligand with an excellent binding rate when binding with VISTA-ECD (extracellular domain). This can significantly promote the proliferation of CD4+ T cells, and induce the production of IL-2 from both CD4+ and CD8+ T cells, and the production of IL-4, IFN-γ and TNF-α from CD8+ T cells, thus exerting potent immune regulation in the mouse model. The security and possibility for clinical application warrants further evaluation [88]. HMBD-002 is a kind of antibody specifically binding to the CC’ loop of the antibody and is involved in inhibiting IFN-γ secretion. HMBD-002 remodels the immune microenvironment and promotes the response of proinflammatory Th1 cells, and increases the number of CD11b+ macrophages, CD11c+ DCs and CD8+ T cells. This shows potent anti-tumor effects in the CT26 colon cancer mouse model, 4T1 breast cancer model, HCT15 colorectal cancer model and A549 lung cancer model, where the tumor growth inhibition rates are 84%, 53%, 65% and 62%, respectively [70].
In the CT26 colon carcinoma mouse model, the combination therapy for anti-VISTA and anti-PD-L1 mAbs (VISTA-Ig and PD-L1 Ig) may induce 80% tumor regression by enhancing the response and increasing the count of T cells, boosting the antigen presentation and T cells’ activation [89], which may be attributed to the synergistic effect of VISTA and PD-1 in tumor immune regulation. Compared to the combination therapy of anti-PD-1 with anti-VISTA, the combination between anti-CTLA-4 and anti-VISTA (MIH63) shows stronger anti-tumor effect in the squamous cell carcinoma cell model, significantly slowing the growth of the tumor by activating and upregulating CD8+ T cells, converting the exhausted cells into effector CD8+T cells, and promoting the secretion of TNF- α and IFN-γ [90, 91].
Immunotherapy resistance
VISTA may be involved in PD1/PD-L1 treatment resistance. About 50% patients develop the anti- PD-L1 resistance during the treatment and this can be attributed to the problem of antigen presentation and T cell exhaustion [92, 93]. It was also found that VISTA raised higher expression after the treatment of anti-PD1 or anti-CTLA-4 [53]. These two phenomena may be linked together and VISTA may contribute to the resistance immunotherapy, and the combination therapy of anti-VISTA and anti-PD1 may delay the progression of resistance. Clinical trial NCT02812875 aims at evaluating the function of CA170 (anti- VISTA/PD-L1) in solid tumor or lymphoma, which has proved its potent antitumor effect and may help to get a higher immune response rate [86]. VISTA may also be involved in PD-1 resistance in NK/T cell lymphoma. Patients with low expression of VISTA show no response to anti-PD-1 therapy but those with high expression show complete remission, which can even be maintained for more than 12 months [80]. VISTA may also induce the anti-PD-1 resistance in metastatic melanoma. Kakavand et al. found that from pretreatment to disease progression, the expression of VISTA (P = 0.009), PD-L1 and FOXP3 all increased during the process. Given the negative immune regulation function of VISTA in tumors, VSITA may be involved in the failure of anti-PD-1 therapy; however, the mechanisms of VISTA in metastatic melanoma immunotherapy resistance are still unclear, and warrant further exploration [94]. The combination therapy of cyclophosphamide, radiation therapy, plus the dual block of PD-1/VISTA, may exert a potent anti-tumor effect in metastatic triple negative breast cancer. This has been proven in the 4T1 tumor mouse model, and, most importantly, this strategy can effectively improve the anti-PD-1 therapy resistance and inhibit lung metastasis by activating CD8 + T cells and decreasing the counts of MDSCs (53.55% vs. 19.9). Further, the combination of radiation therapy with anti-VISTA can also decrease the number of MDSCs in tumors [95].
Prognosis prediction
Both IGSF11 and VISTA can predict the poor prognosis in tumors (Fig. 4). The survival curves with IGSF11 and VISTA for 19 tumors can be found in Supplementary Fig. 1.
At present, the relationship between IGSF11 and tumor prognosis prediction is only reflected in glioma, which may coordinate with PD-1, especially in advanced human gliomas, the high expression of IGSF11 is associated with poor overall survival (P = 0.0004) the high co-expression of PD-1 and VISTA in advanced human gliomas show poorer survival (P < 0.0001, P = 0.0078), the protein level of IGSF11 is not related to tumor grades and histologic type, but IGSF11 is found expressed in tumor samples of all grades, on both glioma cells and tumor-associated inflammatory cells. indicating that IGSF11 may serve as a ligand and receptor simultaneously, to interact with PD-1 and VISTA to create an inhibitory immune microenvironment, which is related to the poor prognosis [71], however, this study only proved the role of IGSF11 in advanced glioma, but the prognostic role of IGSF11 in other tumors has not been demonstrated, and there is a great space for scientific research and exploration. VISTA also predicts the poor prognosis of glioma patients (P = 0.0085); VISTA may co-express with PD1, and high expression of both the immune checkpoints indicate worse survival (P < 0.0001), which is associated with the negative regulation of VISTA in the glioma microenvironment [72].
High expression of VISTA predicts the poor prognosis in following tumors, which is associated with tumor immune microenvironment. In melanoma the co-expression of VISTA and CD33 (MDSC marker) predicts a worse prognosis,the high expression of VISTA is associated with the occurrence of ulceration (P = 0.015), deeper Breslow thickness (P = 0.002), lymph node involvement (P < 0.001) and advanced stage (P = 0.008), specially, in 30% cutaneous melanoma patients, co-expression of VISTA and PD-1 co-mediate immunosuppression, further it proves that the expression of PD-1 can be affected by CD33+ MDSCs, all above indicate that VISTA, PD-1 and MDSCs may co-participate in melanoma immunosuppression and further induce poor prognosis of patients [46]. In NK/T cell lymphoma, high expression of VISTA is correlated with more infiltration of Foxp3+ Tregs, both expressions of VISTA (P = 0.001, HR = 2.05, 95% CI: 1.29–3.25) and PD-L1 (P = 0.005, HR = 1.93, 95% CI: 1.22–3.07), respectively, are risk factors and predict poor PFS (progression free survival) and OS (overall survival), the upregulation of VISTA and PD-L1 inhibit the activation and proliferation of CD8+T cells, which are associated with lymph node metastasis (P = 0.004) and the advanced stage (P = 0.002) [80]. In lung adenocarcinoma, the high expression of VISTA in CD4+ T cells is associated with a reduction in the overall survival (P = 0.03), lymph node metastasis (P = 0.05) and cytokine production inhibition, including IL2/4/10/17, and IFN-γ, the inhibitory tumor immune microenvironment may be associated with the poor prognosis of patients, moreover, the increased infiltration of CD4+ VISTA+ T cells is associated with advanced pathology Node (pN) staging but not for Tumor (pT) staging, however, the role of VISTA in lung squamous carcinoma or other types is still unclear [96]. In testicular germ cell tumors, the low counts of tumor-associated immune cells expressed with PD-L1 and VISTA may be related to stage I patients’ relapse but not for stage II and stage III, the high platelet-to-lymphocyte ratio and low counts of VISTA express tumor-associated immune cells which are the biomarkers to predict worse prognosis of testicular germ cell tumors in patients (HR = 15.56, P = 0.001 and HR = 4.1, P = 0.006, respectively) [97]. In non‑muscle‑invasive bladder cancer, the positive expression of VISTA may indicate the short recurrence of bladder cancer with recurrence-free survival, compared to the negative ones (34.0 vs. 39.9 months, P = 0.03). Moreover, high expression of VISTA is associated with advanced tumor stage (67.7% vs. 32.3%, pT1 vs. pTa and pTis, R = 0.325, P < 0.001), high pathologic grade (71.0% vs. 29.0%, pT1 vs. pTa and pTis, R = 0.438, P < 0.001) and tumor size larger than 3 cm (R = 0.322, P < 0.001) [98].
In some tumors, high expression of VISTA predicts a favorable prognosis with more infiltration of TILs. In soft tissue sarcomas, high expression of VISTA may predict the favorable prognosis of patients (P = 0.043), which can be attributed to the increased TIL, especially for CD3+ cells, however, more frequent VISTA is found in higher FNCLCC grade (G3 vs. G2, P = 0.019), exactly opposite of what is predicted without further explanation [25]. In TNBC (triple-negative breast cancer), Cao et al. indicate that higher expression of VISTA is less associated with lymph node metastasis but more related to the infiltration of TILs, especially for CD4+ TILs, more VISTA+ immune cells are found in stage I/ II patients and basal-like subtype with favorable OS, especially for T1-2N0 stage patients [99]. Similar results can be found in IDC (invasive ductal carcinoma) of the breast, proving that VISTA is accumulated more in the subtypes of EGFR2+, and positively associated with the expression of PD1/PD-L1, as well as stromal CD8+ TILs (P < 0.001), predicting the favorable disease-specific survival and relapse free survival of IDC patients, especially in basal-like, ER− and PR− IDC subtypes, however, high VISTA expression is also found in EGFR2−, EGFR2+ and poorly differentiated subtypes, but not related with prognosis prediction [100]. In all types of malignant mesothelioma, the survival analysis shows that VISTA is an independent favorable prognostic factor (P = 0.008); these results are confirmed by a multivariate survival analysis (P = 0.014, 95% CI = 1.25–7.72) [101], especially, in pleural mesothelioma, VISTA expression predicts the favorable prognosis but PD-L1 expression predicts the worse (P = 0.001, P = 0.002, respectively), but the expression of PD-L1 is infrequent, which may induce the insensitivity of pleural mesothelioma patients to anti-PD-L1 treatment [102]. In pancreatic cancer, high expression of VISTA is found in tumor cells, positively associated with the infiltration of CD19+ B cells, CD3+ T cells and CD68+ macrophages, besides, frequent VISTA expression is a risk factor of tumor grade (HR = 3.911, P = 0.012), T stage (HR = 2.885, P = 0.006), N stage HR = 4.221, P = 0.001) and M stage (HR = 5.57, P = 0.001) but a protective factor for patients survival ( HR = 0.588, P = 0.029) [77]. VISTA improves the prognosis of esophageal adenocarcinoma, especially for T1/T2 tumors with VISTA+ TILs, compared to the tumors without VISTA expression (median overall survival is 21.6 months, 95%CI 13.3–29.9 months), the tumors with high VISTA expression have better prognosis (median overall survival is 202.2 months, 95%CI 32.6–371.8 months), however, it has proved that this benefit is not seen in advanced esophageal adenocarcinoma stages and unclear in other pathological subtypes of esophageal neoplasms [103]. In HCC (Hepatocellular carcinoma), the expression of VISTA is associated with CD8+ TILs (P < 0.001) and higher pathological grades (III-IV) but not related with TNM stages, and the double positive expression of VISTA and CD8 has the most favorable prognosis of HCC [104].
The prognostic role of VISTA is contradictory in colorectal cancer and cervical cancer. In colorectal cancer, hypoxia can induce HIF1α (hypoxia-inducible factor 1-alpha), binding to the promoter of VISTA to increase the expression on MDSCs, enhance the inhibition of MDSCs to T cell function, which can be the reason for high VISTA expression indicating the poor prognosis of colorectal patients, however, the survival analysis is on the foundation of GSE40967, the survival association is confirmed only at the mRNA level but not in protein level [105]. Besides, compared to the patients in the early stage, the mRNA level of VISTA in circulation is significantly upregulated in the advanced stage, which may indicate the poor prognosis of colorectal cancer patients [106]. However, Wu et al. consider that the high expression of VISTA predicts the better prognosis of colorectal cancer patients (P = 0.005), with high lymphocyte infiltration and low tumor stage, high AJCC III-IV stages tumors have lower VISTA expression, the order of VISTA expression is as follows: pT1 > pT2 > pT3 > pT4 [107]. Zong et al. also provide similar results for survival analysis [108]. In a word, the differences in VISTA prediction of colorectal cancer survival can be attributed as follows, the first two studies only complete the survival analysis of VISTA mRNA level but not for protein level, besides, the cutoff of VISTA expression may decide optimized, the median cutoff is not correlated with tumor survival in GSE40967 but optimized cutoff does [105].
The double positive expression of VISTA and Foxp3 indicate the worst prognosis of cervical cancer patients, compared to the single negative group; the survival analysis shows that the single positive expression of VISTA has the worst average survival (35.114 ± 2.828 vs. 51.486 ± 1.403 months). The 3-year survival rate (54.3% vs. 91.4%), similarly, compared to the double negative group, the double positive expression of VISTA and Foxp3 shows the worst average survival (26.813 ± 3.584 vs. 52.929 ± 1.052), especially for patients in higher stage because VISTA and Foxp3 both can be found higher in stage II cervical cancer [54]. In ovarian, cervical, and endometrial cancer, high expression of VISTA is related to the advanced stage (stage II, III, IV) and lymph node metastasis, and positively associated with prolonged survival [42, 109]. Especially, in high‑grade serous ovarian cancer, the expression of the VISTA encoding gene C10orf54 also indicates the prolonged overall survival (P = 0.004) [49]. There is lack of convincing explanation for the differences in the prognosis prediction of VISTA in cervical cancer, which may be attributed to sample heterogeneity.
Perspective
Immunotherapy has become the major treatment for tumors, but the problem of the low response rate and the high immune-related adverse events remain unsolved. Thus, novel immunotherapy targets warrant further exploration. According to our review and bibliometric study, VISTA and its ligands, especially for IGSF11, may be the novel and promising target in tumor immunotherapy [110, 111].
The crystal structure of both IGSF11 and VISTA have been identified, which may provide more targets for antibody design. The V-type and C-type immunoglobulin-like domain of IGSF11 are responsible for binding with VISTA, SG7 is a kind of antibody that inhibits the interaction between IGSF11 and VISTA. An in-depth analysis of the VISTA structure finds the CC’ loop region of VISTA is also a target for antibody design (HMBD-002). Thus, it is of great clinical significance to further explore the IGSF11 structure, which may provide further rationale for anti-IGSF11 targeted drug design.
IGSF11 proved to be highly accumulated in tumor cells and VISTA raised more in both immune cells and malignant cells; the expression of these are modulated by signal pathways and epigenetic regulation. Considering the differences of IGSF11 and VISTA studies in different tumors, we consider that the role of IGSF11 in non-small-cell lung cancer, ovarian cancer and oral squamous cell carcinoma, even in esophageal carcinoma, worth further exploration. Besides, more research focuses on the expression regulation signal pathways and epigenetic regulation of VISTA but less on IGSF11, and studying the corresponding IGSF11 expression regulation mechanism may provide more targets for IGSF11 downregulation and contribute further to tumor immunotherapy.
At present, the high affinity between IGSF11 and VISTA has been proved, however, after IGSF11 binds to VISTA, the related inhibitory signals occur and are involved in immune inhibition, but the specific intracellular mechanisms and signals are still unknown, and warrant further investigation.
Both IGSF11 and VISTA exert an inhibitory function in immune regulation. IGSF11 and VISTA can both inhibit the function of TILs, especially for CD8+ T cells, besides, both promote the production of TGF-β and IL-10, inhibit the production of TNF-α and IFN-γ, and finally exert immunosuppression in tumors. Various studies have proved that VISTA coordinates with PD1/PD-L1 in tumor immune regulation but not for IGSF11 [112]. Even the immune regulation of IGSF11 has just been proved in glioma, but VISTA has been proved to be in many other tumors, thus, the research potential for the mechanisms of IGSF11 in tumor immune regulation is enormous, since the intracellular mechanisms are still unclear. Most studies have found a negative relationship between IGSF11/VISTA and immunity in some tumors, and just exhibit the relationship between gene expression and cell count, but there has been a lack of sufficient cytological trials to prove the positive relationship and thus, this has not been sufficiently convincing.
Both IGSF11 and VISTA may be involved in tumor growth. The downregulation of either IGSF11 or VISTA in various tumor models, significantly inhibits tumor growth, and the mechanisms of VISTA may be attributed to the restoration of T cells, but the mechanisms of IGSF11 are still unknown. We consider that VISTA locates on the membrane and in the cytoplasm of tumors may directly interact with IGSF11 and affect the proliferation of tumors, the discovery of this phenomenon may suggest a new hypothesis: IGSF11 and VISTA exert a double regulation in tumors, which include both immune regulation and proliferation regulation—which is also a novel direction for exploration of the immune checkpoints.
Both IGSF11 and VISTA have the potential to be the novel target in tumor immunotherapy. Currently, three drugs (JNJ-61610588, CA-170 and CI-8993) targeting VISTA are ongoing clinical trials, other VISTA inhibitors like 13F3, αVISTA, 6809‑0223, HMBD-002, MIH63 have tested their therapeutic effect in mouse tumor models, most of them exert by restoring or enhancing the function of immune cells, and promoting the production of cytokines like IL-4, IFN-γ and TNF-α. Compared to VISTA, few studies focus on anti-IGSF11 drugs. SG7 is such a kind of antibody that inhibits the interaction between IGSF11 and VISTA. Further, IGSF11-related peptide drugs have emerged and proved their efficacy in a variety of tumors, but there is still a need to explore and further expand the scope of tumor adaptation, and the corresponding clinical validation is lacking. In addition to monotherapy, combination therapy is also an important research field, and the combination therapy of anti-VISTA/anti-IGSF11 and anti-PD1/PD-L1, may produce better therapeutic effects in tumors [113,114,115]. Current research has found the high expression of VISTA in anti-PD1/PD-L1 resistance samples, thus, combination therapy may simultaneously improve the clinical immunotherapy resistance and the occurrence of irAEs should be emphasized during the process of combination therapy.
Both IGSF11 and VISTA can predict the prognosis in tumors. IGSF11 predicts the favorable prognosis of tumors but VISTA predicts both the favorable and poor prognosis in various tumors, and the expression of this pair of immune checkpoints is associated with other immune checkpoints and immune markers. VISTA is negatively associated with tumor immune regulation but improves the infiltration of TILs in some tumors like soft tissue sarcomas and TNBC, which can be a possible reason that explains the high level of VISTA’s ability to predict favorable prognosis in these tumors.
Taken together, IGSF11 and VISTA are both highly accumulated in tumors, and participate in the regulation of tumor growth, immune microenvironment, therapy resistance and prognosis prediction. As a whole, more research focuses on VISTA and less on IGSF11, but we consider that IGSF11 also plays a strong regulatory role in a variety of tumors, although the specific role and regulatory mechanism are unclear. The present research has proved the predictive role of IGSF11 and VISTA in tumors, but whether the anti-VISTA/anti-IGSF11 treatment is indeed effective and can improve the prognosis of patients is still unknown. There is a lack of data about patient survival and a contributory factor is due to the delays in the targeted drug studies. This may also be due to the fact that the specific mechanism and site of action of IGSF11 and VISTA in tumors have not been effectively developed, thus, relevant drug research and clinical research are needed for further exploration.
Availability of data and materials
The materials that support the conclusion of this review have been included within the article.
Abbreviations
- PD1/PD-L1:
-
Programmed Cell Death 1/ Programmed Cell Death Ligand 1
- CTLA4:
-
Cytotoxic T-Lymphocyte Associated Protein 4
- LAG3:
-
Lymphocyte Activating 3
- irAEs:
-
Immune-related adverse events
- IGSF11:
-
Immunoglobulin superfamily 11 gene
- VISTA:
-
V-domain immunoglobulin suppressor of T cell activation
- PSGL1:
-
P-selectin glycoprotein ligand 1
- SH2 domain:
-
Src homology domain 2
- SH3 domain:
-
Src homology domain 3
- MDSCs:
-
Myeloid-derived suppressor cells
- NSCLC:
-
Non-small-cell lung cancer
- NF-κB:
-
nuclear factor kappa B
- EMT/MET:
-
Epithelial-mesenchymal transition/ mesenchymal-epithelial transition
- FOXD3:
-
Factor Forkhead box D3
- SPR:
-
Surface plasmon resonance
- FACS:
-
Fluorescence-activated cell sorting
- IL-17:
-
Interleukin-17
- CCL3:
-
Chemokine ligand 3
- CXCL11:
-
C-X-C motif chemokine 11
- CCL5:
-
Chemokine ligand 5
- TME:
-
Tumor microenvironment
- TIL:
-
Tumour-infiltrating lymphocyte
- PDAC:
-
Pancreatic ductal adenocarcinoma
- CTLs:
-
Cytotoxic T lymphocytes
- RECIST:
-
Response evaluation criteria in solid tumors
- ECD:
-
Extracellular domain
- PFS:
-
Progression free survival
- TNBC:
-
Triple-negative breast cancer
- IDC:
-
Invasive ductal carcinoma
References
Siegel RL, Jemal A, Wender RC, Gansler T, Ma J, Brawley OW. An assessment of progress in cancer control. CA Cancer J Clin. 2018;68(5):329–39.
Pasello G, Pavan A, Attili I, Bortolami A, Bonanno L, Menis J, et al. Real world data in the era of Immune Checkpoint Inhibitors (ICIs): Increasing evidence and future applications in lung cancer. Cancer Treat Rev. 2020;87:102031.
Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13:211.
Higashine K, Hashimoto K, Tsujimoto E, Oishi Y, Hayashi Y, Miyamoto Y. Promotion of differentiation in developing mouse cerebellar granule cells by a cell adhesion molecule BT-IgSF. Neurosci Lett. 2018;686:87–93.
Pelz L, Purfürst B, Rathjen FG. The cell adhesion molecule BT-IgSF is essential for a functional blood-testis barrier and male fertility in mice. J Biol Chem. 2017;292(52):21490–503.
Eom DS, Inoue S, Patterson LB, Gordon TN, Slingwine R, Kondo S, et al. Melanophore migration and survival during zebrafish adult pigment stripe development require the immunoglobulin superfamily adhesion molecule Igsf11. PLoS Genet. 2012;8(8):e1002899.
Katoh M, Katoh M. IGSF11 gene, frequently up-regulated in intestinal-type gastric cancer, encodes adhesion molecule homologous to CXADR, FLJ22415 and ESAM. Int J Oncol. 2003;23(2):525–31.
Kim H, Takegahara N, Walsh MC, Choi Y. CD44 Can Compensate for IgSF11 Deficiency by Associating with the Scaffold Protein PSD-95 during Osteoclast Differentiation. Int J Mol Sci. 2020;21(7):2646.
Hayano Y, Ishino Y, Hyun JH, Orozco CG, Steinecke A, Potts E, et al. IgSF11 homophilic adhesion proteins promote layer-specific synaptic assembly of the cortical interneuron subtype. Sci Adv. 2021;7(29):eabf1600.
Jang S, Oh D, Lee Y, Hosy E, Shin H, van Riesen C, et al. Synaptic adhesion molecule IgSF11 regulates synaptic transmission and plasticity. Nat Neurosci. 2016;19(1):84–93.
Harada H, Suzu S, Hayashi Y, Okada S. BT-IgSF, a novel immunoglobulin superfamily protein, functions as a cell adhesion molecule. J Cell Physiol. 2005;204(3):919–26.
Chen B, Zhu G, Yan A, He J, Liu Y, Li L, et al. IGSF11 is required for pericentric heterochromatin dissociation during meiotic diplotene. PLoS Genet. 2021;17(9):e1009778.
Wang J, Wu G, Manick B, Hernandez V, Renelt M, Erickson C, et al. VSIG-3 as a ligand of VISTA inhibits human T-cell function. Immunology. 2019;156(1):74–85.
Johnston RJ, Su LJ, Pinckney J, Critton D, Boyer E, Krishnakumar A, et al. VISTA is an acidic pH-selective ligand for PSGL-1. Nature. 2019;574(7779):565–70.
Yuan L, Tatineni J, Mahoney KM, Freeman GJ. VISTA: A Mediator of Quiescence and a Promising Target in Cancer Immunotherapy. Trends Immunol. 2021;42(3):209–27.
Gabr MT, Gambhir SS. Discovery and Optimization of Small-Molecule Ligands for V-Domain Ig Suppressor of T-Cell Activation (VISTA). J Am Chem Soc. 2020;142(38):16194–8.
ElTanbouly MA, Croteau W, Noelle RJ, Lines JL. VISTA: a novel immunotherapy target for normalizing innate and adaptive immunity. Semin Immunol. 2019;42:101308.
Wang G, Tai R, Wu Y, Yang S, Wang J, Yu X, et al. The expression and immunoregulation of immune checkpoint molecule VISTA in autoimmune diseases and cancers. Cytokine Growth Factor Rev. 2020;52:1–14.
Bharaj P, Chahar HS, Alozie OK, Rodarte L, Bansal A, Goepfert PA, et al. Characterization of programmed death-1 homologue-1 (PD-1H) expression and function in normal and HIV infected individuals. PLoS ONE. 2014;9(10):e109103.
Wang L, Le Mercier I, Putra J, Chen W, Liu J, Schenk AD, et al. Disruption of the immune-checkpoint VISTA gene imparts a proinflammatory phenotype with predisposition to the development of autoimmunity. Proc Natl Acad Sci U S A. 2014;111(41):14846–51.
Tang T, Li L, Tang J, Li Y, Lin WY, Martin F, et al. A mouse knockout library for secreted and transmembrane proteins. Nat Biotechnol. 2010;28(7):749–55.
Borggrewe M, Grit C, Den Dunnen WFA, Burm SM, Bajramovic JJ, Noelle RJ, et al. VISTA expression by microglia decreases during inflammation and is differentially regulated in CNS diseases. Glia. 2018;66(12):2645–58.
Borggrewe M, Kooistra SM, Noelle RJ, Eggen BJL, Laman JD. Exploring the VISTA of microglia: immune checkpoints in CNS inflammation. J Mol Med (Berl). 2020;98(10):1415–30.
Broughton TWK, ElTanbouly MA, Schaafsma E, Deng J, Sarde A, Croteau W, et al. Defining the Signature of VISTA on Myeloid Cell Chemokine Responsiveness. Front Immunol. 2019;10:2641.
Albertsmeier M, Altendorf-Hofmann A, Lindner LH, Issels RD, Kampmann E, Dürr HR, et al. VISTA in Soft Tissue Sarcomas: A Perspective for Immunotherapy? Cancers (Basel). 2022;14(4):1006.
Liu D. Cancer biomarkers for targeted therapy. Biomark Res. 2019;7:25.
Xie X, Chen C, Chen W, Jiang J, Wang L, Li T, et al. Structural Basis of VSIG3: The Ligand for VISTA. Front Immunol. 2021;12:625808.
Hosseinkhani N, Derakhshani A, Shadbad MA, Argentiero A, Racanelli V, Kazemi T, et al. The Role of V-Domain Ig Suppressor of T Cell Activation (VISTA) in Cancer Therapy: Lessons Learned and the Road Ahead. Front Immunol. 2021;12:676181.
Mehta N, Maddineni S, Mathews II, Andres Parra Sperberg R, Huang PS, Cochran JR. Structure and Functional Binding Epitope of V-domain Ig Suppressor of T Cell Activation. Cell Rep. 2019;28(10):2509-2516.e5.
Slater BT, Han X, Chen L, Xiong Y. Structural insight into T cell coinhibition by PD-1H (VISTA). Proc Natl Acad Sci U S A. 2020;117(3):1648–57.
Mahoney KM, Freeman GJ. Acidity changes immunology: a new VISTA pathway. Nat Immunol. 2020;21(1):13–6.
Watanabe T, Suda T, Tsunoda T, Uchida N, Ura K, Kato T, et al. Identification of immunoglobulin superfamily 11 (IGSF11) as a novel target for cancer immunotherapy of gastrointestinal and hepatocellular carcinomas. Cancer Sci. 2005;96(8):498–506.
Grelet S, Fréreux C, Obellianne C, Noguchi K, Howley BV, Dalton AC, et al. TGFβ-induced expression of long noncoding lincRNA Platr18 controls breast cancer axonogenesis. Life Sci Alliance. 2022;5(2):e202101261.
Le Mercier I, Chen W, Lines JL, Day M, Li J, Sergent P, et al. VISTA Regulates the Development of Protective Antitumor Immunity. Cancer Res. 2014;74(7):1933–44.
Wang L, Jia B, Claxton DF, Ehmann WC, Rybka WB, Mineishi S, et al. VISTA is highly expressed on MDSCs and mediates an inhibition of T cell response in patients with AML. Oncoimmunol. 2018;7(9):e1469594.
Xu W, Hiếu T, Malarkannan S, Wang L. The structure, expression, and multifaceted role of immune-checkpoint protein VISTA as a critical regulator of anti-tumor immunity, autoimmunity, and inflammation. Cell Mol Immunol. 2018;15(5):438–46.
Deng J, Le Mercier I, Kuta A, Noelle RJ. A New VISTA on combination therapy for negative checkpoint regulator blockade. J Immunother Cancer. 2016;4:86.
Wang C, Feng H, Cheng X, Liu K, Cai D, Zhao R. Potential Therapeutic Targets of B7 Family in Colorectal Cancer. Front Immunol. 2020;11:681.
Xu W, Dong J, Zheng Y, Zhou J, Yuan Y, Ta HM, et al. Immune-Checkpoint Protein VISTA Regulates Antitumor Immunity by Controlling Myeloid Cell-Mediated Inflammation and Immunosuppression. Cancer Immunol Res. 2019;7(9):1497–510.
ElTanbouly MA, Zhao Y, Nowak E, Li J, Schaafsma E, Le Mercier I, et al. VISTA is a checkpoint regulator for naïve T cell quiescence and peripheral tolerance. Science. 2020;367(6475):eaay0524.
Zielinski C, Knapp S, Mascaux C, Hirsch F. Rationale for targeting the immune system through checkpoint molecule blockade in the treatment of non-small-cell lung cancer. Ann Oncol. 2013;24(5):1170–9.
Liao H, Zhu H, Liu S, Wang H. Expression of V-domain immunoglobulin suppressor of T cell activation is associated with the advanced stage and presence of lymph node metastasis in ovarian cancer. Oncol Lett. 2018;16(3):3465–72.
Böger C, Behrens HM, Krüger S, Röcken C. The novel negative checkpoint regulator VISTA is expressed in gastric carcinoma and associated with PD-L1/PD-1: A future perspective for a combined gastric cancer therapy? Oncoimmunology. 2017;6(4):e1293215.
Xie S, Huang J, Qiao Q, Zang W, Hong S, Tan H, et al. Expression of the inhibitory B7 family molecule VISTA in human colorectal carcinoma tumors. Cancer Immunol Immunother. 2018;67(11):1685–94.
Wu L, Deng WW, Huang CF, Bu LL, Yu GT, Mao L, et al. Expression of VISTA correlated with immunosuppression and synergized with CD8 to predict survival in human oral squamous cell carcinoma. Cancer Immunol Immunother. 2017;66(5):627–36.
Choi JW, Kim YJ, Yun KA, Won CH, Lee MW, Choi JH, et al. The prognostic significance of VISTA and CD33-positive myeloid cells in cutaneous melanoma and their relationship with PD-1 expression. Sci Rep. 2020;10(1):14372.
Gao J, Ward JF, Pettaway CA, Shi LZ, Subudhi SK, Vence LM, et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat Med. 2017;23(5):551–5.
Zong L, Zhang M, Wang W, Wan X, Yang J, Xiang Y. PD-L1, B7–H3 and VISTA are highly expressed in gestational trophoblastic neoplasia. Histopathology. 2019;75(3):421–30.
Zong L, Zhou Y, Zhang M, Chen J, Xiang Y. VISTA expression is associated with a favorable prognosis in patients with high-grade serous ovarian cancer. Cancer Immunol Immunother. 2020;69(1):33–42.
Mills AM, Bullock TN, Ring KL. Targeting immune checkpoints in gynecologic cancer: updates & perspectives for pathologists. Mod Pathol. 2022;35(2):142–51.
Xie X, Zhang J, Shi Z, Liu W, Hu X, Qie C, et al. The Expression Pattern and Clinical Significance of the Immune Checkpoint Regulator VISTA in Human Breast Cancer. Front Immunol. 2020;11:563044.
Hernandez-Martinez JM, Vergara E, Zatarain-Barrón ZL, Barrón-Barrón F, Arrieta O. VISTA/PD-1H: a potential target for non-small cell lung cancer immunotherapy. J Thorac Dis. 2018;10(12):6378–82.
Villarroel-Espindola F, Yu X, Datar I, Mani N, Sanmamed M, Velcheti V, et al. Spatially Resolved and Quantitative Analysis of VISTA/PD-1H as a Novel Immunotherapy Target in Human Non-Small Cell Lung Cancer. Clin Cancer Res. 2018;24(7):1562–73.
Li L, Xu XT, Wang LL, Qin SB, Zhou JY. Expression and clinicopathological significance of Foxp3 and VISTA in cervical cancer. Am J Transl Res. 2021;13(9):10428–38.
Mulati K, Hamanishi J, Matsumura N, Chamoto K, Mise N, Abiko K, et al. VISTA expressed in tumour cells regulates T cell function. Br J Cancer. 2019;120(1):115–27.
Zong L, Mo S, Sun Z, Lu Z, Yu S, Chen J, et al. Analysis of the immune checkpoint V-domain Ig-containing suppressor of T-cell activation (VISTA) in endometrial cancer. Mod Pathol. 2022;35(2):266–73.
He M, Abro B, Kaushal M, Chen L, Chen T, Gondim M, et al. Tumor mutation burden and checkpoint immunotherapy markers in primary and metastatic synovial sarcoma. Hum Pathol. 2020;100:15–23.
Wisdom AJ, Mowery YM, Hong CS, Himes JE, Nabet BY, Qin X, et al. Single cell analysis reveals distinct immune landscapes in transplant and primary sarcomas that determine response or resistance to immunotherapy. Nature Commun. 2020;11(1):6410.
Lines JL, Sempere LF, Broughton T, Wang L, Noelle R. VISTA is a novel broad-spectrum negative checkpoint regulator for cancer immunotherapy. Cancer Immunol Res. 2014;2(6):510–7.
Popp FC, Capino I, Bartels J, Damanakis A, Li J, Datta RR, et al. Expression of Immune Checkpoint Regulators IDO, VISTA, LAG3, and TIM3 in Resected Pancreatic Ductal Adenocarcinoma. Cancers (Basel). 2021;13(11):2689.
ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature, 2012. 489(7414):57-74.
ElTanbouly MA, Schaafsma E, Noelle RJ, Lines JL. VISTA: Coming of age as a multi-lineage immune checkpoint. Clin Exp Immunol. 2020;200(2):120–30.
Battista M, Musto A, Navarra A, Minopoli G, Russo T, Parisi S. miR-125b regulates the early steps of ESC differentiation through dies1 in a TGF-independent manner. Int J Mol Sci. 2013;14(7):13482–96.
Parisi S, Battista M, Musto A, Navarra A, Tarantino C, Russo T. A regulatory loop involving Dies1 and miR-125a controls BMP4 signaling in mouse embryonic stem cells. Faseb J. 2012;26(10):3957–68.
Oliveira P, Carvalho J, Rocha S, Azevedo M, Reis I, Camilo V, et al. Dies1/VISTA expression loss is a recurrent event in gastric cancer due to epigenetic regulation. Sci Rep. 2016;6:34860.
Rosenbaum SR, Knecht M, Mollaee M, Zhong Z, Erkes DA, McCue PA, et al. FOXD3 Regulates VISTA Expression in Melanoma. Cell Rep. 2020;30(2):510-524.e6.
Schlichtner S, Yasinska IM, Ruggiero S, Berger SM, Aliu N, Prunk M, et al. Expression of the Immune Checkpoint Protein VISTA Is Differentially Regulated by the TGF-β1 - Smad3 Signaling Pathway in Rapidly Proliferating Human Cells and T Lymphocytes. Front Med (Lausanne). 2022;9:790995.
Yang W, Padkjær SB, Wang J, Sun Z, Shan B, Yang L, et al. Construction of a versatile expression library for all human single-pass transmembrane proteins for receptor pairings by high throughput screening. J Biotechnol. 2017;260:18–30.
Mehta N, Maddineni S, Kelly RL, Lee RB, Hunter SA, Silberstein JL, et al. An engineered antibody binds a distinct epitope and is a potent inhibitor of murine and human VISTA. Sci Rep. 2020;10(1):15171.
Thakkar D, Paliwal S, Dharmadhikari B, Guan S, Liu L, Kar S, et al. Rationally targeted anti-VISTA antibody that blockades the C-C’ loop region can reverse VISTA immune suppression and remodel the immune microenvironment to potently inhibit tumor growth in an Fc independent manner. J Immunother Cancer. 2022;10(2):e003382.
Ghouzlani A, Rafii S, Karkouri M, Lakhdar A, Badou A. The Promising IgSF11 Immune Checkpoint Is Highly Expressed in Advanced Human Gliomas and Associates to Poor Prognosis. Front Oncol. 2020;10:608609.
Ghouzlani A, Lakhdar A, Rafii S, Karkouri M, Badou A. The immune checkpoint VISTA exhibits high expression levels in human gliomas and associates with a poor prognosis. Sci Rep. 2021;11(1):21504.
Cheng H, Zong L, Kong Y, Gu Y, Yang J, Xiang Y. Emerging Targets of Immunotherapy in Gynecologic Cancer. Onco Targets Ther. 2020;13:11869–82.
Lines JL, Pantazi E, Mak J, Sempere LF, Wang L, O’Connell S, et al. VISTA is an immune checkpoint molecule for human T cells. Cancer Res. 2014;74(7):1924–32.
Gorczynski RM, Zhu F. Checkpoint blockade in solid tumors and B-cell malignancies, with special consideration of the role of CD200. Cancer Manag Res. 2017;9:601–9.
Blando J, Sharma A, Higa MG, Zhao H, Vence L, Yadav SS, et al. Comparison of immune infiltrates in melanoma and pancreatic cancer highlights VISTA as a potential target in pancreatic cancer. Proc Natl Acad Sci U S A. 2019;116(5):1692–7.
Hou Z, Pan Y, Fei Q, Lin Y, Zhou Y, Liu Y, et al. Prognostic significance and therapeutic potential of the immune checkpoint VISTA in pancreatic cancer. J Cancer Res Clin Oncol. 2021;147(2):517–31.
Nowak EC, Lines JL, Varn FS, Deng J, Sarde A, Mabaera R, et al. Immunoregulatory functions of VISTA. Immunol Rev. 2017;276(1):66–79.
Wang L, Rubinstein R, Lines JL, Wasiuk A, Ahonen C, Guo Y, et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med. 2011;208(3):577–92.
He HX, Gao Y, Fu JC, Zhou QH, Wang XX, Bai B, et al. VISTA and PD-L1 synergistically predict poor prognosis in patients with extranodal natural killer/T-cell lymphoma. Oncoimmunol. 2021;10(1):1907059.
Yasinska IM, Meyer NH, Schlichtner S, Hussain R, Siligardi G, Casely-Hayford M, et al. Ligand-Receptor Interactions of Galectin-9 and VISTA Suppress Human T Lymphocyte Cytotoxic Activity. Front Immunol. 2020;11:580557.
Flies DB, Han X, Higuchi T, Zheng L, Sun J, Ye JJ, et al. Coinhibitory receptor PD-1H preferentially suppresses CD4+ T cell-mediated immunity. J Clin Invest. 2014;124(5):1966–75.
Topcu KSB, Korucu EN, Menevse E, Kocak N, Duran T. Investigation of the effects of the toll-like receptor 4 pathway on immune checkpoint vista in pancreatic cancer. Invest New Drugs. 2022;40(3):519–28.
Tagliamento M, Bironzo P, Novello S. New emerging targets in cancer immunotherapy: the role of VISTA. ESMO Open. 2020;4(Suppl 3):e000683.
Pan J, Chen Y, Zhang Q, Khatun A, Palen K, Xin G, et al. Inhibition of lung tumorigenesis by a small molecule CA170 targeting the immune checkpoint protein VISTA. Commun Biol. 2021;4(1):906.
Barrueto L, Caminero F, Cash L, Makris C, Lamichhane P, Deshmukh RR. Resistance to Checkpoint Inhibition in Cancer Immunotherapy. Transl Oncol. 2020;13(3):100738.
Yum JI, Hong YK. Terminating Cancer by Blocking VISTA as a Novel Immunotherapy: Hasta la vista, baby. Front Oncol. 2021;11:658488.
Li TT, Jiang JW, Qie CX, Xuan CX, Hu XL, Liu WM, et al. Identification of active small-molecule modulators targeting the novel immune checkpoint VISTA. BMC Immunol. 2021;22(1):55.
Liu J, Yuan Y, Chen W, Putra J, Suriawinata AA, Schenk AD, et al. Immune-checkpoint proteins VISTA and PD-1 nonredundantly regulate murine T-cell responses. Proc Natl Acad Sci U S A. 2015;112(21):6682–7.
Wright Q, Gonzalez Cruz JL, Wells JW, Leggatt GR. PD-1 and beyond to Activate T Cells in Cutaneous Squamous Cell Cancers: The Case for 4–1BB and VISTA Antibodies in Combination Therapy. Cancers (Basel). 2021;13(13):3310.
Kondo Y, Ohno T, Nishii N, Harada K, Yagita H, Azuma M. Differential contribution of three immune checkpoint (VISTA, CTLA-4, PD-1) pathways to antitumor responses against squamous cell carcinoma. Oral Oncol. 2016;57:54–60.
Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–7.
Bai J, Gao Z, Li X, Dong L, Han W, Nie J. Regulation of PD-1/PD-L1 pathway and resistance to PD-1/PD-L1 blockade. Oncotarget. 2017;8(66):110693–707.
Kakavand H, Jackett LA, Menzies AM, Gide TN, Carlino MS, Saw RPM, et al. Negative immune checkpoint regulation by VISTA: a mechanism of acquired resistance to anti-PD-1 therapy in metastatic melanoma patients. Mod Pathol. 2017;30(12):1666–76.
Pilones KA, Hensler M, Daviaud C, Kraynak J, Fucikova J, Galluzzi L, et al. Converging focal radiation and immunotherapy in a preclinical model of triple negative breast cancer: contribution of VISTA blockade. Oncoimmunol. 2020;9(1):1830524.
S Ma, L Qin, X Wang, W Wang, J Li, H Wang, et al. The Expression of VISTA on CD4+ T Cells Associate with Poor Prognosis and Immune Status in Non-small Cell Lung Cancer Patients. Bosn J Basic Med Sci. 2022;7(10):1-5.
Pęksa R, Kunc M, Popęda M, Piątek M, Bieńkowski M, Żok J, et al. Combined Assessment of Immune Checkpoint Regulator VISTA on Tumor-Associated Immune Cells and Platelet-to-Lymphocyte Ratio Identifies Advanced Germ Cell Tumors with Higher Risk of Unfavorable Outcomes. Cancers (Basel). 2021;13(8):1750.
Seo WI, Lee CH, Jung SJ, Lee DS, Park HY, Jeong DH, et al. Expression of VISTA on tumor-infiltrating immune cells correlated with short intravesical recurrence in non-muscle-invasive bladder cancer. Cancer Immunol Immunother. 2021;70(11):3113–22.
Cao X, Ren X, Zhou Y, Mao F, Lin Y, Wu H, et al. VISTA Expression on Immune Cells Correlates With Favorable Prognosis in Patients With Triple-Negative Breast Cancer. Front Oncol. 2020;10:583966.
Zong L, Mo S, Yu S, Zhou Y, Zhang M, Chen J, et al. Expression of the immune checkpoint VISTA in breast cancer. Cancer Immunol Immunother. 2020;69(8):1437–46.
Chung YS, Kim M, Cha YJ, Kim KA, Shim HS. Expression of V-set immunoregulatory receptor in malignant mesothelioma. Mod Pathol. 2020;33(2):263–70.
Muller S, Victoria Lai W, Adusumilli PS, Desmeules P, Frosina D, Jungbluth A, et al. V-domain Ig-containing suppressor of T-cell activation (VISTA), a potentially targetable immune checkpoint molecule, is highly expressed in epithelioid malignant pleural mesothelioma. Mod Pathol. 2020;33(2):303–11.
Loeser H, Kraemer M, Gebauer F, Bruns C, Schröder W, Zander T, et al. The expression of the immune checkpoint regulator VISTA correlates with improved overall survival in pT1/2 tumor stages in esophageal adenocarcinoma. Oncoimmunology. 2019;8(5):e1581546.
Zhang M, Pang HJ, Zhao W, Li YF, Yan LX, Dong ZY, et al. VISTA expression associated with CD8 confers a favorable immune microenvironment and better overall survival in hepatocellular carcinoma. BMC Cancer. 2018;18(1):511.
Deng J, Li J, Sarde A, Lines JL, Lee YC, Qian DC, et al. Hypoxia-Induced VISTA Promotes the Suppressive Function of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Cancer Immunol Res. 2019;7(7):1079–90.
Saleh R, Taha RZ, Toor SM, Sasidharan Nair V, Murshed K, Khawar M, et al. Expression of immune checkpoints and T cell exhaustion markers in early and advanced stages of colorectal cancer. Cancer Immunol Immunother. 2020;69(10):1989–99.
Wu D, Hacking S, Vitkovski T, Nasim M. Superpixel image segmentation of VISTA expression in colorectal cancer and its relationship to the tumoral microenvironment. Sci Rep. 2021;11(1):17426.
Zong L, Yu S, Mo S, Zhou Y, Xiang Y, Lu Z, et al. High VISTA Expression Correlates With a Favorable Prognosis in Patients With Colorectal Cancer. J Immunother. 2021;44(1):22–8.
Kuang L, He Y. Potential value of V-domain Ig suppressor of T-cell activation for assessing progn osis in cervical cancer and as a target for therapy. Int J Clin Exp Pathol. 2020;13(1):26–37.
Ait Boujmia OK. V-domain Ig suppressor of T cell activation (VISTA) inhibition is a new approach to cancer therapy: a Bibliometric study. Naunyn Schmiedebergs Arch Pharmacol. 2021;394(6):1057–65.
Burugu S, Dancsok AR, Nielsen TO. Emerging targets in cancer immunotherapy. Semin Cancer Biol. 2018;52(Pt 2):39–52.
Marin-Acevedo JA, Dholaria B, Soyano AE, Knutson KL, Chumsri S, Lou Y. Next generation of immune checkpoint therapy in cancer: new developments and challenges. J Hematol Oncol. 2018;11(1):39.
Hacking S, Wu D, Lee L, Vitkovski T, Nasim M. Nature and Significance of Stromal Differentiation, PD-L1, and VISTA in GIST. Pathol Res Pract. 2022;229:153703.
E Im, D Y Sim, H J Lee, J E Park, W Y Park, S Ko, et al. Immune functions as a ligand or a receptor, cancer prognosis potential, clinical implication of VISTA in cancer immunotherapy. Semin Cancer Biol. 2021;8:1-10.
Kon E, Benhar I. Immune checkpoint inhibitor combinations: Current efforts and important aspects for success. Drug Resist Updat. 2019;45:13–29.
Acknowledgements
The authors would like to thank Ms. Man-Yu Tang for her excellent assistance with Figure preparation.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82002421; No. 81001041), the Natural Science Basic Research Project of Shaanxi Province (No. 2016JM8087).
Author information
Authors and Affiliations
Contributions
XYT, YLX, XGS and YBZ collected the related papers and drafted the manuscript, APS, KFZ, YJL participated in the design of the review, JBZ, NM and TJ initiated the study and revised and finalized the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: SupplementaryFigure 1.
[The survival curvesof IGSF11 and VISTA in 19 tumors]. The survival curves of IGSF11 and VISTA, 19tumors are included: BLCA (Bladder Urothelial Carcinoma), BRCA (Breast invasivecarcinoma), CESC (Cervical squamous cell carcinoma and endocervicaladenocarcinoma), COAD (Colon adenocarcinoma), DLBC (Lymphoid Neoplasm DiffuseLarge B-cell Lymphoma), ESCA (Esophageal carcinoma), GBM (Glioblastomamultiforme), LAML (Acute Myeloid Leukemia), LGG (Brain Lower Grade Glioma),LIHC (Liver hepatocellular carcinoma), LUAD (Lung adenocarcinoma), LUSC (Lungsquamous cell carcinoma), MESO (Mesothelioma), OV (Ovarian serouscystadenocarcinoma), PAAD (Pancreatic adenocarcinoma), SARC (Sarcoma), SKCM(Skin Cutaneous Melanoma), TGCT (Testicular Germ Cell Tumors), UVM (UvealMelanoma).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tang, XY., Xiong, YL., Shi, XG. et al. IGSF11 and VISTA: a pair of promising immune checkpoints in tumor immunotherapy. Biomark Res 10, 49 (2022). https://doi.org/10.1186/s40364-022-00394-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40364-022-00394-0