Abstract
Background
Bisphenol A (BPA), an endocrine disruptor, is a widely used chemical that has adverse effects on animal development and reproduction. The current research aimed to evaluate the effect of BPA on the in vitro maturation (IVM) and subsequent embryo development of mouse oocytes following in vitro fertilization (IVF).
Methods
IVM was performed in the presence of different concentrations (0, 20, 50, or 100 μg/mL) of BPA. Nuclear maturation, IVF efficiency and embryonic development were determined. The levels of reactive oxygen species (ROS) and glutathione (GSH) in the BPA (50 μg/mL) group were evaluated. We explored the ability of N-acetyl-L-cysteine (NAC) in the IVM medium to rescue the BPA-induced damage by examining changes in nuclear maturation, IVF rate, blastocyst formation, ROS levels and GSH content.
Results
Compared with the control, BPA (50 μg/mL) supplementation during oocyte IVM significantly inhibited nuclear maturation and decreased fertilization and blastocyst formation rates. In addition, BPA exposure increased ROS levels and decreased GSH content in oocytes. The addition of NAC weakened the BPA-induced suppression of nuclear maturation, relieved the BPA-induced downregulation of the fertilization and blastocyst formation rates, and mitigated the increased ROS levels and decreased GSH content.
Conclusion
BPA affects mouse oocyte maturation and subsequent early embryonic developmental competence following IVF by increasing intracytoplasmic oxidative stress in mature oocytes. NAC can reduce these harmful effects to a certain extent.
Similar content being viewed by others
Background
Bisphenol A (BPA), an endocrine disruptor [1, 2], is widely used in the production of polycarbonate beverages or food packaging materials and the metal coating on cans. BPA migration into food and beverages could have a negative effect on human health [3]. BPA has been found in some human fluids [4]. BPA exposure causes detrimental effects on development and reproduction by disturbing the oestrous cycle and hormone levels, causing miscarriage, altering oocyte maturation, and compromising oocyte fertilization [4,5,6,7,8]. These adverse effects of BPA exposure have been a cause of concern worldwide.
BPA supplementation inhibits oocyte maturation mostly through disrupting microtubule organization [9,10,11,12,13,14,15,16]. The influence of oxidative stress on the IVM of oocytes has been in discussion, and the results imply that oxidative stress is related to the inhibition of oocyte maturation [17,18,19]. Liang et al. found that pre-mature ageing of immature oocytes may induce oxidative stress, which inhibits oocyte maturation [20]. The HT-2 toxin induces oxidative stress and inhibits mouse oocyte maturation [21]. Another report showed that BPA exposure could induce oxidative stress, thereby causing DNA damage in INS-1 cells [22]. Few studies have focused on the relationship between the inhibition of oocyte maturation induced by BPA and oxidative stress. One study in which oocytes from mice orally treated with 100 μg/kg body weight (bw)/day BPA and/or 15 or 30 mg/kg bw/day melatonin for 7 days were collected and then cultured for IVM and IVF, it was found that BPA exposure causes oxidative stress and decreases the rate of oocyte maturation and that melatonin protects oocyte maturation [23]. Another study showed that BPA exposure during IVM causes oxidative stress, which is one reason why BPA inhibits porcine oocyte maturation [24]. However, because of differences in the sensitivity of porcine oocytes and mouse oocytes to BPA exposure [24], it is unknown whether BPA addition inhibits the IVM of mouse oocytes by causing oxidative stress.
Furthermore, BPA affects fertility in animal models. Female Sprague–Dawley rats neonatally exposed to 50 μg/50 μL BPA exhibited reduced pup number after mating [25]. Female CD-1 mice treated daily with BPA (0.025 μg/kg bw) perinatally showed reduced fertility and fecundity [26]. Female FVB mice treated with 50 μg/kg bw/day BPA during the perinatal period have smaller litters [27]. In addition, young adult female C57BL/6 J mice exposed to BPA have poorer quality oocytes, leading to reduced fertility following IVF or natural mating [6]. However, whether BPA exposure during mouse oocyte IVM affects subsequent IVF ability and embryonic developmental competence remains unclear.
Reactive oxygen species (ROS) are natural by-products of normal cellular metabolism. Some external environmental factors, such as elevated levels of oxygen tension, increase the cellular production of ROS, which can cause oxidative damage in cells [28, 29]. Excessive ROS can retard oocyte maturation and lead to cell death [30, 31]. Supplementation of the environment with antioxidants could decrease oxidative damage and thus protect cells [32,33,34]. N-Acetyl-L-cysteine (NAC) is less toxic and can increase intracellular glutathione (GSH) levels [35, 36], and GSH plays a key role in preventing oxidative stress in oocytes [37]. It was reported that oral intake of NAC could increase the intact egg rate of female mice [38]. Compared with the control, 1.5 mM NAC increased the blastocyst rate of porcine zygotes cultured in vitro [39]. Moreover, NAC addition could prevent apoptosis, ageing, and male pronucleus formation of oocytes. NAC (1.5 mM) addition during oocyte maturation also improved the blastocyst rate [40]. However, whether NAC can improve oocyte maturity and development after oocytes exposure to BPA during IVM remains unknown.
Therefore, we investigated the effect of BPA exposure during oocyte IVM on polar body emission, subsequent early embryonic development potential after IVF, ROS levels, and GSH content. Furthermore, NAC was added to the maturation medium to lessen the BPA-induced reduction in meiotic maturation.
Methods
Chemicals and culture media
Unless otherwise mentioned, all chemicals used were purchased from Sigma–Aldrich. Dimethyl sulfoxide (DMSO) was used to create BPA stock solutions at different concentrations (20, 50, and 100 μg/mL). The final concentration of DMSO in the culture system was set at 0.1%, and the final BPA dosage used was determined on the basis of our preliminary experiment and a previous report [16]. Ultra-pure water was used to dissolve the NAC stock (200 mM). The final dose of NAC was 100 μM according to our preliminary experiment (see Additional file 1: Figure S1) and a previous report [41]. The ROS Assay Kit was purchased from Beyotime Institute of Biotechnology (Nantong, China), and the GSH detection kit was purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The maturation medium was tissue culture medium-199 (TCM199; Gibco) supplemented with 10% (v/v) foetal bovine serum (Gibco), 24.2 mg/mL sodium pyruvate, 0.05 IU/mL follicle-stimulating hormone, 1 μg/mL 17β-oestradiol, 0.05 IU/mL luteinizing hormone and 10 ng/mL epidermal growth factor. Human tubal fluid (HTF; Merck Millipore) was used for sperm capacitation. KSOM medium (Merck Millipore) was used for embryo culture.
Experimental design
To study the effect of BPA on IVM and the subsequent developmental potential of oocytes after IVF, oocytes were treated with various concentrations of BPA (0, 20, 50, and 100 μg/mL) during maturation. After 14 h, the oocytes were subjected to IVF, and embryo development was allowed to proceed (experiment 1). In experiment 2, ROS and GSH levels were determined after oocyte maturation. In experiment 3, the protective effects of NAC on the BPA-induced decreases in oocyte nuclear maturation and embryonic developmental potential following IVF were evaluated. ROS levels and GSH content in oocytes after IVM and treatment with NAC and BPA were also evaluated.
Animals and oocyte recovery
Female (aged 6–8 weeks) and male (aged 8–10 weeks) Kunming mice (Experimental Animal Centre of Shandong Lvye Pharmaceutical Co., China) were housed in ordinary cages with stainless-steel covers and autoclaved wood bedding. The mice were kept in an air-conditioned room (22 ± 2 °C) with 60 ± 10% humidity under a 12 h/12 h (light/dark) cycle and ad libitum access to food and water. The mice were acclimatized to the housing facility for at least one week before use. At the start of the experiments, female and male mice weighed (mean ± SD) 30 ± 2 and 40 ± 2 g, respectively. 50 mg/mL sodium pentobarbital was diluted 1:10 with sterile water. The mice female and male were anaesthetized by intraperitoneal injection of pentobarbital sodium (50 mg/kg) using a 1-ml plastic syringe. After the mice were asleep, they were killed by cervical dislocation. Fertile male mice were killed to collect spermatozoa from the cauda epididymis for use in IVF. Female mice were killed at 48 h after priming with 10 IU equine chorionic gonadotrophin (Ningbo Hormone Product Co., Ltd., China). The large follicles in the ovary were punctured with M2 medium to release cumulus–oocyte complexes at the germinal vesicle stage. Experimental animals used in this study were cared for in accordance with the standards for laboratory animals. All procedures were approved by the Animal Care of Yantai University.
In vitro maturation
After being washed three times in M2 and once in TCM199, cumulus oocyte complexes (approximately 30 oocytes per group) were transferred TCM199 drops with different concentrations of BPA (0, 20, 50, or 100 μg/mL) and cultured for 14 h at 37 °C and 5% CO2 in humidified air. NAC was added to the IVM medium to observe the effect of antioxidant supplementation. After maturation, cumulus cells were removed from oocytes by pipetting to determine the maturation rate. Oocytes presenting the first polar body were at the metaphase II stage.
IVF assay and embryo culture
Collected spermatozoa were capacitated in HTF for 1 h at 37 °C. Then, oocytes free of cumulus cells were placed in fertilization medium (30–35 oocytes/100 μL drop) covered with mineral oil. Capacitated spermatozoa (final sperm count 1 × 106/mL) were added to the drop of fertilizing HTF medium. At 6 h after insemination, the oocytes were observed using a stereomicroscope. Oocytes with two pronuclei and two polar bodies were considered fertilized. After insemination, the oocytes were cultured in KSOM medium for 96 h to examine their subsequent development.
Evaluation of ROS levels
We carried out the ROS analysis according to Nasr-Esfahani et al. [42]. In brief, after being rinsed three times in serum-free TCM199, denuded oocytes were incubated in M2 with 10 μM dichlorodihydrofluorescein diacetate for 10 min at 37 °C. Then, the oocytes were washed and placed on glass slides (approximately 10 oocytes per slide). Fluorescence signals were measured by a laser scanning confocal microscope (emission wavelength of 488 nm), and the fluorescence intensity was analysed using Leica software.
Evaluation of GSH
Intracellular GSH content was measured as described by Funahashi et al. [43] with modifications. In brief, after being washed with phosphate buffered saline (PBS) three times and rinsed with distilled water three times, denuded oocytes (35 oocytes per group) matured in vitro were evaluated. The freeze-thawed samples were centrifuged (10,000 rpm, 10 min at 4 °C). The supernatant was collected for the GSH assay. The GSH concentration in the oocytes was determined by the 5,5-dithiobis-(2-nitrobenzoic acid)-oxidized GSH reductase recycling assay. The absorbance was measured with a microplate reader at 412 nm. The results are presented as pmol/oocyte.
Statistical analysis
Each experiment was repeated at least three times. Data are reported as the mean ± SEM. All data were analysed by SPSS software (SPSS Inc., Chicago, IL). In the BPA group and the control groups (as shown in Fig. 1 a, b), the data of ROS levels and GSH content in oocytes were compared by an independent-samples T test. Other data were analysed by one-way ANOVA with Dunnett’s post-hocpost hoc test. Statistical significance was considered at P < 0.05.
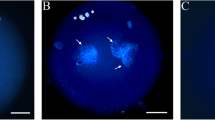
Effect of 50 μg/mL BPA on ROS levels and GSH content in mouse oocytes. a. Intracellular ROS levels in mouse oocytes in the control and treatment groups. Oocytes exposed to 50 μg/mL BPA showed higher dichlorodihydrofluorescein fluorescence than those in the control group. ROS levels are presented as the average fluorescence intensity value, which was calculated from the fluorescence intensity values of multiple oocytes. Each treatment was repeated 3–4 times, with each replicate including approximately 10 oocytes. b. Intracellular GSH content in mouse oocytes in the control and treatment groups. Oocytes in the 50 μg/mL BPA treatment group had a lower GSH content than those in the control group. Each treatment was repeated 3 times, with each replicate containing approximately 25 oocytes. The data were analysed with an independent-samples T test. a, bValues with different letters above the bars differ significantly (P < 0.05). C, control; NAC, 100 μM N-Acetyl-L-cysteine; BPA, 50 μg/mL bisphenol A; NAC + BPA, combined treatment with N-acetyl-L-cysteine (100 μM) and bisphenol A (50 μg/mL). The photographs in C and D show the fluorescence intensity in the control and BPA groups, respectively. Scale bar, 100 μm
Results
BPA disturbed oocyte polar body extrusion
The effects of BPA on oocyte polar body extrusion are shown in Table 1. The low dose of BPA at (20 μg/mL) had no effect on the polar body extrusion rate compared to the control. The percentage of oocytes developing to the MII stage was significantly lower with at 50 and 100 μg/mL BPA treatments than with control.
BPA disrupted the subsequent early embryo development of in vitro matured oocytes
After IVM, the embryo development of oocytes following IVF was studied. The effects of BPA on embryonic developmental competence after IVF are shown in Table 2. No difference in the rates of 4-cell embryos was found among all treated groups. In the BPA (20 μg/mL) group, the rates of fertilization and blastulation were similar to those in the control group. BPA 50 (50 μg/mL) and BPA 100 (100 μg/mL) significantly decreased the fertilization and blastulation rate compared with the control. Based on the results presented in Tables 1 and 2, 50 μg/mL BPA was chosen as the lowest active concentration and used for the following experiments.
BPA increased ROS levels in mouse oocytes
After 14 h of IVM, ROS levels in the oocytes were measured. Compared with the control, BPA significantly elevated ROS levels from 7.0 to 23.0 (Fig. 1 a, c, d).
BPA decreased the intracellular GSH content of mouse oocytes
In the BPA treatment group, the intracellular GSH content was 2.3 ± 0.1, which was lower than that in the control group (3.5 ± 0.2, Fig. 1 b).
NAC ameliorated the BPA-induced decrease in oocyte nuclear maturation
The rate of oocyte nuclear maturation decreased significantly from 95.3 to 72.3% in the BPA-treated group compared with the control group (Fig. 2). The rate of polar body extrusion was 90.8 in the NAC + BPA group, and 72.3 in the BPA group (P < 0.05).
Effect of 100 μM NAC on the inhibition of poly body emission by 50 μg/mL BPA. C, control; NAC, 100 μM N-acetyl-L-cysteine; BPA, 50 μg/mL bisphenol A; NAC + BPA, combined treatment with NAC (100 μM) and BPA (50 μg/mL). The rates of polar body emission (%) in the control, NAC, BPA and NAC + BPA groups were 95.3 ± 1.5, 94.7 ± 1.4, 72.3 ± 3.3 and 90.8 ± 1.9, respectively. After maturation in the presence of 100 μM NAC and,50 μg/mL BPA, the rate of polar body emission in the NAC + BPA co-treated group was somewhat normalized. Each treatment was repeated 3 times, with each replicate containing 30–40 oocytes. a-cValues with different superscripted letters above the bars differ significantly (P < 0.05)
NAC reversed the BPA-induced defect in embryo development after IVF
To investigate the effect of BPA exposure during IVM on the fertilization and developmental competence of the oocytes, we evaluated embryo development following IVF after oocytes matured in medium containing 50 μg/mL BPA alone or in combination with 100 μM NAC. The rates of fertilization and blastocyst formation increased significantly in the NAC + BPA group compared with the BPA group (81.5 vs. 87.0 and 57.0 vs. 45.9, respectively; P < 0.05) (Table 3).
NAC decreased the BPA-induced increase in ROS levels
After IVM, intracellular ROS levels in mature oocytes were significantly lower in the NAC + BPA group than in the BPA group (Fig. 3 a).
Effect of 100 μM NAC on the ROS increase and GSH decrease induced by 50 μg/mL BPA.C, control; NAC, 100 μM N-acetyl-L-cysteine; BPA, 50 μg/mL bisphenol A; NAC + BPA, combined treatment with N-acetyl-L-cysteine (100 μM) and bisphenol A (50 μg/mL). After 14 h of maturation, (a) ROS levels (average fluorescence intensity value) in the control, NAC, BPA and NAC + BPA groups were 7.1 ± 0.2, 4.1 ± 0.4, 22.6 ± 1.2 and 7.9 ± 0.8, respectively. ROS levels significantly decreased in the combination group compared with the BPA group. Each treatment was repeated 3–4 times, with each replicate including approximately 10 oocytes. (b) GSH content significantly increased in the combination group compared with the BPA group. Each treatment was repeated 3 times, with each replicate including approximately 25 oocytes.a-cValues with a different letter above the bars differ significantly (P < 0.05)
NAC reversed the BPA-induced decrease in GSH content
The results (Fig. 3 b) showed that the GSH content was significantly lower in the BPA-treated group (2.1 ± 0.3 pmol/oocyte) than that in the control group (3.5 ± 0.2 pmol/oocyte). With NAC and BPA co-treatment, the intracellular GSH content increased to 3.9 pmol/oocyte, compared with the BPA group. The oocytes co-administration with NAC led to a higher intracellular GSH content in oocytes compared with the BPA-treated group alone (NAC + BPA: 3.9 ± 0.3 pmol/oocyte vs. BPA: 2.1 ± 0.3 pmol/oocyte).
Discussion
The present results showed that BPA inhibited polar body emission, disturbed subsequent fertilization ability and embryo development, increased ROS levels, and decreased intracellular GSH content. NAC could reverse the BPA-mediated inhibition of nuclear maturation and defects in fertilization and embryonic developmental ability. Furthermore, NAC rescued the BPA-induced upregulation of ROS levels and downregulation of intracellular GSH content. To the best of our knowledge, our study is the first to investigate the effect of BPA exposure during IVM on the subsequent embryo development of mouse oocytes following IVF. Moreover, we showed that the reproductive toxicity of BPA can be restored by preventing oxidative stress with NAC supplementation. The study is important in that it identified possible methods to prevent damage induced by BPA exposure during the IVM of mouse oocytes.
Accumulating experimental data have proven that BPA has toxic effects on animal oocyte maturation. Oocyte meiotic progression is an important indicator of high-quality oocytes. In vivo or in vitro BPA exposure causes cell cycle delay in mice [9, 10]. The addition of 50 μM BPA to the culture medium for mouse preantral follicles decreased the maturation rate from 79 to 53% compared with the control [12]. The rate at which porcine oocytes reached the MII stage declined from 81.5 to 53.6% after IVM with 100 μM BPA [13]. BPA (50 and 100 μg/mL) also inhibited the maturation of mouse oocytes cultured for 18 h [16]. Exposure to 20 μM BPA significantly decreased the maturation rate of human oocytes in vitro (19.1%) compared with the control (76.2%) [14]. BPA (30 ng/mL) exposure during bovine IVM decreased the maturation rate from 72.4 to 57.4% compared with the control [15]. These studies suggest that BPA inhibits oocyte maturation, primarily through disrupting microtubule organization. To further clarify the mechanism by which BPA affects oocyte maturation, studies showed that the downregulation of phosphorylated Erk1 (p-Erk1) and phosphorylated CaMKII (p-CaMKII) levels contributes to the decreased oocyte maturation rate [44]. BPA treatment also disrupts porcine oocyte maturation by altering epigenetic modifications and causing oxidative stress and apoptosis/autophagy [24]. A recent study reported that oral administration of BPA (100 μg/kg bw per day for 7 days) decreased the oocyte maturation rate by disrupting spindle organization and chromosome alignment and inducing oxidative stress [25].
Fertilization competence is another important indicator of high-quality oocytes. The current study showed that BPA significantly decreased the rate of IVF. In agreement with a previous study, exposure to BPA (50 μg/kg bw/day) also reduced oocyte fertility after IVF or natural mating in mice [6]. In the current study, BPA addition during oocyte IVM was detrimental to embryo development following IVF. This result agrees with the finding of Ferris et al. [15] that 30 ng/mL BPA supplementation during bovine oocyte IVM could weaken embryonic developmental competence following IVF.
Oxidative stress in oocytes is often determined by measuring ROS levels and GSH content [42, 45, 46]. From the present data, we observed that BPA exposure increased ROS levels and decreased GSH content. These results suggest that BPA exposure results in increased oxidative stress in mouse oocytes. ROS accumulation prevents nuclear maturation and causes cell damage. GSH is an indicator of oocyte cytoplasmic quality. GSH can maintain normal oocyte spindle morphology and help form the male pronucleus. The BPA-induced GSH decrease indicates poor oocyte cytoplasmic quality, which affects subsequent oocyte development. Thus, the decreased developmental potential was possibly caused by oxidative stress resulting from BPA exposure during oocyte maturation. We hypothesized that decreasing oxidative stress during IVM could improve IVF and embryonic developmental competence.
NAC has the ability to facilitate GSH biosynthesis, thus, it can be used as a supplement to replenish GSH during oxidative stress conditions [47]. Mature oocytes need adequate levels of GSH to form a male pronucleus during fertilization [48]. Data indicated that NAC supplementation, specifically during the last 24 h of maturation, in porcine oocyte maturation medium improved male pronucleus formation and blastocyst development [36]. Thus, we propose that NAC could reduce BPA-mediated damage to oocytes. As expected, NAC addition during oocyte IVM largely restored oocyte nuclear maturation and subsequent embryonic development competence following IVF.
Thus, this study showed that BPA impairs mouse oocyte maturation, subsequent fertilization ability and early embryonic developmental competence by increasing intracytoplasmic oxidative stress in mature oocytes, and NAC can mitigate these injuries to a certain extent.
Although this study showed that 100 μM NAC can alleviate BPA-induced damage in mouse oocytes, further study is required to understand the mechanism by which BPA-induced oxidative stress affects nuclear maturation. We hypothesize that the BPA-induced ROS elevation causes oocyte DNA damage and that NAC can alleviate this damage in some way. Moreover, further study is required to evaluate the ability of various antioxidants at different dosages to weaken BPA-induced meiotic arrest, alleviate the disturbance in fertilization and improve the developmental potential of oocytes. In addition, the protective effect of NAC on oxidative stress induced by BPA in mouse oocytes in vivo also needs to be investigated in further studies.
Conclusion
Our research confirms that BPA negatively affects mouse oocyte maturation, subsequent fertilization ability and early embryonic developmental competence; increases ROS levels; and decreases GSH content. Co-administering NAC and BPA improves mouse oocyte maturation, subsequent fertilization ability and early embryonic developmental competence. Oocytes co-treated with NAC and BPA had lower ROS levels and a higher intracellular GSH content. These results indicate that NAC is suitable for preventing oxidative damage induced by BPA in oocytes.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author upon request.
Abbreviations
- BPA:
-
Bisphenol A
- DMSO:
-
Dimethyl sulfoxide
- GSH:
-
Glutathione
- IVF:
-
In vitro fertilization
- IVM:
-
In vitro maturation
- NAC:
-
N-Acetyl-L-cysteine
- PBS:
-
Phosphate buffered saline
- p-CaMKII:
-
Phosphorylated CaMKII
- p-Erk1:
-
Phosphorylated Erk1
- ROS:
-
Reactive oxygen species
References
Rubin BS. Bisphenol a: an endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem Mol Biol. 2011;127:27–34. https://doi.org/10.1016/j.jsbmb.2011.05.002.
Alonso-Magdalena P, Ropero AB, Soriano S, Garcia-Arevalo M, Ripoll C, Fuentes E, Quesada I, Nadal A. Bisphenol-a acts as a potent estrogen via non-classical estrogen triggered pathways. Mol Cell Endocrinol. 2012;355:201–7. https://doi.org/10.1016/j.mce.2011.12.012.
Rochester JR. Bisphenol A and human health: a review of the literature. Reprod Toxicol 2013;42:132–155. doi: https://doi.org/10.1016/j.reprotox.2013.08.008.
Machtinger R, Orvieto R. Bisphenol a oocyte maturation, implantation, and IVF outcome: review of animal and human data. Reprod BioMed Online. 2014;29:404–10. https://doi.org/10.1016/j.rbmo.2014.06.013.
Manfo FP, Jubendradass R, Nantia EA, Moundipa PF, Mathur PP. Adverse effects of bisphenol a on male reproductive function. Rev Environ Contam Toxicol. 2014;228:57–82. https://doi.org/10.1007/978-3-319-01619-1_3.
Moore-Ambriz TR, Acuna-Hernandez DG, Ramos-Robles B, Sanchez-Gutierrez M, Santacruz-Marquez R, Sierra-Santoyo A, Pina-Guzman B, Shibayama M, Hernandez-Ochoa I. Exposure to bisphenol a in young adult mice does not alter ovulation but does alter the fertilization ability of oocytes. Toxicol Appl Pharmacol. 2015;289:507–14. https://doi.org/10.1016/j.taap.2015.10.010.
Santangeli S, Maradonna F, Olivotto I, Piccinetti CC, Gioacchini G, Carnevali O. Effects of BPA on female reproductive function: the involvement of epigenetic mechanism. Gen Comp Endocrinol. 2017;245:122–6. https://doi.org/10.1016/j.ygcen.2016.08.010.
Minguez-Alarcon L, Gaskins AJ. Female exposure to endocrine disrupting chemicals and fecundity: a review. Curr Opin Obstet Gynecol. 2017;29: ;202–211. doi: https://doi.org/10.1097/gco.0000000000000373.
Hunt PA, Koehler KE, Susiarjo M, Hodges CA, Ilagan A, Voigt RC, Thomas S, Thomas BF, Hassold TJ. Bisphenol a exposure causes meiotic aneuploidy in the female mouse. Curr Biol. 2003; 13:546–553. doi:
Can A, Semiz O, Cinar O. Bisphenol-a induces cell cycle delay and alters centrosome and spindle microtubular organization in oocytes during meiosis. Mol Hum Reprod. 2005;11:389–96. https://doi.org/10.1093/molehr/gah179.
Eichenlaub-Ritter U, Vogt E, Cukurcam S, Sun F, Pacchierotti F, Parry J. Exposure of mouse oocytes to bisphenol a causes meiotic arrest but not aneuploidy. Mutat Res. 2008;651:82–92. https://doi.org/10.1016/j.mrgentox.2007.10.014.
Lenie S, Cortvrindt R, Eichenlaub-Ritter U, Smitz J. Continuous exposure to bisphenol a during in vitro follicular development induces meiotic abnormalities. Mutat Res. 2008;651(1–2):71–81. https://doi.org/10.1016/j.mrgentox.2007.10.017.
Mlynarcikova A, Nagyova E, FickovaM, Scsukova S. Effects of selected endocrine disruptors on meiotic maturation, cumulus expansion, synthesis of hyaluronan and progesterone by porcine oocyte-cumulus complexes. Toxicol In vitro . 2009;23:371–377. doi: https://doi.org/10.1016/j.tiv.2008.12.017.
Machtinger R, Combelles CM, Missmer SA, Correia KF, Williams P, Hauser R, Racowsky C. Bisphenol-a and human oocyte maturation in vitro. Hum Reprod. 2013;28:2735–45. https://doi.org/10.1093/humrep/det312.
Ferris J, Favetta LA, King WA. Bisphenol a exposure during oocyte maturation in vitro results in spindle abnormalities and chromosome misalignment in Bos taurus. Cytogenet Genome Res. 2015;145:50–8. https://doi.org/10.1159/000381321.
Nakano K, Nishio M, Kobayashi N, Hiradate Y, Hoshino Y, Sato E, Tanemura K. Comparison of the effects of BPA and BPAF on oocyte spindle assembly and polar body release in mice. Zygote. 2016;24:172–80. https://doi.org/10.1017/s0967199415000027.
Tamura H, Takasaki A, Miwa I, Taniguchi K, Maekawa R, Asada H, Taketani T, Matsuoka A, Yamagata Y, Shimamura K, Morioka H, Ishikawa H, Reiter RJ, Sugino N. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res. 2008;44:280–7. https://doi.org/10.1111/j.1600-079X.2007.00524.x.
Combelles CM, Gupta S, Agarwal A. Could oxidative stress influence the in-vitro maturation of oocytes?. Reprod Biomed Online. 2009;18:864–880. doi:
Kala M, Shaikh MV, Nivsarkar M. Equilibrium between anti-oxidants and reactive oxygen species: a requisite for oocytedevelopment and maturation. Reprod Med Biol. 2016;16:28–35. https://doi.org/10.1002/rmb2.12013.
Liang LF, Qi ST, Xian YX, Huang L, Sun XF, Wang WH. Protective effect of antioxidants on the pre-maturation aging of mouse oocytes. Sci Rep. 2017;7:1434. https://doi.org/10.1038/s41598-017-01609-3.
Zhu CC, Zhang Y, Duan X, Han J, Sun SC. Toxic effects of HT-2 toxin on mouse oocytes and its possible mechanisms. Arch Toxicol. 2016;90:1495–505. https://doi.org/10.1007/s00204-015-1560-3.
Xin F, Jiang L, Liu X, Geng C, Wang W, Zhong L, Yang G, Chen M. Bisphenol a induces oxidative stress-ass ociated DNA damage in INS-1 cells. Mutat Res Genet Toxicol Environ Mutagen. 2014;769:29–33. https://doi.org/10.1016/j.mrgentox.2014.04.019.
Zhang M, Dai X, Lu Y, Miao Y, Zhou C, Cui Z, Liu H, Xiong B. Melatonin protects oocyte quality from bisphenol A-induced deterioration in the mouse. J Pineal Res. 2017;62. https://doi.org/10.1111/jpi.12396.
Wang T, Han J, Duan X, Xiong B, Cui XS, Kim NH, Liu HL, Sun SC. The toxic effects and possible mechanisms of Bisphenol A on oocyte maturation of porcine in vitro. Oncotarget. 2016;7:32554–32565. doi: https://doi.org/10.18632/oncotarget.8689.
Fernandez M, Bourguignon N, Lux-Lantos V, Libertun C. Neonatal exposure to bisphenol a and reproductive and endocrine alterations resembling the polycystic ovarian syndrome in adult rats. Environ Health Perspect. 2010;118:1217–22. https://doi.org/10.1289/ehp.0901257.
Cabaton NJ, Wadia PR, Rubin BS, Zalko D, Schaeberle CM, Askenase MH, Gadbois JL, Tharp AP, Whitt GS, Sonnenschein C, Soto AM. Perinatal exposure to environmentally relevant levels of bisphenol a decreases fertility and fecundity in CD-1 mice. Environ Health Perspect. 2011;119:547–52. https://doi.org/10.1289/ehp.1002559.
Wang W, Hafner KS, Flaws JA. In utero bisphenol a exposure disrupts germ cell nest breakdown and reduces fertility with age in the mouse. Toxicol Appl Pharmacol. 2014;276:157–64. https://doi.org/10.1016/j.taap.2014.02.009.
Goto Y, Noda Y, Mori T, Nakano M. Increased generation of reactive oxygen species in embryos cultured in vitro. Free Radic Biol Med. 1993;15:69–75. doi:
Guerin P, El Mouatassim S, Menezo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update. 2001;7:175–189. doi:
Fiers W, Beyaert R, Declercq W, Vandenabeele P. More than one way to die: apoptosis, necrosis and reactive oxygen damage. Oncogene. 1999;18:7719–30. https://doi.org/10.1038/sj.onc.1203249.
Cetica PD, Pintos LN, Dalvit GC, Beconi MT. Antioxidant enzyme activity and oxidative stress in bovine oocyte in vitro maturation. IUBMB Life. 2001;51:57–64. https://doi.org/10.1080/15216540119253.
Bezerra MES, Gouveia BB, Barberino RS, Menezes VG, Macedo TJS, Cavalcante AYP, Monte APO, Santos JMS, Matos MHT. Resveratrol promotes in vitro activation of ovine primordial follicles by reducing DNA damage and enhancing granulosa cell proliferation via phosphatidylinositol 3-kinase pathway. Reprod Domest Anim. 2018;53:1298–305. https://doi.org/10.1111/rda.13274.
Lecewicz M, Strzezek R, Kordan W. Maj ewska A. Effect of Extender Supplementation with Low-molecular-weight Antioxidants on Selected Quality Parameters of Cryopreserved Canine Spermatozoa J Vet Res. 2018;62:221–7. https://doi.org/10.2478/jvetres-2018-0032.
Dos Santos EC, Varchetta R, de Lima CB, Ispada J, Martinho HS, Fontes PK. Nogueira MFG., Gasparrini B, Milazzotto MP. The effects of crocetin supplementation on the blastocyst outcome, transcriptomic and metabolic profile of in vitro produced bovine embryos. Theriogenology. 2019;123: 30–36. doi: https://doi.org/10.1016/j.theriogenology.2018.08.010.
Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–60. https://doi.org/10.1146/annurev.bi.52.070183.003431.
Atkuri KR, Mantovani JJ, Herzenberg LA, Herzenberg LA. N-acetylcysteine--a safe antidote for cysteine/glutathione deficiency. Curr Opin Pharmacol. 2007;7:355–9. https://doi.org/10.1016/j.coph.2007.04.005.
Kishida R, Lee ES, Fukui Y. In vitro maturation of porcine oocytes using a defined medium and developmental capacity after intracytoplasmic sperm injection. Theriogenology. 2004;62:1663–76. https://doi.org/10.1016/j.theriogenology.2004.03.008.
Liu J, Liu M, Ye X, Liu K, Huang J, Wang L, Ji G, Liu N, Tang X, Baltz JM, Keefe DL, Liu L. Delay in oocyte aging in mice by the antioxidant N-acetyl-L-cysteine (NAC). Hum Reprod. 2012;27:1411–20. https://doi.org/10.1093/humrep/des019.
Whitaker BD, Knight JW. Effects of N-acetyl-cysteine and N-acetyl-cysteine-amide supplementation on in vitro matured porcine oocytes. Reprod Domest Anim. 2010;45:755–9. https://doi.org/10.1111/j.1439-0531.2009.01344.x.
Whitaker BD, Casey SJ. Taupie r R. the effects of N-acetyl-L-cysteine supplementation on in vitro porcine oocyte maturation and subsequent fertilisation and embryonic development. Reprod Fertil Dev. 2012;24:1048–54. https://doi.org/10.1071/rd12002.
Lai FN, Ma JY, Liu JC, Wang JJ, Cheng SF, Sun XF, Li L, Li B, Nyachoti CM, Shen W. The influence of N-acetyl-l-cysteine on damage of porcine oocyte exposed to zearalenone in vitro. Toxicol Appl Pharmacol. 2015;289:341–8. https://doi.org/10.1016/j.taap.2015.09.010.
Nasr-Esfahani MH, Aitken JR, Johnson MH. Hydrogen peroxide levels in mouse oocytes and early cleavage stage embryos developed in vitro or in vivo. Development. 1990;109:501–507. doi.
Funahashi H, Cantley TC, Stumpf TT, Terlouw SL, Day BN. Use of low-salt culture medium for in vitro maturation of porcine oocytes is associated with elevated oocyte glutathione levels and enhanced male pronuclear formation after in vitro fertilization. Biol Reprod. 1994;51:633–9. https://doi.org/10.1002/mrd.1080250208.
Wang X, Jiang SW, Wang L, Sun Y, Xu F, He H, Wang S, Zhang Z, Pan X. Interfering effects of bisphenol a on in vitro growth of preantral follicles and maturation of oocyes. Clin Chim Acta. 2018;485:119–25. https://doi.org/10.1016/j.cca.2018.06.041.
Boerjan ML, de Boer P. First cell cycle of zygotes of the mouse derived from oocytes aged postovulation in vivo and fertilized in vivo. Mol Reprod Dev. 1990;25:155–63. https://doi.org/10.1146/annurev.bi.52.070183.003431.
Zhou P, Lian HY, Cui W, Wei DL, Li Q, Liu YX, Liu XY, Tan JH. Maternal-restraint stress increases oocyte aneuploidy by impairing metaphase I spindle assembly and reducing spindle assembly checkpoint proteins in mice. Biol Reprod. 2012;86:83. https://doi.org/10.1095/biolreprod.111.095281.
Wu W, Goldstein G, Adams C, Matthews RH, Ercal N. Separation and quantification of N-acetyl-l-cysteine and N-acetyl-cysteine-amide by HPLC with fluorescence detection. Biomed Chromatogr. 2006;20:415–22. https://doi.org/10.1002/bmc.583.
Yoshida M, Ishigaki K, Nagai T, Chikyu M, Pursel VG. Glutathione concentration during maturation and after fertilization in pig oocytes: relevance to the ability of oocytes to form male pronucleus. Biol Reprod. 1993;49:89–94. doi:
Acknowledgments
None.
Funding
This work was supported by the Research Fund of Shandong Provincial Education Department (jyxm2016033). The funder had no role in the design of this study, data acquisition, data interpretation, writing of the manuscript, or decision to submit results.
Author information
Authors and Affiliations
Contributions
QL and ZZ contributed to the study design, data acquisition, and writing the manuscript. QL contributed to data analysis and data interpretation. Both authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Experimental animals used in the study were cared for in accordance with the standards for laboratory animals. The experimental protocol was approved by the Committee of Animal Care of Yantai University.
Consent for publication
Not applicable.
Competing interests
The author declares that there are no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Figure S1. Effect of NAC on the inhibition of poly body emission by 50 μg/mL BPA. C, control; NAC, 100 μM N-acetyl-L-cysteine; BPA, 50 μg/mL bisphenol A; 50 μM NAC + BPA, combined treatment with N-acetyl-L-cysteine (50 μM) and bisphenol A (50 μg/mL); 100 μM NAC + BPA, combined treatment with N-acetyl-L-cysteine (100 μM) and bisphenol A (50 μg/mL); 200 μM NAC + BPA, combined treatment with N-acetyl-L-cysteine (200 μM) and bisphenol A (50 μg/mL); The rates of polar body emission (%) in the control, NAC, BPA, 50 μM NAC + BPA, 100 μM NAC + BPA and 200 μM NAC + BPA groups were 94.7 ± 1.2, 94.6 ± 2.2, 73.9 ± 2.5, 82.1 ± 2.0, 92.7 ± 1.2, and 91.6 ± 2.9, respectively. Each treatment was repeated 3 times with each replicate containing 30–40 oocytes. a-cValues with different letters in their superscripts above the bars differ significantly (P < 0.05). (DOC 1317 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Li, Q., Zhao, Z. Influence of N-acetyl-L-cysteine against bisphenol a on the maturation of mouse oocytes and embryo development: in vitro study. BMC Pharmacol Toxicol 20, 43 (2019). https://doi.org/10.1186/s40360-019-0323-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40360-019-0323-9