Abstract
Background
Cancer patients may receive a high number of medications with the potential to prolong QT interval and subsequent TdP (torsades de pointes). This study aimed to identify the prevalence of QT prolonging drugs, their TdP risk, QT prolonging drug-drug interactions (QT-DDIs), levels, predictors, and TdP risk of drugs involved in QT-DDIs.
Methods
This multicenter study included cancer patients from three major tertiary care hospitals of Khyber-Pakhtunkhwa, Pakistan. Micromedex DrugReax® was used for identification of QT-DDIs. TdP risks were identified by AZCERT (Arizona Center for Education and Research on Therapeutics) classification. Logistic regression analysis was performed to identify predictors of QT-DDIs.
Results
Of 555 patients, 51% were females. Mean age was 46.9 ± 15.7 years. Total 28 distinct QT prolonging drugs were identified in 92.6% of the patients. Overall 21.8% patients were presented with QT-DDIs. Of total 288 identified QT-DDIs, all were of major-severity and fair-documentation. According to AZCERT classification, 59.9% of the interacting drugs were included in list-1 (known risk of TdP), 4.7% in list-2 (possible risk of TdP) and 6.8% in list-3 (conditional risk of TdP). Univariate logistic regression analysis showed significant results for various predictors such as, 8–9 prescribed medications (p < 0.001) and ≥10 medications (p < 0.001), 2 QT drugs (p < 0.001) and ≥3 QT drugs (p < 0.001), breast cancer (p = 0.03), gastrointestinal cancer (p = 0.03), 4–5 supportive care drugs (p < 0.001), 6–8 supportive care drugs (p < 0.001) and >8 supportive care drugs (p < 0.001).
Conclusions
A high prevalence of QT prolonging drugs and QT-DDIs was reported in oncology. Appropriate precautions are needed to prevent harmful consequences of these interactions.
Similar content being viewed by others
Background
In developed world, cancer and cardiac disease play a major role in causing morbidity and mortality [1]. Due to recent therapeutic advancements, 5-year survival for early stage breast cancer increased from 79% to 88% during the last two decades [2,3,4,5]. Similarly, survival rates have also been increased in other solid and hematological cancers as well as non-hodgkin lymphoma and testicular cancer [6].
Numerous drugs are administered to the patients with advanced cancer in order to treat their malignancy, its related ailments (e.g., pain), comorbid illnesses (e.g., heart disease, diabetes, dyslipidemia), and mitigate adverse effects induced by chemotherapy (e.g., nausea and vomiting). Certainly, multiple therapies make cancer patients vulnerable to potentially unsafe drug-drug interactions (DDIs) and it can be worsened in the presence of aberrant organ function (heart, liver, and kidney) [7].
Since last few years, cancer patients have been predisposed to substantial medical complications in the form of heart diseases [8]. A distinctive range of cardiovascular anomalies including, myocardial toxicity, ischemia, hypertension and arrhythmia [9,10,11,12,13] either directly or indirectly (inappropriate lifestyle) have been associated with new cancer therapies [1]. Moreover, anticancer agents and supportive care therapy may cause various cardiac rhythm disorders and most remarkable feature is prolonged QT interval which can ultimately lead to ventricular arrhythmias. Concomitant use of supportive care therapy and cancer medications may cause prolongation of QT interval [1].
The QT interval on an electrocardiography (ECG) rhythm strip indicates phases of ventricular depolarization and consequent repolarization and its measurement is taken from the point where QRS complex begins to the end of T wave [7]. A delay in the cardiac repolarization phase leads to the electrophysiological disturbances and subsequent torsades de pointes (TdP) [14, 15]. TdP is a rare form of fatal polymorphic ventricular tachycardia that is often illustrated by the twisting of points on an ECG [7]. Currently, pharmacoepidemiologic data regarding prevalence and nature of QT prolonging drug-drug interactions (QT-DDIs) in cancer patients is limited and there are certain areas which need to be explored. Issue of QT-DDIs in cancer patients is a poorly addressed area. To the best of our knowledge, there is no specific study regarding the prevalence of QT-DDIs in oncology settings. There are some studies which have worked on the prevalence and nature of overall potential DDIs in cancer patients [16,17,18]. As the main aims of these studies were to explore all types of DDIs in a generalized manner, therefore limited considerations have been given to QT-DDIs. All of these studies have elaborated in their discussions that proper attention should be given to QT-DDIs and their associated negative consequences in cancer patients [16,17,18]. Therefore, specific work is needed in cancer patients to explore the prevalence of QT-DDIs, possible risk factors, extent of the risk of QTc prolongation and possible predictors. Lack of scientific evidence regarding prescribing pattern of QT prolonging medications, QT-DDIs and QTc prolongation may predispose cancer patients to TdP. Such studies will be helpful to improve clinical practice and ensure patients’ safety.
Aim of the study
The aim of this study was to investigate the frequency of QT prolonging drugs and their TdP risk; and QT-DDIs, their levels of severity and documentation, predictors and TdP risk of drugs involved in QT-DDIs.
Methods
Study design and settings
This was a multicenter cross-sectional retrospective study conducted in three tertiary care hospitals, Medical Teaching Institute, Ayub Teaching Hospital (ATH), Abbottabad, North West General Hospital and Research Center, Peshawar and Medical Teaching Institute, Hayatabad Medical Complex (HMC), Peshawar, Pakistan.
Data source
The study included data of all consecutive patients, aged >18 years, who received treatment for cancer during a one-year period, Jan-2014 to Dec-2014. Approval was obtained from hospitals’ administrations to access patients’ data in order to collect all relevant information needed for the study. Data were collected regarding patients’ age, gender, cancer type, comorbidities and prescribed medications.
Data analysis
For each patient, medication lists were analyzed for the presence of QT-DDIs using an online database, Micromedex Drug-Reax® [19]. QT-DDIs were classified on the basis of severity and documentation according to the Micromedex Drug-Reax® classification system [19]. The Arizona Center for Education and Research on Therapeutics (AZCERT) QT drug list [20] was used for identifying QT prolonging drugs. The AZCERT classification system categorizes QT prolonging drugs in to list-1 (known risk of TdP), list-2 (possible risk of TdP), and list-3 (conditional risk of TdP). Therapeutic classes of drugs involved in QT-DDIs were coded according to Anatomical Therapeutic Chemical (ATC) index of the World Health Organization (WHO) [21].
Statistical analyses
Categorical data were presented as frequencies and percentages. While continuous data were presented as mean ± SD. Logistic regression analysis was used to calculate the odds ratios (OR) for predictors of QT-DDIs. A p-value ≤0.05 was considered statistically significant. SPSS (IBM SPSS statistics version 23) was used for all statistical analyses.
Results
Patients’ demographic and clinical characteristics are listed in Table 1. Total 555 patients were included in this study, of which 274 (49%) were males and 281 (51%) were females. Mean age of the patients was 46.9 ± 15.7 years, whereas majority of the patients were in the age range > 50 years (39.5%). Average number of prescribed medications were 8.4 ± 3.6, while in 35.9% of the cases, ≥10 drugs were prescribed. The most frequent diagnoses were breast cancer (15.3%), non-hodgkin lymphoma (15.1%), gastrointestinal cancer (12.8%), gynecologic cancer (5.9%), acute lymphoblastic leukemia (5.2%), and genitourinary cancer (4.1%). The most frequent comorbid illnesses were diabetes mellitus (4.9%), hypertension (4.1%), hepatitis B (0.5%) and hepatitis C (0.5%).
Total 993 QT prolonging drugs were identified in 92.6% of the patients (Table 2). Among them 46.5% were females while 46.1% were males. The cancer patients were frequently encountered with antiemetics (n = 571), proton pump inhibitors (145), antimicrobials (126), anticancer drugs (51) and antineoplastic agents (30) which carried the potential for QT prolongation (Table 2). Total 28 distinct QT prolonging drugs were used in cancer patients. Among them, the most prevalent were ondansetron (n = 278), metoclopramide (152), tropisetron (139), ciprofloxacin (90), omeprazole (87), capecitabine (46) and oxaliplatin (30).
Table 3 shows the highly prevalent (n > 5) QT prolonging drugs used in various types of cancer, such as, breast cancer: ondansetron (54), ciprofloxacin (32), tropisetron (20), and metoclopramide (18); gastrointestinal cancer: ondansetron (46), capecitabine (25), metoclopramide (21), and oxaliplatin (20); and non-hodgkin lymphoma: ondansetron (39), tropisetron (33), metoclopramide (25), and esomeprazole (16). A full presentation of all QT prolonging drugs stratified with respect to various types of cancer has been given in Additional file 1: Table S1.
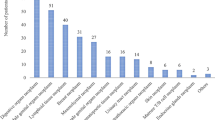
Of 555 patients, 21.8% were presented with QT-DDIs (Fig. 1). Prevalence of QT-DDIs was higher in females (11.3%) as compared with males (10.5%) (p = 0.7) and in age group >50 years (8.5%) as compared with other age groups (p = 0.4). Similarly, prevalence of QT-DDIs was significantly higher in breast cancer (5.8%) and gastrointestinal cancer (5%) compared with other cancers (p < 0.001) and in solid malignancy (17.8%) compared with hematological malignancy (4%) (p < 0.001) (Fig. 1).
Total 288 QT-DDIs were identified, of which, all were of major severity and fair documentation (Table 4). According to AZCERT classification, 59.9% of the interacting drugs were included in list-1 (known risk of TdP), 4.7% in list-2 (possible risk of TdP) and 6.8% in list-3 (conditional risk of TdP) (Table 4). As far as therapeutic classes are concerned, antimicrobials (36.3%), antiemetic (34.7%) and antipsychotics (27.3%) were more common. Table 5 shows top 20 QT-DDIs, their AZCERT classification, [20] therapeutic classes, severity and documentation levels. Of the total QT-DDIs, 76 QT-DDIs involved both the interacting drugs from the AZCERT QT drugs list-1 (known risk of TdP). The most common drug interacting pairs involved in QT-DDIs were ondansetron-prochlorperazine (n = 88), ciprofloxacin-ondansetron (71), ciprofloxacin-prochlorperazine (64), ciprofloxacin-metronidazole (10) and ciprofloxacin-dolasetron (6). Drugs frequently involved in QT-DDIs were ondansetron (n = 174), ciprofloxacin (157), prochlorperazine (157), metronidazole (30), dolasetron (21) and fluconazole (8).
Table 6 shows the highly prevalent (n > 2) QT-DDIs in various types of cancer, such as, breast cancer: ciprofloxacin-Ondansetron (31), ciprofloxacin-prochlorperazine (31), and ondansetron-prochlorperazine (31); gastrointestinal cancer: ondansetron-prochlorperazine (26), ciprofloxacin-ondansetron (13), and ciprofloxacin-prochlorperazine (12); and gynecologic cancer: ciprofloxacin-ondansetron (8), ciprofloxacin-prochlorperazine (6), and Ondansetron-Prochlorperazine (6). The entire result has been provided in Additional file 2: Table S2 which shows frequency of all QT-DDIs along with their levels and TdP risks of drugs involved in these QT-DDIs stratified with respect to various types of cancer.
In univariate logistic regression analysis (Table 7), a significant association of QT-DDIs with 8–9 prescribed medications (OR = 8.9; 95% CI = 2.6–30.3; p < 0.001), ≥10 prescribed medications (OR = 25.2; 95% CI = 7.7–82.2; p < 0.001), 2 QT prolonging drugs (OR = 25.4; 95% CI = 11.2–57.5; p < 0.001) and ≥3 QT prolonging drugs (OR = 21; 95% CI = 9.2–48; p < 0.001). There was significant association of the occurrence of QT-DDIs with breast cancer (OR = 3.7; 95% CI = 1.2–11.6; p = 0.03), gastrointestinal cancer (OR = 4; 95% CI = 1.3–13; p = 0.02), 4–5 supportive care drugs (OR = 4.3; 95% CI = 1.9–9.5; p < 0.001), 6–8 supportive care drugs (OR = 8.1; 95% CI = 3.7–17.7; p < 0.001) and >8 supportive care drugs (OR = 12.2; 95% CI = 4.9–30.5; p < 0.001).
Discussion
This is the first study in oncology which specifically and extensively determined various drug related factors having potential of QT interval prolongation. In this study, we detected a high prevalence of QT prolonging drugs and QT-DDIs, which is of particular concern. Several important findings have emerged from our analysis. The patients with breast cancer and gastrointestinal cancer are at increased risk of TdP due to frequent use of high risk QT interval prolonging medications and QT-DDIs involving both drugs from AZCERT list-1 (known risk of TdP). Proper considerations should be given to monitor the effects of these medications and QT-DDIs in high risk patients. Polypharmacy was the major issue in cancer patients, which might be responsible for such a high prevalence of QT prolonging drugs and QT-DDIs.
The most frequent QT prolonging drugs used in cancer patients were ondansetron, metoclopramide, tropisetron, ciprofloxacin, capecitabine and oxaliplatin which are also responsible for high prevalence of QT-DDIs. While the most common drugs involved in QT-DDIs were ondansetron, metoclopramide, quinolones, capecitabine, oxaliplatin and domperidone. Domperidone is associated with QTc prolongation, subsequent TdP and sudden cardiac death [22]. The published data suggest that ondansetron, metoclopramide and fluoroquinolones may significantly prolong the QT interval causing serious arrhythmias and mortality [23,24,25]. The monitoring of arrhythmogenic risks associated with these medications is mandatory to avoid life threatening situations.
The data regarding the prevalence of QT interval prolonging drugs and QT-DDIs in oncology settings are scarce. Over the past few years, a limited number of studies investigated the prevalence of QT-DDIs among cancer patients [16,17,18]. We identified 288 QT-DDIs in contrast to 45–110 QT-DDIs reported by those studies [16,17,18]. The lack of consistency in results might be due to a variety of reasons. The study design and various tools used for screening QT-DDIs were different. Moreover, the scope and nature of these studies regarding the prevalence of QT-DDIs was limited.
Previous studies [16, 18] screened QT-DDIs using AZCERT drug list, [20] which demonstrates that they considered only pharmacodynamic interactions whereas both pharmacokinetic and pharmacodynamic drug interactions were taken in to account in our analysis. The latest and updated tool, Micromedex Drug-Reax® [19] was used for screening QT-DDIs along with AZCERT QT drug lists [20]. A cross-sectional study considered oral anticancer drugs only while we included all drugs in our study [16]. Variations in prescribing patterns and clinical profile of the patients might me some other factors responsible for these inconsistencies in results. It is quite obvious from our findings that QT-DDIs and their monitoring protocols should be given appropriate consideration in clinical practice.
The prevalence of QT prolonging drugs and QT-DDIs in various types of cancer has not been the subject of studies conducted in past. These parameters were considered in the current study. We identified a high prevalence of QT prolonging drugs and QT-DDIs in breast cancer, gastrointestinal cancer, gynecologic cancer and non-hodgkin lymphoma. The high prevalence of QT-DDIs among cancer patients was due to the frequent use of the QT prolonging drugs. Appropriate considerations are needed to avoid any detrimental effects associated with QT interval prolonging drugs and QT-DDIs. We identified that the potential risk of QT-DDIs increases with rising number of all prescribed medications, QT interval prolonging drugs and supportive care drugs. The patients with breast cancer and gastrointestinal cancer are significantly exposed to QT-DDIs.
Concomitant use of QT prolonging drugs, possibly leading to fatal outcomes, should be avoided [26]. Several drugs involved in QT-DDIs represented a variety of therapeutic classes such as anticancer, antimicrobials, antiemetics and antipsychotics. QT-DDIs involving these drug classes potentiate the drug induced QTc prolongation and subsequent TdP. There is scarcity of information to guide physicians about the risks of QT-DDIs and this study would definitely help them about this critical area. It is difficult to guess the magnitude of knowledge of health care professionals about the use of QT drugs and QT-DDIs and whether or not they had made any attempts to avoid such drugs or their combinations.
One of the limitations of this study was the lack of ECG data. Consequently, we could not investigate the prevalence of the QTc interval prolongation among cancer patients. This is quite possible that these factors were not considered in routine clinical practice in oncology. In this study, Micromedex DrugReax® was used as a screening tool while other tools are also available and published literature have reported several inconsistencies among these tools [27].
Conclusion
The present study shows a high prevalence of QT-DDIs in cancer patients. Various anticancer and supportive care drugs associated with QTc prolongation and TdP are often prescribed concomitantly in oncology, which may lead to lethal arrhythmias. Future studies should further explore the clinical outcomes of QT-DDIs such as QTc prolongation and TdP.
Recommendations
The study findings suggest that the QT interval prolongation and subsequent risk of TdP should be considered as an essential component of the patients’ monitoring plan in the clinical practice. Moreover, an ECG should be done before starting a QT prolonging drug, 8–12 h after administration of QT prolonging drug or after increasing its dose, as recommended by American College of Cardiology Foundation (ACCF) and American Heart Association (AHA) [28]. The physicians should be aware of the arrhythmogenic risks associated with the QT interval prolonging drugs and QT-DDIs in oncology ward. In certain cases, where it is inevitable to avoid a QT prolonging drug or its combination, appropriate precautions such as ECG monitoring, dosage adjustment and rectifying the electrolyte imbalance should be undertaken to prevent the potential harmful consequences. The patients with breast cancer and gastrointestinal cancer are considerably exposed to the harmful effects of the QT-DDIs and need special attention. The QT-DDIs involving both the high-risk medications (known risk of TdP) should be particularly avoided. The updated drug information sources such as the AZCERT QT drugs lists [20] and the Micromedex DrugReax [19] can be helpful to clinicians regarding the drug selection in oncology.
Abbreviations
- ACCF:
-
American college of cardiology foundation
- AHA:
-
American heart association
- ATC:
-
Anatomical therapeutic chemical
- ATH:
-
Ayub teaching hospital
- AZCERT:
-
Arizona center for education and research on therapeutics
- DDIs:
-
Drug-drug interactions
- ECG:
-
Electrocardiogram
- HMC:
-
Hayatabad medical complex
- QT-DDIs:
-
QT prolonging drug-drug interactions
- SPSS:
-
Statistical package for the social sciences
- TdP:
-
Torsades de pointes
- WHO:
-
World health organization
References
Curigliano G, Cardinale D, Dent S, Criscitiello C, Aseyev O, Lenihan D, et al. Cardiotoxicity of anticancer treatments: epidemiology, detection, and management. CA Cancer J Clin. 2016;66(4):309–25. doi:10.3322/caac.21341.
Howlader N, Ries LA, Mariotto AB, Reichman ME, Ruhl J, Cronin KA. Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst. 2010;102(20):1584–98. doi:10.1093/jnci/djq366.
Jemal A, Ward E, Hao Y, Thun M. Trends in the leading causes of death in the United States, 1970-2002. JAMA. 2005;294(10):1255–9. doi:10.1001/jama.294.10.1255.
Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220–41. https://doi.org/10.3322/caac.21149.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29.
Jemal A, Ward E, Thun M. Declining death rates reflect progress against cancer. PLoS One. 2010;5(3):e9584. doi:10.1371/journal.pone.0009584.
Barni S, Petrelli F, Cabiddu M. Cardiotoxicity of antiemetic drugs in oncology: an overview of the current state of the art. Crit Rev Oncol Hematol. 2016;102:125–34. https://doi.org/10.1016/j.critrevonc.2016.04.012.
Salvatorelli E, Menna P, Cantalupo E, Chello M, Covino E, Wolf FI, et al. The concomitant management of cancer therapy and cardiac therapy. Biochim Biophys Acta. 2015;1848(10 Pt B):2727–37. doi:10.1016/j.bbamem.2015.01.003.
Ewer MS, Ewer SM. Cardiotoxicity of anticancer treatments: Nature Reviews Cardiology. 2015;12(9):547-58. doi:10.1038/nrcardio.2015.65.
Ali MK, Ewer MS, Gibbs HR, Swafford J, Graff KL. Late doxorubicin-associated cardiotoxicity in children. The possible role of intercurrent viral infection. Cancer. 1994;74(1):182–8.
Hahn VS, Lenihan DJ, Ky B. Cancer therapy-induced cardiotoxicity: basic mechanisms and potential cardioprotective therapies. J Am Heart Assoc. 2014;3(2):e000665. doi:10.1161/JAHA.113.000665.
Suter TM, Ewer MS. Cancer drugs and the heart: importance and management. Eur Heart J. 2013;34(15):1102–11. doi:10.1093/eurheartj/ehs181.
Procter M, Suter TM, de Azambuja E, Dafni U, van Dooren V, Muehlbauer S, et al. Longer-term assessment of trastuzumab-related cardiac adverse events in the Herceptin adjuvant (HERA) trial. J Clin Oncol. 2010;28(21):3422–8. doi:10.1200/JCO.2009.26.0463.
Shah RR. Drugs, QT interval prolongation and ICH E14: the need to get it right. Drug Saf. 2005;28(2):115–25.
Shah RR. Drugs, QTc interval prolongation and final ICH E14 guideline : an important milestone with challenges ahead. Drug Saf. 2005;28(11):1009–28.
van Leeuwen RW, Brundel DH, Neef C, van Gelder T, Mathijssen RH, Burger DM, et al. Prevalence of potential drug-drug interactions in cancer patients treated with oral anticancer drugs. Br J Cancer. 2013;108(5):1071–8. doi:10.1038/bjc.2013.48.
van Leeuwen RW, Jansman FG, van den Bemt PM, de Man F, Piran F, Vincenten I, et al. Drug-drug interactions in patients treated for cancer: a prospective study on clinical interventions. Ann Oncol. 2015;26(5):992–7. doi:10.1093/annonc/mdv029.
van Leeuwen RW, Swart EL, Boven E, Boom FA, Schuitenmaker MG, Hugtenburg JG. Potential drug interactions in cancer therapy: a prevalence study using an advanced screening method. Ann Oncol. 2011;22(10):2334–41. doi:10.1093/annonc/mdq761.
Micromedex Drug-Reax®. Greenwood Village, CO: Truven Health Analytics. http://www.micromedexsolutions.com/micromedex2/librarian/ Accessed 26 Feb 2017.
Arizona Center for Education and Research on Therapeutics (AZCERT) QTdrugs List, AZCERT Inc. 1822 Innovation Park Dr., Oro Valley, AZ 85755. https://www.crediblemeds.org/index.php/. Accessed 10 Feb 2017.
WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD index. http://www.whocc.no/atc_ddd_index/ Accessed 25 Feb 2017.
van Noord C, Dieleman JP, van Herpen G, Verhamme K, Sturkenboom MC. Domperidone and ventricular arrhythmia or sudden cardiac death: a population-based case-control study in the Netherlands. Drug Saf. 2010;33(11):1003–14. doi:10.2165/11536840-000000000-00000.
Benedict CR, Arbogast R, Martin L, Patton L, Morrill B, Hahne W. Single-blind study of the effects of intravenous dolasetron mesylate versus ondansetron on electrocardiographic parameters in normal volunteers. J Cardiovasc Pharmacol. 1996;28(1):53–9.
Bentsen G, Stubhaug A. Cardiac arrest after intravenous metoclopramide - a case of five repeated injections of metoclopramide causing five episodes of cardiac arrest. Acta Anaesthesiol Scand. 2002;46(7):908–10.
Briasoulis A, Agarwal V, Pierce WJ. QT prolongation and torsade de pointes induced by fluoroquinolones: infrequent side effects from commonly used medications. Cardiology. 2011;120(2):103–10. doi:10.1159/000334441.
Woosley RL, Chen Y, Freiman JP, Gillis RA. Mechanism of the cardiotoxic actions of terfenadine. JAMA. 1993;269(12):1532–6.
Roblek T, Vaupotic T, Mrhar A, Lainscak M. Drug-drug interaction software in clinical practice: a systematic review. Eur J Clin Pharmacol. 2015;71(2):131–42. doi:10.1007/s00228-014-1786-7.
Drew BJ, Ackerman MJ, Funk M, Gibler WB, Kligfield P, Menon V, et al. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2010;55(9):934–47. doi:10.1016/j.jacc.2010.01.001.
Acknowledgements
We are very grateful to administrations of all hospitals, physicians and all other staff for their cooperation in this study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
All authors contributed substantially to the work presented in this paper, read and approved the submission of the final manuscript. QK designed all the work under the supervision of MI, analyzed and interpreted resulting data and drafted the manuscript. SK collected the patients’ data from the participating hospitals. MI designed the research theme, contributed substantially with data analysis, results interpretations and manuscript editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by Institutional Review and Ethical Board, Post Graduate Medical Institute, Peshawar. All ethical considerations were observed and patients’ personal information was kept confidential.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1: Table S1.
Prevalence of QT prolonging drugs along with their TdP risks stratified with respect to various types of cancer. (PDF 234 kb)
Additional file 2: Table S2.
Frequency of QT-DDIs along with their levels and TdP risks of drugs involved in these QT-DDIs stratified with respect to various types of cancer. (PDF 170 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Khan, Q., Ismail, M. & Khan, S. Frequency, characteristics and risk factors of QT interval prolonging drugs and drug-drug interactions in cancer patients: a multicenter study. BMC Pharmacol Toxicol 18, 75 (2017). https://doi.org/10.1186/s40360-017-0181-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40360-017-0181-2