Abstract
Background
As the most common chronic disease in childhood, asthma displays a major public health problem worldwide with the incidence of those affected rising. As there is currently no cure for allergic asthma, it is mandatory to get a better understanding of the underlying molecular mechanism.
Main body
By producing IgE antibodies upon allergen contact, B cells play a pivotal role in allergic asthma. Besides that, IL-10-secreting B cell subsets, namely regulatory B cells (Bregs), are reported in mice and humans to play a role in allergic asthma. In humans, several Breg subsets with distinct phenotypic and functional properties are identified among B cells at different maturational and differentiation stages that exert anti-inflammatory functions by expressing several suppressor molecules. Emerging research has focused on the role of Bregs in allergic asthma as well as their role for future diagnostic and preventive strategies.
Conclusion
Knowledge about the exact function of human Bregs in allergic asthma is still very limited. This review aims to summarize the current knowledge on Bregs. We discuss different human Breg subsets, several ways of Breg induction as well as the mechanisms through which they exert immunoregulatory functions, and their role in (childhood) allergic asthma.
Similar content being viewed by others
Background
Asthma is an inflammatory airway disease, affecting both children and adults, being the most common chronic disease in childhood. It is characterized by airway hyperresponsiveness, acute and chronic bronchoconstriction, airway edema, and mucus plugging [1]. There are two main forms of asthma: allergic and non-allergic asthma [2]. The World Health Organization (WHO) estimated that 262 million people were affected by asthma in 2019 [3], and with current trends rising, it is expected to reach 400 million by 2025 [4]. To this end, understanding the underlying disease mechanisms for further treatment is of great importance. Currently, there is no cure for asthma and therapy, which is focused on inhaled corticosteroids and bronchodilators, suppresses symptoms rather than changing the natural history of the disease [5]. Additional asthma therapies, also approved for children, use biologicals such as Omalizumab or Dupilumab which target IgE or asthma-associated cytokines such as IL-4 and IL-13, respectively [6]. Nevertheless, depending on the severity, asthma results in a diminished quality of life and an important economic burden on public health care systems, thus representing a major public health problem worldwide with a high socio-economic impact [7].
The immune system has several mechanisms to defend itself against viral, bacterial, fungal, and protozoal infections. For this, the mammalian immune system consists of the innate and the adaptive immune system. The latter is highly specific and relies on diverse antigen-specific receptors expressed on the surface of T- and B-lymphocytes [8]. For healthy immune regulation and to avoid excessive responses, the immune system needs to be tolerant against self and external innocuous antigens, referred to as immune tolerance. In healthy individuals, there is a subtle balance between anti-inflammatory (prevention of chronic inflammation and tissue damage) and pro-inflammatory (counter infections) responses [9]. Dysregulation can result in asthma, allergy, and autoimmune diseases [10].
The peripheral B cell compartment comprises a heterogeneous population of cells at different maturational stages along the lineage, each with distinctive functional properties [11]. They produce antibodies, cytokines, and act as antigen-presenting cells (APCs). In addition, B cells have the capability to regulate immune responses through regulatory B cells (Bregs), which are B cells with anti-inflammatory functions [12]. They exhibit their anti-inflammatory role by producing mainly anti-inflammatory cytokines, e.g., IL-10, transforming growth factor (TGF)-β, and IL-35, as well as by induction of other regulatory cells [13, 14]. In humans, Bregs are found among B-lymphocytes at different stages of maturation and differentiation, ranging from early transitional B cells to highly differentiated plasmablasts [15, 16].
Knowledge about the impact of B cells and Bregs on asthma is still rather limited. IgE production upon allergen contact is the main described role of B cells in allergic asthma, which is mandatory for the initiation of the allergic cascade [17]. Habener et al. revealed that regulatory B cells control airway hyperresponsiveness and airway remodeling in a murine asthma model, suggesting a potential role for B cells in future diagnostic and preventive strategies in asthma [18]. They also showed differences in human B cell populations between asthma and controls as well as between mild-moderate and severe asthma [19]. Moreover, Breg numbers contribute to the regulation of the immune system, which was confirmed by multiple human studies showing lower frequencies of some Breg subsets in allergic asthmatics [20,21,22,23]. This review aims to summarize the current knowledge about Bregs and their role in childhood allergic asthma to get new mechanistic insights into childhood asthma development and therapeutic and preventive strategies.
Immune mechanisms underlying childhood allergic asthma
Allergic asthma is the predominant form of asthma in childhood, which has been characterized by sensitization to specific allergens, high IgE levels, eosinophilia, a type 2 shifted immune response, and decreased innate immunity gene expression [24]. Both the innate and the adaptive immune system play a role in type 2 immune response and involve several immune cells such as T-helper (Th) 2 cells, group 2 innate lymphoid cells (ILCs), B cells, natural killer (NK) cells, NK T cells, basophils, eosinophils, and mast cells, as well as their major cytokines [25].
Repetitive exposure of children to environmental allergens such as house dust mites (HDM) results in the development of allergen-specific memory T and B cell responses following expression of inflammatory mediators to recruit immune effector cells such as T and B cells [26, 27]. Upon allergen presentation by APCs to naïve CD4+ T cells, they get activated and release type 2 cytokines such as IL-3 and IL-5, which drive the proliferation and differentiation of basophils, eosinophils, and mast cells, which in turn contribute to asthmatic bronchial hyperresponsiveness. In addition, IL-4 and IL-13 are released, which play a key role in IgE production of B cells. IgE in turn binds to high-affinity IgE surface receptors (FcεRI) on immune cells such as dendritic cells (DCs), basophils, and mast cells [28, 29]. Upon cross-linking of the IgE-FcεRI complexes by the allergen, it activates the cells resulting in the release of inflammatory mediators like histamine, leukotrienes, and the type 2 cytokines IL-4, IL-5, and IL-13. Consequently, an influx of CD4+ T cells and eosinophils into the airways maintains type 2 inflammation. To this end, higher levels of lymphocytes, eosinophils, basophils, and mast cells are found in the airways of allergic asthmatics [28, 30]. Moreover, higher levels of airway epithelial desquamation, goblet cell hyperplasia, and thicker basement membranes are characteristics of asthmatics [29]. Since sensitization to allergens plays a key role in the development of allergic asthma [31], targeting of allergen sensitization seems to be promising in allergic asthma therapy [32].
B cells
As part of the adaptive immune system, B cells play a pivotal role in the protection against pathogens. Moreover, they play important roles in several diseases such as autoimmune disorders [33, 34], cancer [35], allergy [36], and asthma [37, 38].
The peripheral B cell compartment consists of several cells at different maturational stages with different functions [11]. In humans, B cells develop in the bone marrow from hematopoietic precursors derived from the fetal liver [39]. Early B cell development includes (early and late) pro-B, pre-B, and immature B cells in which specific surface markers are expressed and are characterized by rearrangements of the immunoglobulin (Ig) heavy and light gene segments to generate diverse antigen receptors [40]. Immature B cells then exist in the bone marrow for several days until they enter the circulation as transitional B cells [41]. Depending on their surface markers and function, transitional B cells can be subdivided into T1, T2, and T3 B cells [42]. Depending on signals received through the B cell receptor (BCR) and other receptors, transitional B cells further differentiate into either follicular (FO) or marginal zone (MZ) B cells, now considered mature or naïve B cells [39, 43]. After specific antigen-recognition via BCR, selected B cells become either antibody-producing plasmablasts or plasma cells, or memory B cells [39]. Memory B cells are B cells, which circulate through the body [44]. Upon binding of the specific antigen, that originally activated the parent B cell that led to the production of the memory B cell, to the BCR, a strong and more rapid antibody response is initiated by the memory B cell [45]. In addition to the generation of immunological memory through memory B cells [45], B cells also contribute to immune responses through cytokine production [46], and antigen presentation, as well as through antibody production, which is their main contribution to allergy [47]. These multifaceted roles of B cells were also shown in several studies using allergic mouse models of asthma [48,49,50].
Regulatory B cells (Bregs)
B cells can also regulate immune responses by other mechanism than antibody production, antigen presentation, and cytokine production. In the 1970s, allergen sensitization studies described an immunosuppressive potential of B cells for the first time [51, 52]. At that time, the precise mechanisms of suppression were not identified, and for more than 20 years, the study of immunosuppressive B cells did not receive significant attention [9, 53]. However, during the past two decades, the knowledge of regulatory B cell (Breg) phenotype and function increased [12]. In 2002, Mitzoguchi et al. described interleukin 10 (IL-10) producing B cells with regulatory functions that are characterized by CD1d upregulation in murine models of intestinal inflammation [54]. Other studies in murine models also showed an essential role of IL-10-producing B cells in controlling autoimmunity [55] and the prevention of arthritis [56]. Regulatory B cells (Bregs) were not only described in mice but they were also identified in humans: In 1998, Akdis et al. described IL-10-producing B cells in humans in the context of high-allergen exposure models and allergen immunotherapy (AIT). In AIT, high doses of the allergen are administered, which significantly changed IL-10 production after 7 days of bee venom (BV)-AIT in epitope-specific T cells, but not in B cells [57]. In 2007, Goetz et al. and Dass et al. demonstrated that therapy with Rituximab, an anti-CD20-antibody that depletes B cells, leads to severe exacerbation of ulcerative colitis and new onset of psoriasis in humans. Furthermore, they showed an association with the suppression of local IL-10 production suggesting an important anti-inflammatory role of IL-10-producing B cells in humans [58, 59].
Human Breg subsets
Several murine and human disease models were used to study the phenotype of Bregs as well as the underlying molecular mechanisms of Breg-mediated immune suppression. This resulted in the identification of different Breg subsets in both mice and humans with distinct phenotypic and functional properties [9]. Until now, there is no clear consensus on the classification and definition of Bregs. Here, we discuss the major Breg subsets that were identified in humans (Table 1).
Human Bregs are found among B-lymphocytes at different stages of maturation and differentiation: Human CD27+CD24hi B10 cells produce IL-10 and thereby regulate cytokine production by monocytes after in vitro stimulation with lipopolysaccharide (LPS) and CpG, indicating a functional link between Bregs and the innate immune system. The mean frequencies of these cells are significantly increased in patients with autoimmune diseases [60]. Human CD19+CD24hiCD38hi immature transitional B cells also have regulatory functions. After stimulation with CD40, these cells produce IL-10 thereby suppressing the differentiation of naïve T cells into TH1 and TH17 cells while inducing the conversion into Tregs [15, 61]. Moreover, this Breg subset was shown to regulate T cell immunity in chronic HBV (CHB) infection [62]. Human CD19+CD25+CD71+CD73− BR1 cells are characterized by high expression of IL-10 and suppression of antigen-specific CD4+ T cell proliferation. Also, anti-inflammatory IgG4 antibody production was shown by these BR1 cells [63]. Lindner et al. described IL-21-induced granzyme B-expressing B cells (GrB+ B cells) that are found in the microenvironment of solid tumors next to IL-21-providing T cells. They are characterized by a CD19+CD38+CD1d+IgM+CD147+ expression signature and express regulatory molecules such as GrB, IL-10, CD25, and indolamine-2,3-dioxygenase (IDO). It was shown that they suppress T cell proliferation through GrB-dependent T cell receptor (TCR) degradation [65]. Another human Breg subset was described in 2017 by Brosseau et al. showing that CD9+ B cells induce effector T cell apoptosis in both mice and humans by IL-10 secretion. Moreover, they showed that these CD9+ B cells with regulatory properties are reduced in patients with severe asthma [66]. Human CD19+CD27intCD38+ plasmablasts produce IL-10 and secrete IgM. Interestingly, Matsumoto et al. showed that human CD19+CD27intCD38+ plasmablasts derived from naïve and especially immature B cells, but not human CD19+CD27hiCD38+ plasmablasts derived from memory B cells, are the major IL-10-producing B cells [16]. For human IgA+PD-L1+IL-10+ plasma cells, suppression of anti-tumor immunity was described [64]. Patients with tuberculosis were shown to have an increased number of CD5+Cd1d+ B cells with a stronger suppressive activity by inhibiting Th17 cell activation and IL-22 production [67, 68].
Due to this heterogeneity of Breg subsets, it was not possible until now to identify a Breg-specific transcription factor [9]. Since this is an emerging field of research, many questions still need to be answered with regard to plasticity, ontogeny, and the Breg-mediated suppression mechanism.
Several ways of Breg induction
The heterogeneity of Breg subsets leads to the assumption that there are either various distinct Breg lineages or that IL-10 can be induced by external stimuli at different stages of B cell development [9]. The precise mechanisms and required signals for Breg differentiation still need to be elucidated. However, several murine and human studies revealed that B cells of different maturational and differentiation stages are able to differentiate into Bregs upon antigen recognition and/or several kinds of stimuli [69,70,71]. CpG stimulation results in the generation of human CD19+CD27intCD38+ plasmablasts. Additionally treating B cells with IL-2, IL-6, and especially with the type I interferon IFN-α results in the generation of IL-10 secreting human CD19+CD27intCD38+ plasmablasts [16]. IFN-α was described to induce CD38+ expression on human naïve B cells [72] and promote differentiation into plasma cells [73]. In this regard, it is likely that IFN receptor signaling is required for IL-10-producing human plasmablasts [16]. Indeed, treatment with IFN-β, which is another type I IFN, increases IL-10 expression of human B cells after BCR and CD40 ligation [74]. Another study in mice revealed that stimulation with IL-1β, IL-6, and anti-CD40 in combination promotes the differentiation into IL-10-producing Bregs. Interestingly, B cell stimulation with TNF-α or IL-17 with or without anti-CD40 has no effect on IL-10 production [70]. It was also demonstrated that the TLR-MyD88-STAT3 pathway not only leads to antibody production of human B cells, but also regulates IL-10 production of human B cells by TLR7/8, which is enhanced by IFN-α [75]. The BATF/IRF-4/IRF-8-axis was also shown to play a role in IL-10 and IL-35 expression of murine regulatory B cells [76]. Matsumoto et al. also showed that IRF4 is required for the differentiation into IL-10-expressing murine plasmablasts, both in vitro and in vivo [16]. Moreover, IL-21 was described to induce IL-10 expression of murine B10 cells [71] and human GrB+ Bregs [65]. Also, in vitro stimulation of murine B cells with other molecules such as LPS, phorbol myristate acetate (PMA), and ionomycin generates IL-10-expressing B cells [77,78,79]. Menon et al. revealed that also plasmacytoid dendritic cells (pDCs) can drive the differentiation of human CD19+CD24hiCD38hi Bregs and plasmablasts that express IL-10, IL-6, and TNF-α [69].
As another mechanism for Breg induction, overexpression of IL-10 in primary human B cells was shown to upregulate suppressor of cytokine signaling 3 (SOCS3), glycoprotein A repetitions predominant (GARP), IL-2 receptor α chain (CD25), and programmed cell death ligand 1 (PD-L1). Moreover, IL-10 overexpressing human B cells secrete less pro-inflammatory cytokines such as TNF-α or IL-8, whereas production of anti-inflammatory IL-1 receptor antagonists (IL-1RN) and vascular endothelial growth factor (VEGF) is increased [80]. In addition, the aryl hydrocarbon receptor (AhR) was shown to regulate the differentiation and function of IL-10-producing murine Bregs [81] and A proliferation-inducing ligand (APRIL) drives the differentiation of naïve human B cells to IL-10-producing Bregs [82]. Human type 3 innate lymphoid cells (ILC3s) were also shown to play a role in the induction of human IL-10-producing immature transitional Bregs [83], whereas IL-35 was described to induce IL-35-producing human Bregs (IL-35+ Bregs) through activation of STAT1/STAT3 [84].
Breg suppressor molecules
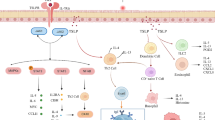
Regulatory B cells mainly act by secreting immune-modulatory cytokines. Although research mainly focused on the role of IL-10 as the hallmark cytokine of Bregs [54,55,56, 63, 85], three main cytokines were identified to be expressed by Bregs as suppressor molecules: IL-10, TGF-β [86], and IL-35 [87, 88] (Fig. 1). The anti-inflammatory cytokine IL-10 is involved in the maintenance of homeostasis by dampening inflammatory responses and can be produced by many cell types such as monocytes, T cells, DCs, NK cells, macrophages, mast cells, and B cells. It induces immune tolerance in patients with chronic inflammatory diseases such as allergy, autoimmunity, organ transplantation, and tumor tolerance and has direct and indirect suppressive effects on cytokine production and proliferation of effector T cells [89]. Moreover, IL-10 has an effect on B cells, including B cell survival, class-switch recombination, and plasma cell differentiation [25, 89]. IL-10 is also thought to have a positive effect on asthma pathophysiology by suppressing the IgE-mediated allergic cascade and decreasing airway inflammation [37]. Production of IL-10 was shown to be negatively regulated by B cell lymphoma-3 (Bcl-3) [90, 91], which is an atypical member of the inhibitor of NF-κB (IκB) protein family and regulates NF-κB-mediated gene expression, thereby regulating TLR signaling [92, 93]. TGF-β is an anti-inflammatory cytokine that has an important role in immune regulation, wound healing, and tissue remodeling and is involved in the conversion of naïve CD4+ T cells into functional regulatory T cells (Tregs) [25, 89]. IL-35 is primarily produced by Tregs and induces Treg proliferation while suppressing TH17 and TH1 responses [25, 84].
Breg suppressor molecules and their effect on other immune cells. Bregs mainly secrete the anti-inflammatory cytokines IL-10, IL-35, and TGF-β that in turn have a suppressive function on TH1 and TH17 cells. In addition, the conversion of CD4+ T cells into Tregs is induced. The transmembrane protein PD-L1 regulates cell expansion and differentiation of TFH cells. Also, other surface-bound molecules such as MHC-II, CD19, CD73, CD39, FasL, and TIM1 as well as AhR and GrB are described to play a role in Breg-mediated immunosuppressive effects. IL, interleukin; TGF-β, transforming growth factor β; TH, T helper cell; Treg, regulatory T cell; PD-L1, programmed cell death ligand 1; TFH, follicular helper T cells, MHC-II, major histocompatibility complex II; FasL, Fas ligand; TIM1, T cell immunoglobulin and mucin domain 1; AhR, aryl hydrocarbon receptor; GrB, granzyme B
In addition to the expression of these anti-inflammatory cytokines, other anti-inflammatory molecules such as PD-L1 are associated with suppressive effects of Bregs. PD-L1, also known as CD274, is a transmembrane protein that regulates induced Treg cell function, development, and maintenance [94]. Bregs expressing PD-L1, described as PD-L1hi B cells, regulate cell expansion and differentiation of follicular helper T cells (TFH), thereby suppressing autoimmune disease [95]. Also other surface-bound molecules like the Fas ligand (FasL) [96], CD39 [97], CD73 [97], CD19 [78], major histocompatibility complex II (MHC-II) [71], T cell immunoglobulin, and mucin domain 1 (TIM1) [98] as well as GrB [65], AhR [99], and intracellular signaling molecules such as STAT3 and MyD88 [100] are described to play a role in Breg-mediated immunosuppressive effects. This shows that there is a huge variety of suppressor molecules that are expressed by Bregs to fulfill their immunosuppressive function (Fig. 1).
Role of Bregs in (childhood) allergic asthma
Recent research has concentrated on the role of Bregs in allergic asthma [26]. As mentioned above, Bregs express the anti-inflammatory cytokine IL-10, which is thought to have a positive effect on asthma pathophysiology by suppressing the IgE-mediated allergic cascade and decreasing airway inflammation [37]. Parasite infection in allergic mice models for asthma reveals protection from lung inflammation and airway hyperresponsiveness through IL-10-producing Bregs [37, 85]. Moreover, in human studies a higher number of allergen-specific, IL-10-producing Bregs is found upon allergen immunotherapies for cow milk and bee venom, indicating that higher Breg numbers are characteristic for induced tolerance to allergens [63, 101]. Braza et al. showed that upon allergen exposure the number of IL-10-producing Bregs is decreased in the lungs of asthmatic mice, indicating that the homeostasis of Bregs is altered by asthma [102]. Recently, Qian et al. showed that asthmatic patients have higher levels of IL-10 but lower levels of Bcl-3, suggesting that they have an important role in asthma pathogenesis. They also revealed that mice lacking Bcl-3 have increased eosinophilic airway inflammation, augmented airway goblet cell hyperplasia, elevated airway hyperresponsiveness, and increased levels of epithelial chemokines in the lungs after stimulation with HDM compared to control mice. These data demonstrate that Bcl-3 limits the IL-10 expression during allergic sensitization, thereby preventing lung inflammation and asthma pathogenesis in HDM-induced mice [103]. Since it was already shown that Bcl-3 is a negative regulator for IL-10 [91], these results indicate that Bcl-3 is a critical inhibitor of IL-10 in allergic asthma and targeting the Bcl-3/IL-10 axis may be a promising approach for allergic asthma therapy [103].
In adults, patients with allergic asthma show a lower percentage and absolute number of CD19+CD24hiCD27+ circulating Bregs [20, 21]. Also, these Bregs express less IL-10 upon LPS [104], but not CpG [105] stimulation. Moreover, frequencies of CD5+ and Cd1d+CD5+ Bregs are decreased in adult allergic asthmatics [22]. Recently, Sheehan et al. revealed that pediatric patients with allergic asthma also have significantly lower levels of circulating IL-10 expressing CD24+CD38+ Bregs, compared to the healthy control group. This could indicate that the lower Breg levels are associated with suboptimal control of allergic inflammation leading to asthma development or excess morbidity from asthma. To this end, it seems that Bregs are important for both children and adults with allergic asthma and that the appropriate number and function of Bregs may be mandatory to control asthma by releasing suppressive signals to decrease TH2 inflammation [23].
Conclusion
During the past two decades, several Breg subsets were described in mice and humans, each with distinct phenotypical and functional characteristics. However, in contrast to regulatory T cells, which can be clearly defined by the expression of the transcription factor Foxp3, the identification of specific markers or transcription factors that clearly define Bregs is still missing. In addition, the precise mechanism and required signals for Breg differentiation are not completely understood. The way by which Bregs mainly exert their immunosuppressive function is through anti-inflammatory cytokines such as IL-10, TGF-β, and IL-35. Other anti-inflammatory molecules such as PD-L1, FasL, or intracellular signaling molecules like STAT3 and MyD88 are also associated with Breg function; however, this varies between different human Breg subsets. Several studies showed that IL-10 expression has a positive effect on asthma pathophysiology and that higher Breg numbers are characteristic of allergen tolerance. Moreover, it has been shown that asthma alters the homeostasis of Bregs: in adult as well as pediatric patients with allergic asthma, a lower percentage and absolute number of several IL-10 expressing Breg subsets are found. This suggests that lower Breg levels are associated with suboptimal control of allergic inflammation, which in turn may lead to the development of asthma. Since this is an emerging field of research, many questions still need to be answered with regard to plasticity, ontogeny, and Breg-mediated suppression mechanism as well as their exact role especially in childhood allergic asthma.
Availability of data and materials
Not applicable.
Abbreviations
- AA:
-
Allergic asthma
- AhR:
-
Aryl hydrocarbon receptor
- AIT:
-
Allergen immunotherapy
- APC:
-
Antigen-presenting cell
- APRIL:
-
A proliferation-inducing ligand
- BATF:
-
Basic leucine zipper transcription factor
- Bcl-3:
-
B cell lymphoma 3
- BCR:
-
B cell receptor
- Breg:
-
Regulatory B cell
- CD:
-
Cluster of differentiation
- CHB:
-
Chronic HBV
- DC:
-
Dendritic cell
- FasL:
-
Fas ligand
- FcεRI:
-
High-affinity IgE receptor
- FO:
-
Follicular
- GARP:
-
Glycoprotein A repetitions predominant
- GrB:
-
Granzyme B
- HDM:
-
House dust mite
- IDO:
-
Indolamine-2,3-dioxygenase
- IFN:
-
Interferon
- Ig:
-
Immunoglobulin
- IgE:
-
Immunoglobulin E
- IκB:
-
Inhibitor of NF-κB
- IL:
-
Interleukin
- IL-1RN:
-
IL-1 receptor antagonist
- ILC:
-
Innate lymphoid cell
- ILC3:
-
Type 3 innate lymphoid cell
- IRF:
-
Immunoregulatory factor
- LPS:
-
Lipopolysaccharide
- MHC-II:
-
Major histocompatibility complex II
- MyD88:
-
Myeloid differentiation primary response 88
- MZ:
-
Marginal zone
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NK:
-
Natural killer
- PD-L1:
-
Programmed cell death ligand 1
- pDCs:
-
Plasmacytoid dendritic cells
- PMA:
-
Phorbol myristate acetate
- SOCS3:
-
Suppressor of cytokine signaling 3
- STAT:
-
Signal transducer and activator of transcription
- TCR:
-
T cell receptor
- TFH :
-
Follicular helper T cells
- TGF-β:
-
Transforming growth factor β
- TH :
-
T helper cell
- TIM1:
-
T cell immunoglobulin and mucin domain 1
- TLR:
-
Toll-like receptor
- TNF:
-
Tumor necrosis factor
- Treg:
-
Regulatory T cell
- VEGF:
-
Vascular endothelial growth factor
- WHO:
-
World Health Organization
References
Castagnoli R, Brambilla I, Giovannini M, Marseglia GL, Licari A (2023) New approaches in childhood asthma treatment. Curr Opin Allergy Clin Immunol 23(4):319–326
Fainardi V, Esposito S, Chetta A, Pisi G (2022) Asthma phenotypes and endotypes in childhood. Minerva Med 113(1):94–105
Vos T et al. (2020) Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396(10258):1204–22
Masoli M, Fabian D, Holt S, Beasley R (2004) The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 59(5):469–478
El-Husseini ZW, Gosens R, Dekker F, Koppelman GH (2020) The genetics of asthma and the promise of genomics-guided drug target discovery. Lancet Respir Med 8(10):1045–1056
Salvermoser M, Zeber K, Boeck A, Klucker E, Schaub B (2021) Childhood asthma: novel endotyping by cytokines, validated through sensitization profiles and clinical characteristics. Clin Exp Allergy 51(5):654–665
Bousquet J, Hellings PW, Agache I, Bedbrook A, Bachert C, Bergmann KC et al (2016) ARIA 2016: care pathways implementing emerging technologies for predictive medicine in rhinitis and asthma across the life cycle. Clin Transl Allergy 6:47
Janeway C (2005) Immunobiology: the immune system in health and disease, 6th edn. Garland Science, New York
van de Veen W, Stanic B, Wirz OF, Jansen K, Globinska A, Akdis M (2016) Role of regulatory B cells in immune tolerance to allergens and beyond. J Allergy Clin Immunol 138(3):654–665
Akdis M, Akdis CA (2009) Therapeutic manipulation of immune tolerance in allergic disease. Nat Rev Drug Discov 8(8):645–660
Pieper K, Grimbacher B, Eibel H (2013) B-cell biology and development. J Allergy Clin Immunol 131(4):959–971
Jansen K, Cevhertas L, Ma S, Satitsuksanoa P, Akdis M, van de Veen W (2021) Regulatory B cells. A to Z Allergy 76(9):2699–2715
Mauri C, Bosma A (2012) Immune regulatory function of B cells. Annu Rev Immunol 30:221–241
Mauri C, Nistala K (2014) Interleukin-35 takes the ‘B’ line. Nat Med 20(6):580–581
Blair PA, Noreña LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR et al (2010) CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity 32(1):129–140
Matsumoto M, Baba A, Yokota T, Nishikawa H, Ohkawa Y, Kayama H et al (2014) Interleukin-10-producing plasmablasts exert regulatory function in autoimmune inflammation. Immunity 41(6):1040–1051
Akdis CA, Arkwright PD, Brüggen M-C, Busse W, Gadina M, Guttman-Yassky E et al (2020) Type 2 immunity in the skin and lungs. Allergy 75(7):1582–1605
Habener A, Happle C, Grychtol R, Skuljec J, Busse M, Dalüge K et al (2021) Regulatory B cells control airway hyperreactivity and lung remodeling in a murine asthma model. J Allergy Clin Immunol 147(6):2281-2294.e7
Habener A, Grychtol R, Gaedcke S, DeLuca D, Dittrich AM, Happle C et al (2022) IgA+ memory B-cells are significantly increased in patients with asthma and small airway dysfunction. Eur Respir J 60(5):2102130
Oliveria J-P, El-Gammal AI, Yee M, Obminski CD, Scime TX, Watson RM et al (2018) Changes in regulatory B-cell levels in bone marrow, blood, and sputum of patients with asthma following inhaled allergen challenge. J Allergy Clin Immunol 141(4):1495-1498.e9
Miyajima S, Shigehara K, Kamekura R, Takaki H, Yabe H, Ikegami I et al (2020) Activated circulating T follicular helper cells and skewing of T follicular helper 2 cells are down-regulated by treatment including an inhaled corticosteroid in patients with allergic asthma. Allergol Int 69(1):66–77
Wiest M, Upchurch K, Hasan MM, Cardenas J, Lanier B, Millard M et al (2019) Phenotypic and functional alterations of regulatory B cell subsets in adult allergic asthma patients. Clin Exp Allergy 49(9):1214–1224
Sheehan WJ, Maghzian N, Rastogi D, Bollard CM, Lin AA (2023) Decreased regulatory B cells in pediatric patients with asthma. Ann Allergy Asthma Immunol 131(1):120–121
Krusche J, Basse S, Schaub B (2020) Role of early life immune regulation in asthma development. Semin Immunopathol 42(1):29–42
Akdis M, Aab A, Altunbulakli C, Azkur K, Costa RA, Crameri R et al (2016) Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: receptors, functions, and roles in diseases. J Allergy Clin Immunol 138(4):984–1010
Oliveria JP, Agayby R, Gauvreau GM (2021) Regulatory and IgE+ B cells in allergic asthma. Methods Mol Biol 2270:375–418
Akdis CA, Akdis M (2015) Mechanisms of allergen-specific immunotherapy and immune tolerance to allergens. World Allergy Organ J 8(1):17
Wills-Karp M (1999) Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol 17:255–281
Wills-Karp M (2004) Interleukin-13 in asthma pathogenesis. Immunol Rev 202:175–190
Smith SG, Gauvreau GM (2007) IL-13 is a novel therapeutic target in allergic asthma. Expert Rev Clin Immunol 3(5):671–675
Stoltz DJ, Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Gern JE et al (2013) Specific patterns of allergic sensitization in early childhood and asthma & rhinitis risk. Clin Exp Allergy 43(2):233–241
Moon H-G, Kim S-J, Lee MK, Kang H, Choi HS, Harijith A et al (2020) Colony-stimulating factor 1 and its receptor are new potential therapeutic targets for allergic asthma. Allergy 75(2):357–369
Matsushita T, Yanaba K, Bouaziz J-D, Fujimoto M, Tedder TF (2008) Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest 118(10):3420–3430
Watanabe R, Ishiura N, Nakashima H, Kuwano Y, Okochi H, Tamaki K et al (2010) Regulatory B cells (B10 cells) have a suppressive role in murine lupus: CD19 and B10 cell deficiency exacerbates systemic autoimmunity. J Immunol 184(9):4801–4809
Jurlander J, Lai C-F, Tan J, Chou C-C, Geisler CH, Schriber J et al (1997) Characterization of interleukin-10 receptor expression on B-cell chronic lymphocytic leukemia cells. Blood 89(11):4146–4152
Hulse KE, Norton JE, Suh L, Zhong Q, Mahdavinia M, Simon P et al (2013) Chronic rhinosinusitis with nasal polyps is characterized by B-cell inflammation and EBV-induced protein 2 expression. J Allergy Clin Immunol 131(4):1075–83 (1083.e1-7)
Amu S, Saunders SP, Kronenberg M, Mangan NE, Atzberger A, Fallon PG (2010) Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J Allergy Clin Immunol 125(5):1114-1124.e8
Gould HJ, Sutton BJ (2008) IgE in allergy and asthma today. Nat Rev Immunol 8(3):205–217
Eibel H, Kraus H, Sic H, Kienzler A-K, Rizzi M (2014) B cell biology: an overview. Curr Allergy Asthma Rep 14(5):434
Ghia P, ten Boekel E, Rolink AG, Melchers F (1998) B-cell development: a comparison between mouse and man. Immunol Today 19(10):480–485
Chung JB, Silverman M, Monroe JG (2003) Transitional B cells: step by step towards immune competence. Trends Immunol 24(6):343–349
Palanichamy A, Barnard J, Zheng B, Owen T, Quach T, Wei C et al (2009) Novel human transitional B cell populations revealed by B cell depletion therapy. J Immunol 182(10):5982–5993
Batten M, Groom J, Cachero TG, Qian F, Schneider P, Tschopp J et al (2000) BAFF mediates survival of peripheral immature B lymphocytes. J Exp Med 192(10):1453–1466
Seifert M, Küppers R (2016) Human memory B cells. Leukemia 30(12):2283–2292
Good KL, Avery DT, Tangye SG (2009) Resting human memory B cells are intrinsically programmed for enhanced survival and responsiveness to diverse stimuli compared to naive B cells. J Immunol 182(2):890–901
Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM et al (2000) Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol 1(6):475–482
Allen CDC (2022) Features of B cell responses relevant to allergic disease. J Immunol 208(2):257–266
Wypych TP, Marzi R, Wu GF, Lanzavecchia A, Sallusto F (2018) Role of B cells in TH cell responses in a mouse model of asthma. J Allergy Clin Immunol 141(4):1395–1410
Dullaers M, Schuijs MJ, Willart M, Fierens K, van Moorleghem J, Hammad H et al (2017) House dust mite-driven asthma and allergen-specific T cells depend on B cells when the amount of inhaled allergen is limiting. J Allergy Clin Immunol 140(1):76-88.e7
Korsgren M, Erjefält JS, Korsgren O, Sundler F, Persson CG (1997) Allergic eosinophil-rich inflammation develops in lungs and airways of B cell-deficient mice. J Exp Med 185(5):885–892
Katz SI, Parker D, Turk JL (1974) B-cell suppression of delayed hypersensitivity reactions. Nature 251(5475):550–551
Neta R, Salvin SB (1974) Specific suppression of delayed hypersensitivity: the possible presence of a suppressor B cell in the regulation of delayed hypersensitivity. J Immunol 113(6):1716–1725
Palomares O, Akdis M, Martín-Fontecha M, Akdis CA (2017) Mechanisms of immune regulation in allergic diseases: the role of regulatory T and B cells. Immunol Rev 278(1):219–236
Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK (2002) Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity 16(2):219–230
Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM (2002) B cells regulate autoimmunity by provision of IL-10. Nat Immunol 3(10):944–950
Mauri C, Gray D, Mushtaq N, Londei M (2003) Prevention of arthritis by interleukin 10-producing B cells. J Exp Med 197(4):489–501
Akdis CA, Blesken T, Akdis M, Wüthrich B, Blaser K (1998) Role of interleukin 10 in specific immunotherapy. J Clin Invest 102(1):98–106
Goetz M, Atreya R, Ghalibafian M, Galle PR, Neurath MF (2007) Exacerbation of ulcerative colitis after rituximab salvage therapy. Inflamm Bowel Dis 13(11):1365–1368
Dass S, Vital EM, Emery P (2007) Development of psoriasis after B cell depletion with rituximab. Arthritis Rheum 56(8):2715–2718
Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM et al (2011) Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 117(2):530–541
Flores-Borja F, Bosma A, Ng D, Reddy V, Ehrenstein MR, Isenberg DA et al (2013) CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci Transl Med 5(173):173ra23
Das A, Ellis G, Pallant C, Lopes AR, Khanna P, Peppa D et al (2012) IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. J Immunol 189(8):3925–3935
van de Veen W, Stanic B, Yaman G, Wawrzyniak M, Söllner S, Akdis DG et al (2013) IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol 131(4):1204–1212
Shalapour S, Font-Burgada J, Di Caro G, Zhong Z, Sanchez-Lopez E, Dhar D et al (2015) Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature 521(7550):94–98
Lindner S, Dahlke K, Sontheimer K, Hagn M, Kaltenmeier C, Barth TFE et al (2013) Interleukin 21-induced granzyme B-expressing B cells infiltrate tumors and regulate T cells. Cancer Res 73(8):2468–2479
Brosseau C, Durand M, Colas L, Durand E, Foureau A, Cheminant M-A et al (2018) CD9+ regulatory B cells induce T cell apoptosis via IL-10 and are reduced in severe asthmatic patients. Front Immunol 9:3034
Zhang M, Zheng X, Zhang J, Zhu Y, Zhu X, Liu H et al (2012) CD19(+)CD1d(+)CD5(+) B cell frequencies are increased in patients with tuberculosis and suppress Th17 responses. Cell Immunol 274(1–2):89–97
Zhang M, Zeng G, Yang Q, Zhang J, Zhu X, Chen Q et al (2014) Anti-tuberculosis treatment enhances the production of IL-22 through reducing the frequencies of regulatory B cell. Tuberculosis (Edinb) 94(3):238–244
Menon M, Blair PA, Isenberg DA, Mauri C (2016) A regulatory feedback between plasmacytoid dendritic cells and regulatory B cells is aberrant in systemic lupus erythematosus. Immunity 44(3):683–697
Rosser EC, Oleinika K, Tonon S, Doyle R, Bosma A, Carter NA et al (2014) Regulatory B cells are induced by gut microbiota-driven interleukin-1β and interleukin-6 production. Nat Med 20(11):1334–1339
Yoshizaki A, Miyagaki T, Dilillo DJ, Matsushita T, Horikawa M, Kountikov EI et al (2012) Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature 491(7423):264–268
Giordani L, Sanchez M, Libri I, Quaranta MG, Mattioli B, Viora M (2009) IFN-alpha amplifies human naive B cell TLR-9-mediated activation and Ig production. J Leukoc Biol 86(2):261–271
Jego G, Palucka AK, Blanck J-P, Chalouni C, Pascual V, Banchereau J (2003) Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity 19(2):225–234
Ramgolam VS, Sha Y, Marcus KL, Choudhary N, Troiani L, Chopra M et al (2011) B cells as a therapeutic target for IFN-β in relapsing-remitting multiple sclerosis. J Immunol 186(7):4518–4526
Liu B-S, Cao Y, Huizinga TW, Hafler DA, Toes REM (2014) TLR-mediated STAT3 and ERK activation controls IL-10 secretion by human B cells. Eur J Immunol 44(7):2121–2129
Yu C-R, Choi JK, Uche AN, Egwuagu CE (2018) Production of IL-35 by Bregs is mediated through binding of BATF-IRF-4-IRF-8 complex to il12a and ebi3 promoter elements. J Leukoc Biol 104(6):1147–1157
Yanaba K, Bouaziz J-D, Haas KM, Poe JC, Fujimoto M, Tedder TF (2008) A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 28(5):639–650
Yanaba K, Bouaziz J-D, Matsushita T, Tsubata T, Tedder TF (2009) The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol 182(12):7459–7472
Haas KM, Watanabe R, Matsushita T, Nakashima H, Ishiura N, Okochi H et al (2010) Protective and pathogenic roles for B cells during systemic autoimmunity in NZB/W F1 mice. J Immunol 184(9):4789–4800
Stanic B, van de Veen W, Wirz OF, Rückert B, Morita H, Söllner S et al (2015) IL-10-overexpressing B cells regulate innate and adaptive immune responses. J Allergy Clin Immunol 135(3):771–80.e8
Piper CJM, Rosser EC, Oleinika K, Nistala K, Krausgruber T, Rendeiro AF et al (2019) Aryl hydrocarbon receptor contributes to the transcriptional program of IL-10-producing regulatory B cells. Cell Rep 29(7):1878-1892.e7
Fehres CM, van Uden NO, Yeremenko NG, Fernandez L, Franco Salinas G, van Duivenvoorde LM et al (2019) APRIL induces a novel subset of IgA+ regulatory B cells that suppress inflammation via expression of IL-10 and PD-L1. Front Immunol 10:1368
Komlósi ZI, Kovács N, van de Veen W, Kirsch AI, Fahrner HB, Wawrzyniak M et al (2018) Human CD40 ligand-expressing type 3 innate lymphoid cells induce IL-10-producing immature transitional regulatory B cells. J Allergy Clin Immunol 142(1):178-194.e11
Wang R-X, Yu C-R, Dambuza IM, Mahdi RM, Dolinska MB, Sergeev YV et al (2014) Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat Med 20(6):633–641
van der Vlugt LEPM, Labuda LA, Ozir-Fazalalikhan A, Lievers E, Gloudemans AK, Liu K-Y et al (2012) Schistosomes induce regulatory features in human and mouse CD1d(hi) B cells: inhibition of allergic inflammation by IL-10 and regulatory T cells. PLoS ONE 7(2):e30883
Parekh VV, Prasad DVR, Banerjee PP, Joshi BN, Kumar A, Mishra GC (2003) B cells activated by lipopolysaccharide, but not by anti-Ig and anti-CD40 antibody, induce anergy in CD8+ T cells: role of TGF-beta 1. J Immunol 170(12):5897–5911
Rosser EC, Mauri C (2015) Regulatory B cells: origin, phenotype, and function. Immunity 42(4):607–612
Shen P, Roch T, Lampropoulou V, O’Connor RA, Stervbo U, Hilgenberg E et al (2014) IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 507(7492):366–370
Palomares O, Martín-Fontecha M, Lauener R, Traidl-Hoffmann C, Cavkaytar O, Akdis M et al (2014) Regulatory T cells and immune regulation of allergic diseases: roles of IL-10 and TGF-β. Genes Immun 15(8):511–520
Reißig S, Tang Y, Nikolaev A, Gerlach K, Wolf C, Davari K et al (2017) Elevated levels of Bcl-3 inhibits Treg development and function resulting in spontaneous colitis. Nat Commun 8:15069
Riemann M, Endres R, Liptay S, Pfeffer K, Schmid RM (2005) The IkappaB protein Bcl-3 negatively regulates transcription of the IL-10 gene in macrophages. J Immunol 175(6):3560–3568
Carmody RJ, Ruan Q, Palmer S, Hilliard B, Chen YH (2007) Negative regulation of toll-like receptor signaling by NF-kappaB p50 ubiquitination blockade. Science 317(5838):675–678
Zhang X, Wang H, Claudio E, Brown K, Siebenlist U (2007) A role for the IκB family member Bcl-3 in the control of central immunologic tolerance. Immunity 27(3):438–452
Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK et al (2009) PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med 206(13):3015–3029
Khan AR, Hams E, Floudas A, Sparwasser T, Weaver CT, Fallon PG (2015) PD-L1hi B cells are critical regulators of humoral immunity. Nat Commun 6:5997
Klinker MW, Reed TJ, Fox DA, Lundy SK (2013) Interleukin-5 supports the expansion of fas ligand-expressing killer B cells that induce antigen-specific apoptosis of CD4(+) T cells and secrete interleukin-10. PLoS ONE 8(8):e70131
Figueiró F, Muller L, Funk S, Jackson EK, Battastini AMO, Whiteside TL (2016) Phenotypic and functional characteristics of CD39high human regulatory B cells (Breg). Oncoimmunology 5(2):e1082703
Ding Q, Yeung M, Camirand G, Zeng Q, Akiba H, Yagita H et al (2011) Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest 121(9):3645–3656
Rosser EC, Piper CJM, Matei DE, Blair PA, Rendeiro AF, Orford M et al (2020) Microbiota-derived metabolites suppress arthritis by amplifying aryl-hydrocarbon receptor activation in regulatory B cells. Cell Metab 31(4):837-851.e10
Neves P, Lampropoulou V, Calderon-Gomez E, Roch T, Stervbo U, Shen P et al (2010) Signaling via the MyD88 adaptor protein in B cells suppresses protective immunity during Salmonella typhimurium infection. Immunity 33(5):777–790
Noh J, Lee JH, Noh G, Bang SY, Kim HS, Choi WS et al (2010) Characterisation of allergen-specific responses of IL-10-producing regulatory B cells (Br 1) in Cow Milk Allergy. Cell Immunol 264(2):143–149
Braza F, Chesne J, Durand M, Dirou S, Brosseau C, Mahay G et al (2015) A regulatory CD9(+) B-cell subset inhibits HDM-induced allergic airway inflammation. Allergy 70(11):1421–1431
Qian G, Jiang W, Sun D, Sun Z, Chen A, Fang H et al (2023) B-cell-derived IL-10 promotes allergic sensitization in asthma regulated by Bcl-3. Cell Mol Immunol 20(11):1313–1327
van der Vlugt LEPM, Mlejnek E, Ozir-Fazalalikhan A, Janssen Bonas M, Dijksman TR, Labuda LA et al (2014) CD24(hi)CD27(+) B cells from patients with allergic asthma have impaired regulatory activity in response to lipopolysaccharide. Clin Exp Allergy 44(4):517–528
Wirz OF, Głobińska A, Ochsner U, van de Veen W, Eller E, Christiansen ES et al (2019) Comparison of regulatory B cells in asthma and allergic rhinitis. Allergy 74(4):815–818
Acknowledgements
Not applicable.
Funding
Open access funding enabled and organized by Projekt DEAL. This work was supported by the DFG grants Sch 997/10–1 (CK) and DFG SCHA 997/11–1 (BS).
Author information
Authors and Affiliations
Contributions
C.K. and B.S. conceived the study. C.K. drafted the manuscript; C.K. and B.S. have revised and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kliem, C.V., Schaub, B. The role of regulatory B cells in immune regulation and childhood allergic asthma. Mol Cell Pediatr 11, 1 (2024). https://doi.org/10.1186/s40348-023-00174-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40348-023-00174-2