Abstract
Human milk contains more than 150 different oligosaccharides, which together are among to the quantitatively predominant solid components of breast milk. The oligosaccharide content and composition of human milk show large inter-individual differences. Oligosaccharide content is mostly influenced by genetic variants of the mother’s secretor status. Oligosaccharides in human milk are utilized by infants’ intestinal bacteria, affecting bacterial composition and metabolic activity. Maternal secretor status, and respective differing fucosylated oligosaccharide content, has been associated both with reduced and increased risk of infection in different populations of breastfed infants, possibly due to environmental conditions and the infant’s genotype. There are no safety concerns regarding the addition of previously approved oligosaccharides to infant formula; however, no firm conclusions can be drawn about clinically relevant benefits either. Therefore, infant formulas with synthetic oligosaccharide additives are currently not preferentially recommended over infant formulas without such additives. We consider the use of terms such as “human milk oligosaccharides” and corresponding abbreviations such as “HMO” in any advertising of infant formula to be an inappropriate idealization of infant formula. Manufacturers should stop this practice, and such marketing practices should be prevented by responsible supervisory authorities. Pediatricians should inform families that infant formulas supplemented with synthetic oligosaccharides do not resemble the complex oligosaccharide composition of human milk.
Similar content being viewed by others
Background
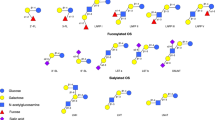
Human milk contains lactose as a digestible carbohydrate and various oligosaccharides as indigestible carbohydrates. In mature human milk, the total content of oligosaccharides is 5–15 g/L. Together with lactose, fat, and protein, they are one of the major solid components of human milk [1, 2]. Oligosaccharides in human milk (known as “breast milk oligosaccharides,” “human milk oligosaccharides,” or “HMOs”) are made up of five building blocks, namely galactose, glucose, fucose, N-acetyl glucosamine, and N-acetyl neuraminic acid [3]. Beginning with lactose, the complexity of the diverse structures increases through one or multiple extensions with lacto-N-biose or lactosamine and additional modifications with fucose and/or sialic acid. The activity of various short- and long-chain components of mammalian milk has been characterized [4]. Of these, about two-thirds is neutral, and one-third is acidic (containing sialic acid) oligosaccharides. There are 15 predominant oligosaccharides that account for 80–90% of the total content of oligosaccharides in human milk.
Individual variations and genetic predisposition
The oligosaccharide patterns in human milk show very large inter-individual differences which are partly genetically determined. In humans, certain clusters can be distinguished by the presence or absence of certain glycosyltransferases, such as fucosyltransferases FUT2 and FUT3 [5, 6]. FUT2 mediates the synthesis of the neutral oligosaccharides such as 2′-FL and lacto-N-fucopentaose-I (LNFP-I). FUT3 is crucial for the formation of lacto-N-fucopentaose-II (LNFP-II). FUT2-positive mothers have higher concentrations of oligosaccharides in their milk than FUT2-negative mothers [7]. Mothers who express FUT2 are referred to as “secretors” because α1-2-fucosylated oligosaccharides are detectable in their milk. In contrast, such components are absent in nonsecretors (inactive FUT2 gene). In Europe, about 70–80% of the population are secretors, and 20–30% are nonsecretors [8].
The biological significance of the differences in human milk composition between secretors and nonsecretors (of 2′-FL) is a matter of debate. Lack of FUT2 activity has been associated with relative resistance to rotavirus and norovirus infections [9,10,11] but increased colonization rate with group B streptococci [12]. Divergent effects have been reported in different populations. Studies from North America showed a lower incidence of diarrhea in breastfed children of secretors than in breastfed children of nonsecretors [13, 14], while breastfed children of secretors in the UK, Bangladesh, Peru, and Tanzania showed increased diarrhea incidence [15, 16]. An association of the level of 2′-FL in milk with excessive weight gain in infants has also been reported [17]. The effects of secretor status may differ depending on environmental conditions and pathogen exposure. In addition to the composition of human milk, the infant’s secretor status also seems to be important. Infant FUT2 and FUT3 positivity were associated with a marked risk reduction by almost 30% for all-cause diarrhea [15]. However, further data from clinical studies are required to potentially support conclusive inferences.
Biological functions of oligosaccharides in human milk
Oligosaccharides pass undigested through the small intestine but are metabolized by gut bacteria. They can affect metabolic activity and proliferation of the intestinal microbiota, similar to the effects of undigested lactose and fiber. With regard to the structural variety and the sometimes very high content of certain oligosaccharides in human milk, structure-specific effects have also been ascribed to them [1, 2, 18, 19]. An ever-increasing number of ex vivo and animal studies indicates potential gastrointestinal and systemic effects. Effects on the composition of the intestinal microbiome have been the most studied so far. Oligosaccharides conveyed through human milk seem to be preferentially metabolized by certain commensal bacteria, in particular Bifidobacteria and Bacteroides species. Bacteria that utilize certain oligosaccharides (“cross-feeding”) and receptor-analogous effects of oligosaccharides may influence intestinal colonization and the composition of the microbiota (through, for example, the formation of short-chain fatty acids). An infant’s immune system could be influenced directly or indirectly via the composition of the microbiota. Furthermore, certain oligosaccharides interfere with the lectin-mediated binding of certain pathogenic bacteria or viruses to the intestinal mucosa [12, 20]. Influences on intestinal permeability and intestinal cell maturation are also debated [1, 21].
The total amount of oligosaccharides in milk does not differ between mothers of preterm infants with and without necrotizing enterocolitis (NEC) [22, 23]. However, human milk fed to preterm infants who developed NEC had less disialyllacto-N-tetraose (DSLNT) than milk fed to control infants in studies conducted in South Africa [24], North America [22], and the UK [25], whereas NEC was associated with less milk lacto-N-difucohexaose I and lower diversity of oligosaccharides in a Swedish cohort [23]. In randomized controlled trials, pasteurized human milk has been shown to reduce the risk of NEC in preterm infants [26]. It is conceivable that human milk oligosaccharides which are not affected by pasteurization might contribute to the observed risk reduction for NEC.
Since small amounts of oligosaccharides can be taken up systemically, leukocyte-endothelium interactions detected in vitro or the effect on lymphocytes with the subsequent production of specific cytokines is also conceivable in vivo [27]. There is also some evidence to suggest that oligosaccharides may influence the gut-brain axis. In rodents and pigs, the use of oligosaccharides had a positive effect on the development of brain functions [28, 29]. However, it is currently unclear whether these experimental animal data reflect the situation in human infants.
Oligosaccharides in cow’s milk and goat’s milk
In cow’s milk, which serves as the basis for the production of infant formula, there are only a few mainly acidic oligosaccharides, present in very low concentrations. The total content in mature cow’s milk is about 0.03–0.06 g/L. In goat’s milk, which is also used for producing infant formula [30], concentrations of 0.06–0.35 g/L are slightly higher than in cow’s milk [31].
Addition of synthetic oligosaccharides to infant formula
Oligosaccharides have been added to some infant formulas. Galactooligosaccharides (GOS) are galactose oligomers synthesized from lactose. GOS including 3′-galactosyllactose (3′-GL) are found in human milk only in small amounts [32,33,34]. Fructooligosaccharides (FOS), also called oligofructose, are fructose polymers which have a sweetening effect. They are absent in human milk. In clinical studies, the addition of short-chain GOS and long-chain FOS in a ratio of 9:1 [30, 35], which is approved in Europe, at a concentration of 0.8 g/100 mL led to softer stool consistency and an increase in the proportion of bifidobacteria in infants’ stool [36]. No conclusive data are available for any other effect [36]. The European Food Safety Authority (EFSA) did not find evidence for any cause-effect relationship between the intake of GOS or FOS and reductions in gastrointestinal discomfort or potentially pathogenic microorganisms [37, 38].
Advances in the production of oligosaccharides, including the use of genetically modified microorganisms, have made it possible to produce some of the oligosaccharides found in human milk on an industrial scale [21, 39, 40]. However, only simple, short-chain oligosaccharides are currently used, mostly because of financial costs. EFSA and US Food and Drug Administration (FDA) have evaluated several synthetic oligosaccharides also found in human milk as novel food ingredients (2′-fucosyllactose, 2′-FL; lacto-N-neotetraose, LNnT; lacto-N-tetraose, LNT; 2′-FL + difucosyllactose, DFL; 3′-sialyllactose, 3′-SL; and 6′-sialyllactose, 6′-SL) [41,42,43,44,45,46]. Table 1 shows the maximum levels of synthetic oligosaccharides or combinations of oligosaccharides permitted for addition to infant formulas.
Recent clinical studies on infant formula supplemented with synthetic oligosaccharides
At present, there are only a few clinical studies in which the supplementation of infant formula with 2′-FL alone or in combination with LNnT or other nondairy oligosaccharides (GOS) has been investigated [27, 47,48,49].
Marriage et al. reported in 2015 that the supplementation of infant formula with 2′-FL (control 2.4 g GOS; experimental infant formula 1: 2.2 g GOS + 0.2 g 2′-FL; experimental infant formula 2: 1.4 g GOS + 1.0 g/l 2′-FL) did not lead to significant differences in head circumference, height or weight of the infants in the experimental groups, compared to breastfed infants, over the first 4 months. In addition, the authors state that the supplemented formula was well tolerated, and that the amount of 2′-FL detected in blood was comparable to that in breastfed infants [47].
Two randomized studies with infant formulas to which 2′-FL [50] or 2′-FL and LNnT [48] had been added showed no adverse effects on infant growth or tolerance to the formula. As a secondary endpoint, fewer respiratory infections and less use of antipyretics and antibiotics in the first year of life were reported when using infant formula enriched with 2′-FL and LnNT compared to non-supplemented formula [48]. These findings require further verification. In a further clinical study on an infant formula supplemented with different concentrations of GOS, with or without the addition of 2′-FL, the authors describe a lower inflammatory cytokine profile in the first 4 months of life that is comparable to that of exclusively breastfed children [27]. The addition of 2′-FL + LNnT to infant formula has also been reported to affect bacterial populations in infants’ stool [49].
In summary, no disadvantages in terms of infant growth have been observed in infants fed infant formulas supplemented with individual oligosaccharides previously approved by EFSA. Reported effects on the infant’s gut microbiota and the defense against infections require confirmation in further studies. As reported above, some oligosaccharides such as 2′-FL are absent from human milk in 20–30% of mothers in Europe. Both advantages and disadvantages with regard to risk of infections in breastfed infants of nonsecretory mothers have been described in different studies. It is unknown whether the addition of fucosylated oligosaccharides to infant formula could analogously induce both potential benefits and risks. However, the existence of individual oligosaccharides in human milk alone is not a sufficient justification for an assumed additional benefit of structurally identical synthetic oligosaccharides in infant formula. The oligosaccharide fraction in human milk is highly complex and has an individualized composition. Whether these differences affect the health of the infant cannot be assessed at this time. Moreover, the complexity of the oligosaccharides in human milk currently cannot be emulated in infant formula [51]. Overall, existing data on supplementation of infant formula with synthetic oligosaccharides are considered too limited to make general recommendations for its use.
Marketing of infant formulas fortified with synthetic oligosaccharides
In their marketing to consumers, manufacturers of infant formulas and follow-on formulas enriched with synthetic oligosaccharides suggest a similarity with breastfeeding. They do this by using terms such as “breast milk oligosaccharides” or “human milk oligosaccharides” (“HMO”) on product packaging, on their websites, through sponsored blogs, and in magazine articles. The use of this term suggests to consumers that the oligosaccharide composition in infant formula is similar to that of human milk. This is not correct and can lead to consumer deception, because the addition of simple, short-chain oligosaccharides does not lead to a similarity with the complex composition of hundreds of short- and long-chain oligosaccharides in human milk.
The Committee on Nutrition regards this kind of marketing as a violation of applicable European and German law. The European Union directive on infant formula and follow-on formula states that communication on infant formula “should not undermine the promotion of breastfeeding.” Furthermore, “use of the terms ‘humanised,’ ‘maternalised,’ ‘adapted,’ or similar terms is prohibited” [52]. The German regulation of dietetic foods prohibits “idealized wording” in the labelling of infant formula. Accordingly, when labelling infant formula and follow-on formula, the use of the terms “humanized,” “maternalised,” “adapted,” or similar terms is prohibited [53]. The Commission for Nutrition considers terms such as “breast milk oligosaccharides” or “human milk oligosaccharides” and respective abbreviations such as “HMO” in relation to infant formula to be misleading. Idealization of infant formula with the term “humanized” and similar terms is considered to be equally unlawful and undermine the promotion of breastfeeding.
Additional information
The German version of this consensus article can be found as an additional file attached to this article.
Conclusions
-
The Commission for Nutrition of the German Society for Child and Adolescent Medicine does not see any safety concerns when supplementing infant formulas with the synthetic oligosaccharides previously approved in Europe in the specified maximum amounts.
-
The few studies on infants available to date do not allow any reliable conclusions to be drawn about clinically relevant advantages of synthetic oligosaccharide additives.
-
Preferential use of infant formulas with synthetic oligosaccharide additives is therefore not recommended on the basis of currently available data.
-
The use of terms such as “human milk oligosaccharides” and abbreviations such as “HMO” in promoting infant and follow-on formula represents an unacceptable idealization, which suggests a nonexistent similarity with human milk and can thus undermine the priority of breastfeeding promotion.
-
The Committee on Nutrition urges infant formula manufacturers to end the current unacceptable idealized promotion of infant formula. It calls on supervisory authorities to stop possible violations of the existing legal restrictions on the marketing of infant formula.
-
Pediatricians should inform families that synthetic oligosaccharides in infant formula do not match the complex composition of oligosaccharides found in human milk.
Change history
23 July 2022
A Correction to this paper has been published: https://doi.org/10.1186/s40348-022-00147-x
References
Bode L (2012) Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology 22:1147–1162. https://doi.org/10.1093/glycob/cws074
Rudloff S, Kunz C (2015) Oligosaccharide in Frauenmilch. Monatsschr Kinderheilkd 163:790–795. https://doi.org/10.1007/s00112-014-3292-5
Asadpoor M, Peeters C, Henricks PAJ et al. (2020) Anti-pathogenic functions of non-digestible oligosaccharides in vitro. Nutrients 12.https://doi.org/10.3390/nu12061789
Urashima T, Hirabayashi J, Sato S et al (2018) Human milk oligosaccharides as essential tools for basic and application studies on galectins. Trends Glycosci Glycotechnol 30:SE51–SE65. https://doi.org/10.4052/tigg.1734.1SE
Prieto PA (2012) Profiles of human milk oligosaccharides and production of some human milk oligosaccharides in transgenic animals. Adv Nutr 3:456S-S464. https://doi.org/10.3945/an.111.001529
Lefebvre G, Shevlyakova M, Charpagne A et al (2020) Time of lactation and maternal fucosyltransferase genetic polymorphisms determine the variability in human milk oligosaccharides. Front Nutr 7:574459. https://doi.org/10.3389/fnut.2020.574459
Kunz C, Meyer C, Collado MC et al (2017) Influence of gestational age, secretor, and Lewis blood group status on the oligosaccharide content of human milk. J Pediatr Gastroenterol Nutr 64:789–798. https://doi.org/10.1097/MPG.0000000000001402
McGuire MK, Meehan CL, McGuire MA et al (2017) What’s normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically. Am J Clin Nutr 105:1086–1100. https://doi.org/10.3945/ajcn.116.139980
Nordgren J, Sharma S, Bucardo F et al (2014) Both Lewis and secretor status mediate susceptibility to rotavirus infections in a rotavirus genotype-dependent manner. Clin Infect Dis 59:1567–1573. https://doi.org/10.1093/cid/ciu633
Payne DC, Currier RL, Staat MA et al (2015) Epidemiologic association between FUT2 secretor status and severe rotavirus gastroenteritis in children in the United States. JAMA Pediatr 169:1040–1045. https://doi.org/10.1001/jamapediatrics.2015.2002
Ramani S, Stewart CJ, Laucirica DR et al (2018) Human milk oligosaccharides, milk microbiome and infant gut microbiome modulate neonatal rotavirus infection. Nat Commun 9:5010. https://doi.org/10.1038/s41467-018-07476-4
Andreas NJ, Al-Khalidi A, Jaiteh M et al (2016) Role of human milk oligosaccharides in group B Streptococcus colonisation. Clin Transl Immunology 5. https://doi.org/10.1038/cti.2016.43
Morrow AL, Ruiz-Palacios GM, Altaye M et al (2004) Human milk oligosaccharides are associated with protection against diarrhea in breast-fed infants. J Pediatr 145:297–303. https://doi.org/10.1016/j.jpeds.2004.04.054
Stepans MBF, Wilhelm SL, Hertzog M et al (2006) Early consumption of human milk oligosaccharides is inversely related to subsequent risk of respiratory and enteric disease in infants. Breastfeed Med 1:207–215. https://doi.org/10.1089/bfm.2006.1.207
Colston JM, Francois R, Pisanic N et al (2019) Effects of child and maternal histo-blood group antigen status on symptomatic and asymptomatic enteric infections in early childhood. J Infect Dis 220:151–162. https://doi.org/10.1093/infdis/jiz072
Muthumuni D, Miliku K, Wade KH et al (2021) Enhanced protection against diarrhea among breastfed infants of nonsecretor mothers. Pediatr Infect Dis J 40:260–263. https://doi.org/10.1097/INF.0000000000003014
Larsson MW, Lind MV, Laursen RP et al (2019) Human milk oligosaccharide composition is associated with excessive weight gain during exclusive breastfeeding-an explorative study. Front Pediatr 7:297. https://doi.org/10.3389/fped.2019.00297
Sakanaka M, Gotoh A, Yoshida K et al. (2019) Varied pathways of infant gut-associated bifidobacterium to assimilate human milk oligosaccharides: prevalence of the gene set and its correlation with bifidobacteria-rich microbiota formation. Nutrients 12https://doi.org/10.3390/nu12010071
Davis EC, Dinsmoor AM, Wang M et al (2020) Microbiome composition in pediatric populations from birth to adolescence: impact of diet and prebiotic and probiotic interventions. Dig Dis Sci 65:706–722. https://doi.org/10.1007/s10620-020-06092-x
Ackerman DL, Doster RS, Weitkamp J-H et al (2017) Human milk oligosaccharides exhibit antimicrobial and antibiofilm properties against group B Streptococcus. ACS Infect Dis 3:595–605. https://doi.org/10.1021/acsinfecdis.7b00064
Cheng L, Akkerman R, Kong C et al. (2020) More than sugar in the milk: human milk oligosaccharides as essential bioactive molecules in breast milk and current insight in beneficial effects. Crit Rev Food Sci Nutr:1–17. https://doi.org/10.1080/10408398.2020.1754756.
Autran CA, Kellman BP, Kim JH et al (2018) Human milk oligosaccharide composition predicts risk of necrotising enterocolitis in preterm infants. Gut 67:1064–1070. https://doi.org/10.1136/gutjnl-2016-312819
Wejryd E, Martí M, Marchini G et al. (2018) Low diversity of human milk oligosaccharides is associated with necrotising enterocolitis in extremely low birth weight infants. Nutrients 10https://doi.org/10.3390/nu10101556
van Niekerk E, Autran CA, Nel DG et al (2014) Human milk oligosaccharides differ between HIV-infected and HIV-uninfected mothers and are related to necrotizing enterocolitis incidence in their preterm very-low-birth-weight infants. J Nutr 144:1227–1233. https://doi.org/10.3945/jn.113.187799
Masi AC, Embleton ND, Lamb CA et al (2020) Human milk oligosaccharide DSLNT and gut microbiome in preterm infants predicts necrotising enterocolitis. Gut. https://doi.org/10.1136/gutjnl-2020-322771
Quigley M, Embleton ND, McGuire W (2019) Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev 7:CD002971. https://doi.org/10.1002/14651858.CD002971.pub5
Goehring KC, Marriage BJ, Oliver JS et al (2016) Similar to those who are breastfed, infants fed a formula containing 2'-fucosyllactose have lower inflammatory cytokines in a randomized controlled trial. J Nutr 146:2559–2566. https://doi.org/10.3945/jn.116.236919
Vazquez E, Barranco A, Ramirez M et al (2016) Dietary 2'-fucosyllactose enhances operant conditioning and long-term potentiation via gut-brain communication through the vagus nerve in rodents. PLoS ONE 11:e0166070. https://doi.org/10.1371/journal.pone.0166070
Fleming SA, Mudd AT, Hauser J et al (2020) Human and bovine milk oligosaccharides elicit improved recognition memory concurrent with alterations in regional brain volumes and hippocampal mRNA expression. Front Neurosci 14:770. https://doi.org/10.3389/fnins.2020.00770
European-Commission (2016). Commission Delegated Regulation (EU) 2016/127 of 25 September 2015 supplementing Regulation (EU) No 609/2013 of the European Parliament and of the Council as regards the specific compositional and information requirements for infant formula and follow-on formula and as regards requirements on information relating to infant and young child feeding. Off J Eur Union: L 25/21/1–29.
van Leeuwen SS, Te Poele EM, Chatziioannou AC et al (2020) Goat milk oligosaccharides: their diversity, quantity, and functional properties in comparison to human milk oligosaccharides. J Agric Food Chem 68:13469–13485. https://doi.org/10.1021/acs.jafc.0c03766
Barile D, Rastall RA (2013) Human milk and related oligosaccharides as prebiotics. Curr Opin Biotechnol 24:214–219. https://doi.org/10.1016/j.copbio.2013.01.008
Austin S, Bénet T (2018) Quantitative determination of non-lactose milk oligosaccharides. Anal Chim Acta 1010:86–96. https://doi.org/10.1016/j.aca.2017.12.036
Eussen SRBM, Mank M, Kottler R et al. (2021) Presence and levels of galactosyllactoses and other oligosaccharides in human milk and their variation during lactation and according to maternal phenotype. Nutrients. 13https://doi.org/10.3390/nu13072324
EFSA-Panel-on-Dietetic-Products (2014) Scientific opinion on the essential composition of infant and follow-on formulae. EFS2 12:3760. https://doi.org/10.2903/j.efsa.2014.3760
Skórka A, Pieścik-Lech M, Kołodziej M et al (2018) Infant formulae supplemented with prebiotics: are they better than unsupplemented formulae? An updated systematic review. Br J Nutr 119:810–825. https://doi.org/10.1017/S0007114518000120
EFSA (2011) Scientific opinion on the substantiation of health claims related to fructo-oligosaccharides (FOS) and decreasing potentially pathogenic gastro-intestinal microorganisms (ID 781) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J 9:2222
EFSA (2011) Scientific opinion on the substantiation of health claims related to galacto oligosaccharides (GOS) and reduction of gastro intestinal discomfort (ID 763) and decreasing potentially pathogenic microorganisms (ID 765) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J 9:2060
Bode L, Contractor N, Barile D et al (2016) Overcoming the limited availability of human milk oligosaccharides: challenges and opportunities for research and application. Nutr Rev 74:635–644. https://doi.org/10.1093/nutrit/nuw025
Sprenger GA, Baumgärtner F, Albermann C (2017) Production of human milk oligosaccharides by enzymatic and whole-cell microbial biotransformations. J Biotechnol 258:79–91. https://doi.org/10.1016/j.jbiotec.2017.07.030
EFSA (2015) Safety of 2′-O-fucosyllactose as a novel food ingredient pursuant to Regulation (EC) No 258/97. EFS2 13. https://doi.org/10.2903/j.efsa.2015.4184
EFSA (2015) Safety of lacto-N-neotetraose as a novel food ingredient pursuant to Regulation (EC) No 258/97. EFS2 13. https://doi.org/10.2903/j.efsa.2015.4183
Turck D, Castenmiller J, de Henauw S et al (2019) Safety of 2'-fucosyllactose/difucosyllactose mixture as a novel food pursuant to Regulation (EU) 2015/2283. EFS2. 17:e05717. https://doi.org/10.2903/j.efsa.2019.5717
Turck D, Castenmiller J, de Henauw S et al (2019) Safety of lacto-N-tetraose (LNT) as a novel food pursuant to Regulation (EU) 2015/2283. EFS2 17:e05907. https://doi.org/10.2903/j.efsa.2019.5907
Turck D, Castenmiller J, Henauw S de et al. (2020) Safety of 3’-sialyllactose (3’-SL) sodium salt as a novel food pursuant to Regulation (EU) 2015/2283. EFS2 18. https://doi.org/10.2903/j.efsa.2020.6098
Turck D, Castenmiller J, Henauw S de et al. (2020) Safety of 6′-sialyllactose (6′-SL) sodium salt as a novel food pursuant to Regulation (EU) 2015/2283. EFS2 18. https://doi.org/10.2903/j.efsa.2020.6097
Marriage BJ, Buck RH, Goehring KC et al (2015) Infants fed a lower calorie formula with 2'FL show growth and 2'FL uptake like breast-fed infants. J Pediatr Gastroenterol Nutr 61:649–658. https://doi.org/10.1097/MPG.0000000000000889
Puccio G, Alliet P, Cajozzo C et al (2017) Effects of infant formula with human milk oligosaccharides on growth and morbidity: a randomized multicenter trial. J Pediatr Gastroenterol Nutr 64:624–631. https://doi.org/10.1097/MPG.0000000000001520
Berger B, Porta N, Foata F et al. (2020) Linking human milk oligosaccharides, infant fecal community types, and later risk to require antibiotics. mBio 11. https://doi.org/10.1128/mBio.03196-19
Storm HM, Shepard J, Czerkies LM et al (2019) 2'-Fucosyllactose is well tolerated in a 100% whey, partially hydrolyzed infant formula with Bifidobacterium lactis: a randomized controlled trial. Glob Pediatr Health 6:2333794X19833995. https://doi.org/10.1177/2333794X19833995
Bode L (2019) Human milk oligosaccharides: next-generation functions and questions. Nestle Nutr Inst Workshop Ser 90:191–201. https://doi.org/10.1159/000490306
Kommission E (1999) RICHTLINIE 1999/21/EG DER KOMMISSION vom 25. März 1999 über diätetische Lebensmittel für besondere medizinische Zwecke. Amtsblatt der Europäischen Kommission L 091:29–36
Bundesministerium der Justiz und für Verbraucherschutz (2017) Verordnung über diätetische Lebensmittel (Diätverordnung). https://www.gesetze-im-internet.de/di_tv/BJNR004150963.html.
Author information
Authors and Affiliations
Contributions
This manuscript was developed by the Committee on Nutrition of the German Society of Paediatrics and Adolescent Medicine (DGKJ). All authors contributed to writing and revising of the manuscript. All authors read and approved the final manuscript version.
Corresponding author
Ethics declarations
Competing interests
C. Bührer acted as a consultant/expert for public courts. He was a member of the scientific advisory boards at the Fresenius, the WIdO, and the IQTIG. CB received fees from the Chiesi and Nestlé for lectures and training courses. The BMBF supported his research projects with third-party funds.
R. Ensenauer receives an expense allowance for her work for the Thieme Publishers Pediatrics Up2Date. The BMBF, the Innovation Fund of the G-BA, the DFG, and the foundations Sternstunden e.V., Willi-Althof-Stiftung, EKFS, and Stiftung Kardiovaskuläre Prävention LMU München supported RE’s research projects with third-party funds.
F. Jochum is consultant/expert for the DKG and the G-BA, from which he receives contributions. His employer has carried out contract research with the department of Pediatric Nutrition Research on different aspects of infant formulae for Fonterra, Humana, Nestlé and Hipp. They took place under the leadership of FJ. FJ is a member of the advisory board of the Nestlé Nutrition Institute. He received fees for giving lectures at the intensive care training DHZB, the Med. Hochschule Brandenburg, and the University Greifswald. He has also authored/co-authored several publications focusing on clinical nutrition or treatment of pediatric patients including neonates. He conducted research projects focusing on the nutrition of newborns, for which he received a fee as well as compensations for travel expenses. FJ owns two patents relating to newborns.
H. Kalhoff received fees from the Duleve Mederau and Stallergenes for giving lectures and training courses.
B. Koletzko served as a consultant for the German Research Foundation in the grant review board for clinical trials. He received remunerations for educational and review activities and travel cost reimbursement from Annenberg, Barilla, Bayer, Cogitando, Cheplapharm, Danone, DGC, DSM, Hipp, Nestle, and Reckitt and the nonprofit organization Family Larsson Rosenquist Foundation. He was a member of the scientific advisory boards of the Family Larsson Rosenquist Foundation and the Task Force on Dietary Fat Quality of the International Union of Nutritional Sciences. He is author/co-author and editor for the Karger Publishers, Springer Publishers, and Thieme Publishers. His employer received support for scientific and educational project from the European Commission, the European Research Council, the German Research Council, the German Federal Government, the Government of Bavaria, the US National Institutes of Health, the Ministry of Primary Industries, New Zealand, the Else Kröner Fresenius Foundation, the Larsson Rosenquist Foundation, the University of Amsterdam, and the companies Danone, DGC, Hipp, and Nestle. The employer of BK holds the rights of two patents filed by BK concerning a method for fatty acid analysis and for a device for collecting buccal cell samples.
B. Lawrenz receives fees for his consulting/expert work for the GSK, KVWL Consult GmbH, MSD, Pfizer, and Sanofi. He also works in advisory boards for the GSK, MSD, Pfizer, and Sanofi, from which he receives contributions. He took on paid lectures/training courses for the BVKJ, the BVKJ Service GmbH, GSK, KVWL Consult Life Science, MSD, RG, and Sanofi. He is an author/co-author for the Hansisches Verlagskontor and the Marseille Publishers.
W. A. Mihatsch received contributions for his work in the scientific advisory boards for the human milk research and feeding Prize Committee of the company Nutricia. He has given lectures and training courses for the ÖGKJ, DGPE, GNPI, ESPGHAN, ESPR, and DGKJ. He conducts research projects and clinical studies for the Gießen University, University of Madrid, Ulm University, and the University of Applies Sciences Neu Ulm and gives lectures on breastfeeding encouragement.
C. Posovszky acted as a consultant/expert for the Nestlé Health Science GmbH, Germany, from which he received a fee. He worked in the scientific advisory boards of the Shire Austria and Shire Deutschland GmbH, Takeda (Switzerland), Nutricia Milupa, and Pharmacosmos and received contributions from them. CP received monetary contributions for giving lectures/training courses for the AbbVie Deutschland GmbH, Nutricia GmbH, Nutricia Milupa SA, Takeda (Switzerland), Gebro Pharma GmbH Austria, Publicare AG, and FomF GmbH. The Shire International GmbH and FresuCare AG support CP’s research projects with third-party funds.
S. Rudloff received fees for her lectures/training courses for the DGKJ, HIPP, Nutricia, and the Heiner-Brunner-Seminar of the GPGE.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: authors would like to correct the article title to 'Infant formulas with synthetic oligosaccharides and respective marketing practices: Position Statement of the German Society for Child and Adolescent Medicine e.V. (DGKJ), Commission for Nutrition.'
Supplementary Information
Additional file 1.
The German version of the article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bührer, C., Ensenauer, R., Jochum, F. et al. Infant formulas with synthetic oligosaccharides and respective marketing practices: Position Statement of the German Society for Child and Adolescent Medicine e.V. (DGKJ), Commission for Nutrition. Mol Cell Pediatr 9, 14 (2022). https://doi.org/10.1186/s40348-022-00146-y
Accepted:
Published:
DOI: https://doi.org/10.1186/s40348-022-00146-y