Abstract
Background
Circadian rhythm disturbances overlap with the symptoms of mood episodes and may trigger or prolong mood symptoms. There is limited research on the role of circadian disturbances in mood disorders in young people and/or first episode cases of unipolar and bipolar disorders.
Methods
Actigraphy was undertaken for about 14 days in 63 post-pubertal individuals aged 13–25 years with a recent onset of a mood disorder meeting recognised diagnostic criteria. We examined associations between three easily interpretable markers of circadian rhythm activity (amplitude, acrophase and rhythmicity index) and demography and clinical characteristics. Then, circadian markers were compared between diagnostic groups, controlling for potential confounders.
Results
Longer duration of illness was correlated with reduced circadian rhythmicity and lower levels of activity over 24 h. A delay in the timing of maximum activity was associated with the level of manic but not depressive symptoms. The circadian rhythmicity index differentiated unipolar from bipolar cases, and in bipolar but not unipolar disorder, the rhythmicity was less robust in those with more severe manic or depressive symptoms.
Conclusions
Less robust circadian rhythmicity, especially associated with increasing symptom severity, may represent a more specific or a trait marker of young people with mood disorders who are at higher risk of a bipolar course of illness.
Similar content being viewed by others
Background
Circadian rhythm disturbances are of considerable interest to mood disorder researchers as many of the phenomena that accompany disruptions in the sleep–wake cycle overlap with the symptoms of mood episodes or may trigger or prolong mood symptoms (e.g. McClung 2013; Robillard et al. 2013a). However, most investigations of circadian rhythm disruptions in mood disorders are focused on older adult samples with established unipolar (UP) or bipolar disorders (BD). Often these individuals have a history of multiple mood episodes, accompanied by a high prevalence of mental and physical comorbidities, complex treatment regimens and/or elevated body mass index (BMI) (Geoffroy et al. 2015). As such, it is difficult to disentangle which abnormalities represent causes and which are consequences of mood episodes (Bellivier et al. 2015). There is limited research on the role of circadian disturbances in UP and BD in young people, especially related to first episode onset or the first treated episode (e.g. Ritter et al. 2011; Castro et al. 2015; Ng et al. 2015).
One of the problems in making comparisons between studies undertaken in young people with emerging mood disorders is that the sampling strategies and the parameters measured vary considerably between studies. For example, some studies focus on individuals at high risk of developing psychotic or mood disorders (identified by the presence of attenuated symptoms or temperamental style), whilst others recruit mixed samples of symptomatic and asymptomatic offspring of parents with UP or BD (e.g. Jones et al. 2006; Adan et al. 2010; Hidalgo et al. 2009; Castro et al. 2015; Park et al. 2015). Most of these studies use self-report assessments of sleep patterns or self-ratings of circadian rhythm such as the Morning–Eveningness Questionnaire (MEQ) (Horne and Ostberg 1976); the studies that do use objective measures such as actigraphy often focus on more traditional measures of sleep, such as sleep latency, waking after sleep onset and total sleep time (e.g. Castro et al. 2015). Studies measuring circadian parameters and using actigraphy have often recruited young people with a wide range of disorders (anxiety, UP, BD, psychosis), but failed to take into account in the analyses the other variables known to influence circadian rhythms, such as age, pubertal status or BMI, etc., (Adan et al. 2010; Mansour et al. 2005; Caci et al. 2009; Kim et al. 2010; Tranel et al. 2015).
Despite the methodological heterogeneity (noted above), there is a growing research literature on actigraphic measures of circadian rhythms in youth. However, the clinical translation of some of these studies is reduced because many clinicians are unfamiliar with the relevance of some measures and/or are uncertain how to interpret some of the circadian parameters (e.g. alpha, beta, mesor) (Bellivier et al. 2015). As such, it can be helpful to select circadian markers that have shown differences between young people with mood disorders (compared to healthy control or other cases with mental disorders), and that can be easily understood by clinicians. The three obvious circadian parameters are amplitude, acrophase and circadian rhythmicity index. Several studies report lower 24-hour activity rhythms (amplitude) or changes in dim light melatonin onset for UP and BD (Teicher et al. 1993; Armitage et al. 2004; Robillard et al. 2013b; 2015), and for individuals at risk of BD (Bullock and Murray 2014; Castro et al. 2015). The timing of maximal activity within a 24-h period (acrophase) has shown mixed results in UP and BD (Teicher et al. 1993; Robillard et al. 2013a; 2015), but there is emerging evidence of lower robustness of circadian rhythmicity in broad populations of young patients compared to healthy controls (e.g. Carpenter et al. 2015). The latter could be an interesting avenue for youth research as Gonzalez and colleagues (2014) recently demonstrated that a lower circadian rhythmicity index was a useful marker of illness severity in adults with BD and was correlated with total manic symptom score and with some individual manic symptoms. However, rhythmicity in comparable populations of UP and BD cases remains unexplored.
This study examines the role of selected markers of circadian rhythmicity in emerging mood disorders in young people aged 13–25 years who presented for the first time to clinical services. We focused on three key, easily interpretable parameters of circadian regulation, namely amplitude, acrophase and the circadian rhythmicity index. The aims were (a) to examine the associations between circadian parameters and age, gender, BMI, duration of illness and symptom severity in the total sample; (b) to compare these circadian parameters in BD and UP (after controlling for potential confounders).
Methods
Participants
With the approval of the Human Research Ethics Committee of The University of Sydney, individuals attending the local youth mental health services in and around Sydney, Australia (Youth Mental Health Clinic at the Brain & Mind Research Institute, at The University of Sydney, and headspace in Campbelltown) were invited to participate in a series of studies of mental and physical health, including research on sleep and actigraphy.
Individuals included in this study were aged 13–25 years, had a recent onset of a mood disorder meeting DSM-IV R criteria (APA. 2000), and were willing and able to give written informed consent to participate and to comply with study procedures. In those aged <16 years, additional written consent was obtained from their parents or legal guardians. The exclusion criteria were (i) clinically assessed IQ <70 or intellectual impairment, and/or history of head injury, (ii) mood disorder secondary to a medical condition or psychotic disorder, (iii) primary substance or alcohol misuse disorder, (iv) risk of suicide or self-harm, (v) regular use of medications that affect sleep, melatonin, circadian rhythms or alertness, (vi) evidence of other sleep (e.g. sleep apnoea, narcolepsy), neurological (e.g. epilepsy) or primary medical conditions that could explain the current depression and/or contribute to sleep–wake dysfunction, (vii) recent trans-meridian travel (i.e. potential for jet lag) or regular shift work.
Assessments
As described previously, participants completed a detailed clinical assessment conducted with a structured clinical proforma (Hickie et al. 2014; Scott et al. 2014, 2015). For this study, the following data were used:
-
1.
Demographics—current age and gender.
-
2.
Clinical history—a research psychologist or psychiatrist established that the individual had a UP or BD that met DSM-IV R diagnostic criteria. Although the cases were presenting to clinical services for the first time with syndromal illness episodes, this was not necessarily the first experience of clinical symptoms or sub-threshold syndromes. As such, we included a measure of time since onset of any symptoms, which is referred to as the duration of illness (and was estimated as current age minus age at first onset of any psychiatric symptoms) (Scott et al. 2014). Basic details of current medications (class of medications prescribed) and body mass index (BMI) were recorded.
-
3.
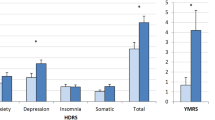
Symptom severity—observer ratings of mood symptoms were undertaken using the 17-item version of the Hamilton Rating Scale for Depression (HRSD; Hamilton, 1960) and the 11-item version of the Young Mania Rating Scale (YMRS) (Young et al. 1978) in all participants.
-
4.
Sleep profile—self-report and observer-rated assessments of sleep were undertaken for other studies by the research group, but are not reported here (for details see Robillard et al. 2014).
In this study, objective recordings of sleep profile were undertaken for between 5 and 14 days (median 10 days) using an actiwatch (Actiwatch-64/L/2/Spectrum, Philips Respironics, USA) and sleep–wake detection was conducted automatically with Actiware 5.0 software (Philips Respironics) using the medium sensitivity threshold.
To characterise the circadian profile of the activity-rest cycle, individual actigraphy datasets were fitted to an extended Cosinor model using GraphPad Software. In this study, we estimated three key parameters, namely:
-
(i)
Amplitude: a measure of the range of activity levels across the 24-h period,
-
(ii)
Acrophase: a phase marker that can be used as a measure of an advance or delay in time of maximum activity, and
-
(iii)
Circadian Rhythmicity Index (R2): an indicator of the strength or robustness of circadian rhythms, and also referred to as R2 or the coefficient of determination. The circadian rhythmicity index is a ‘goodness of fit’ measure and higher values indicate smaller discrepancies between actigraphy data and values predicted by the Cosinor model and higher scores represent more robust rhythms (for an explanation of how to undertake the analysis see Marler et al. 2006).
Statistical analyses
All analyses used an alpha of 0.05 and were conducted using SPSS (version 21).
Clinical and demographic characteristics for the UP and BD groups were compared using univariate analyses (t-tests, Mann–Whitney U and Fisher’s exact test). Pearson’s product moment correlational analyses were used to examine any associations between each circadian parameter (amplitude, acrophase and R2) and continuous variables measuring demographic and illness characteristics i.e. current age, duration of illness, BMI, HRSD and YMRS in the total sample. Next, we conducted an analysis of variance (ANOVA) for each circadian parameter according to mood disorder subtype (UP or BD) with gender, current age, duration of illness, BMI, HRSD and YMRS as covariates.
Results
As shown in Table 1, the groups were well matched for current age and gender and there were no statistically significant differences in duration of illness, BMI or symptom severity scores. The YMRS scores were not normally distributed, but the higher median score in the BD group was not significantly greater than in the UP group. Most cases of both UP and BP were being prescribed only one medication, and the majority of the sample received an antidepressant. Some BD cases were co-prescribed a mood stabilizer or atypical antipsychotic, but prescribing patterns did not differ significantly between the diagnostic groups.
For the total sample, the mean amplitude was 2.04 (SD 0.76), the mean acrophase was 16.19 h (SD 1.65) and the mean R2 was 0.42 (SD 0.13); there were no significant differences according to gender. As shown in Table 2, current age was significantly negatively correlated with R2 (r −0.40; p = 0.001), whilst duration of illness was significantly negatively correlated with amplitude (r −0.25; p = 0.045) and with R2 (r −0.33; p = 0.01). The BMI was not associated with any circadian parameters. With regard to current symptom severity, the HRSD was not significantly associated with circadian parameters, but the YMRS score was significantly positively correlated with acrophase (r 0.28; p = 0.03).
The ANOVA demonstrated that, after taking into account potential confounders (age, gender, duration of illness, BMI, HRSD, YMRS), differences between the diagnostic groups were significant for R2 (mean UP v. BD = 0.43 v 0.40; F = 2.34; df = 6, 56; p = 0.044), but not for amplitude (mean = 2.14 v. 1.84; F = 1.51; p = 0.09) or acrophase (mean = 16.07 v 16.30 h; F = 1.17; p = 0.33). As shown in Figs. 1 and 2, symptom severity and diagnostic group were the main to the significant model for R2. The regression slopes for the correlations between R2 and HRSD or R2 and YMRS are different in BD compared to UP cases. There is little variation in R2 across different levels of symptom severity in UP, but higher levels of either manic or depressive symptoms are associated with a significantly lower circadian rhythmicity index in BD cases.
Discussion
The study set out to explore two aspects of circadian rhythms in youth with mood disorders. First, the associations between the selected circadian parameters and basic demographic and clinical characteristics (independent of mood disorder subtype), and second, whether any specific parameters were more likely to be disturbed in UP compared to BD.
The correlational analyses undertaken in this study show a relationship between the selected circadian rhythm parameters, the first treated episode of mood disorders in youth and current age. First, longer duration of illness (defined as the time since the first onset of any symptoms) was associated with a reduced circadian rhythmicity index and lower levels of activity over 24 h. Second, there was a significant inverse correlation between increasing age and robustness of circadian rhythmicity. Third, acrophase was associated with current mental state, being significantly correlated with the level of manic but not depressive symptoms.
Taken together, these findings could suggest that weakened rhythmicity and lower 24-h activity are markers of vulnerability to developing syndromal episodes of UP or BD. We cannot state categorically that they are trait markers for UP or BD as, even in this first-treated sample, many individuals had a history of subsyndromal symptoms. So, the circadian disturbances could be a consequence of longer duration of symptoms. However, we can hypothesis that these circadian parameters are indicators that mood symptoms will meet the diagnostic threshold for a full syndromal episode (Bullock and Murray 2014; Castro et al. 2015). In contrast, the acrophase findings suggest that the timing of maximum activity may be a state marker of manic symptom severity. This finding is in line with some but not all previous studies. Several studies in young people failed to show an association between acrophase and depression (Teicher et al. 1993; Robillard et al. 2015), whilst several studies in older adults have noted an association between mania and acrophase (although findings are inconsistent with regard to phase advance or delay) (Salvatore et al. 2008; Ng et al. 2015).
We suggest that the findings regarding current age are important as they emphasise that there is a developmental trajectory for the variability in 24-h rhythms that should be taken into account when planning studies of sleep and mood disorders (Mansour et al. 2005; Caci et al. 2009; Kim et al. 2010). This is especially important in studies that focus on populations of adolescents and young adults, as age-matching may be needed to avoid misinterpreting any circadian disruption as an association with illness characteristics as compared to demography (Fares et al. 2015). Similarly, previous studies have demonstrated an association between gender and/or increased BMI and circadian disturbance in adolescents and young adults (e.g. Arora and Taheri 2014; Tranel et al. 2015), and we suggest that these factors also need to be routinely reported in future studies. The lack of significant associations between gender and circadian disturbance may be due to recruiting a post-pubertal sample (or the sample size); but the lack of association between BMI and circadian disturbance was somewhat unexpected. It may be because the sample did not manifest high levels of obesity (the mean BMI was <25) or it may be a consequence of the relatively small sample size (Boudebesse et al. 2015).
The examination of mood disorder subtypes suggests that the circadian rhythmicity index demonstrates a stronger association with illness episodes in BD as compared to UP. After taking into account possible confounders, we found that rhythmicity in BD was lower than in UP. Furthermore, whilst there were little apparent differences in rhythmicity across all levels of symptom severity in UP, reduced rhythmicity was associated with both higher HRSD and higher YMRS scores in BD. A difference between UP and BD might be anticipated for R2 and YMRS scores, as we would not anticipate high levels of manic-like symptoms in the UP group and also, previous publications suggest that there is heightened variability in circadian markers across mood phases in BD (Novakova et al. 2015). However, the finding that the BD group, but not the UP group, also showed lower rhythmicity in association with higher levels of depressive symptoms is an important finding that warrants replication. The findings may indicate that weakened circadian rhythms, especially associated with increasing severity of depressive symptoms, is a more specific marker of BD than of UP in youth.
The current study has a number of limitations. First, the sample size limits the number of statistical analyses that could be planned and performed. Also, it meant we could not justify undertaking any further sub-group analyses to determine if any specific constellations of manic or depressive symptoms were associated with weakened rhythmicity (as reported by Gonzalez et al. 2014). Second, although the age range was limited to the post-pubertal and early adult period, there were trends towards significant differences between the UP and BD groups in mean age (p = 0.06) and the BD group had a longer mean duration of symptoms. Without specific age and gender matching, we cannot rule out the possibility that these differences contributed to some extent to the findings in this study. Third, although the cases were presenting to the clinical services for the first time, the study participants were nearly all receiving medication by the time they undertook the actigraphy recording of their sleep–wake patterns. Although there were no significant differences in medications received by the UP and BD cases, it is possible that some findings might be associated with, or the findings were enhanced by any effects associated with the medications prescribed. Lastly, although symptom levels were in the mild to moderate range, it does make it difficult to distinguish state and trait abnormalities in circadian rhythms.
Conclusions
In conclusion, the study selected circadian markers (acrophase, amplitude and circadian rhythmicity) that are easy to interpret clinically and that capture a holistic picture of circadian rhythm disruption in mood disorders. The findings provide evidence for circadian disturbances in youth with mood disorders that warrant further investigation, especially the possibility that the circadian rhythmicity index is lower in BD compared to UP and is less robust in the face of increasing levels of manic or depressive symptoms.
Abbreviations
- ANOVA:
-
analysis of variance
- BMI:
-
body mass index
- BD:
-
bipolar
- DSM-IV-R:
-
Diagnostic and Statistical Manual of Mental Disorders Fourth Edition Revised
- HRSD:
-
Hamilton Rating Scale for Depression
- IQ:
-
intelligence quotient
- MEQ:
-
morning–eveningness questionnaire
- R2:
-
circadian rhythmicity index
- UP:
-
unipolar
- YMRS:
-
Young mania rating scale
References
Adan A, Lachica J, Caci H, Natale V. Circadian typology and temperament and character personality dimensions. Chronobiol Int. 2010;27(1):181–93.
APA. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. revised. Washington: American Psychiatric Press; 2000.
Armitage R, Hoffmann R, Emslie G, Rintelman J, Moore J, Lewis K. Rest-activity cycles in childhood and adolescent depression. J Am Acad Child Adolesc Psychiatry. 2004;43(6):761–9.
Arora T, Taheri S. Associations among late chronotype, body mass index and dietary behaviors in young adolescents. Int J Obes Relat Metab Disord. 2014;39(1):39–44.
Bellivier F, Geoffroy P, Etain B, Scott J. Sleep- and circadian rhythm-associated pathways as therapeutic targets in bipolar disorder. Expert Opin Ther Targets. 2015;19(6):747–63.
Boudebesse C, Geoffroy P, Henry C, Germain A, Scott J, Lajnef M, et al. Links between sleep and body mass index in bipolar disorders: an exploratory study. Eur Psychiatry. 2015;30(1):89–93.
Bullock B, Murray G. Reduced amplitude of the 24 hour activity rhythm: a biomarker of vulnerability to bipolar disorder? Clin Psychol Sci. 2014;2(1):86–96.
Caci H, Deschaux O, Adan A, Natale V. Comparing three morningness scales: age and gender effects, structure and cut-off criteria. Sleep Med. 2009;10(2):240–5.
Carpenter J, Robillard R, Lee R, Hermens D, Naismith S, White D, et al. The relationship between sleep-wake cycle and cognitive functioning in young people with affective disorders. PLoS One. 2015;10(4):e0124710.
Castro J, Zanini M, Gonçalves B, Coelho F, Bressan R, Bittencourt L, et al. Circadian rest–activity rhythm in individuals at risk for psychosis and bipolar disorder. Schizophr Res. 2015;168(1–2):50–5.
Fares S, Hermens D, Naismith S, White D, Hickie I, Robillard R. Clinical correlates of chronotypes in young persons with mental disorders. Chronobiol Int. 2015;32:1183–91.
Geoffroy P, Scott J, Boudebesse C, Lajnef M, Henry C, Leboyer M, et al. Sleep in patients with remitted bipolar disorders: a meta-analysis of actigraphy studies. Acta Psychiat Scand. 2015;131(2):89–99.
Gonzalez R, Tamminga C, Tohen M, Suppes T. The relationship between affective state and the rhythmicity of activity in bipolar disorder. J Clin Psychiatry. 2014;75(04):e317–22.
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56.
Hickie I, Scott E, Glozier N. headspace—Australia’s innovation in youth mental health: who are the clients and why are they presenting? Med J Aust. 2014;200(8):452–4.
Hidalgo M, Caumo W, Posser M, Coccaro S, Camozzato A, Chaves M. Relationship between depressive mood and chronotype in healthy subjects. Psychiatry Clin Neurosci. 2009;63(3):283–90.
Horne J, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97–110.
Jones S, Tai S, Evershed K, Knowles R, Bentall R. Early detection of bipolar disorder: a pilot familial high-risk study of parents with bipolar disorder and their adolescent children. Bipolar Disord. 2006;8(4):362–72.
Kim S, Lee Y, Kim H, Cho I, Lee J, Cho S. Age as a moderator of the association between depressive symptoms and morningness-eveningness. J Psychosom Res. 2010;68(2):159–64.
Mansour H, Wood J, Chowdari K, Dayal M, Thase M, Kupfer D, et al. Circadian phase variation in bipolar I disorder. Chronobio Int. 2005;22(3):571–84.
Marler M, Gehrman P, Martin J, Ancoli-Israel S. The sigmoidally transformed cosine curve: a mathematical model for circadian rhythms with symmetric non-sinusoidal shapes. Stat Med. 2006;25(22):3893–904.
McClung C. How might circadian rhythms control mood? Let me count the ways. Biol Psychiatry. 2013;74(4):242–9.
Ng T, Chung K, Ho F, Yeung W, Yung K, Lam T. Sleep-wake disturbance in inter-episode bipolar disorder and high-risk individuals: a systematic review and meta-analysis. Sleep Med Rev. 2015;20:46–58.
Novakova M, Prasko J, Latalova K, Sladek M, Sumova A. The circadian system of patients with bipolar disorder differs in episodes of mania and depression. Bipolar Disord. 2015;17(3):303–14.
Park C, An S, Kim H, Koh M, Namkoong K, Kang J, et al. Relationships between chronotypes and affective temperaments in healthy young adults. J Affect Disord. 2015;175:256–9.
Ritter P, Marx C, Bauer M, Lepold K, Pfennig A. The role of disturbed sleep in the early recognition of bipolar disorder: a systematic review. Bipolar Disord. 2011;13(3):227–37.
Robillard R, Naismith S, Hickie I. Recent advances in sleep-wake cycle and biological rhythms in bipolar disorder. Curr Psychiatry Rep. 2013a;15(10):402.
Robillard R, Naismith SL, Rogers NL, Scott EM, Ip TK, Hermens DF, Hickie IB. Sleep-wake cycle and melatonin rhythms in adolescents and young adults with mood disorders: comparison of unipolar and bipolar phenotypes. Eur Psychiatry. 2013b;28(7):412–6.
Robillard R, Naismith S, Smith K, Rogers N, White D, Terpening Z, et al. Sleep-wake cycle in young and older persons with a lifetime history of mood disorders. PLoS One. 2014;9(2):e87763.
Robillard R, Hermens D, Naismith S, White D, Rogers N, Ip T, et al. Ambulatory sleep-wake patterns and variability in young people with emerging mental disorders. J Psychiatry Neurosci. 2015;40(1):28–37.
Salvatore P, Ghidini S, Zita G, Panfilis C, Lambertino S, Maggini C, et al. Circadian activity rhythm abnormalities in ill and recovered bipolar I disorder patients. Bipolar Disord. 2008;10(2):256–65.
Scott E, Hermens D, White D, Naismith S, GeHue J, Whitwell B, et al. Body mass, cardiovascular risk and metabolic characteristics of young persons presenting for mental healthcare in Sydney, Australia. BMJ Open. 2015;5(3):e007066.
Scott J, Scott E, Hermens D, Naismith S, Guastella A, White D, et al. Functional impairment in adolescents and young adults with emerging mood disorders. Br J Psychiatry. 2014;205(5):362–8.
Teicher M, Glod C, Harper D, Magnus E, Brasher C, Wren F, et al. Locomotor activity in depressed children and adolescents: I. circadian dysregulation. J Am Acad Child Adolesc Psychiatry. 1993;32(4):760–9.
Tranel H, Schroder E, England J, Black W, Bush H, Hughes M, et al. Physical activity, and not fat mass is a primary predictor of circadian parameters in young men. Chronobiol Int. 2015;32(6):832–41.
Young R, Biggs J, Ziegler V, Meyer D. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133(5):429–35.
Authors’ contributions
IH, SN, DH, ES were involved in the design and planning of the studies on youth mental health. JS, AG and IH identified the hypotheses for the current study. AG carried out the literature review and drafted the main sections of the manuscript. JS undertook the statistical analyses and assisted in writing the preliminary draft. All authors read and approved the final manuscript.
Competing interests
AG has received grant funding from the National Health and Medical Research Council in Australia. IH is a Commissioner in Australia’s National Mental Health Commission; a Member of the Medical Advisory Panel for Medibank; a Board Member of Psychosis Australia Trust. IH has received honoraria for presentations of his own work at educational seminars supported by a number of non-government organisations and by the pharmaceutical industry (including Servier, Pfizer, AstraZeneca and Eli Lilly). The University of Sydney (Principal Investigator: IH) received funding from Servier for a study of major depression and sleep disturbance in primary care settings. Other relevant funding for IH in relation to this study includes ‘Testing and delivering early interventions for young people with depression’ (APP ID: 1046899). SN has received grant funding from the National Health and Medical Research Council including for research on sleep and actigraphy. DH has received honoraria for educational seminars from Janssen-Cilag and Eli Lilly. DH has received grant funding from the NSW Health, Mental Health and Drug & Alcohol Office. ES has received honoraria for educational seminars from Servier. JS is a visiting professor at the Brain & Mind Centre at The University of Sydney. JS has received UK grant funding from the Medical Research Council (including for projects on actigraphy and bipolar disorders) and from the Research for Patient Benefit programme (PB-PG-0609-16166: Early identification and intervention in young people at risk of mood disorders).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Grierson, A.B., Hickie, I.B., Naismith, S.L. et al. Circadian rhythmicity in emerging mood disorders: state or trait marker?. Int J Bipolar Disord 4, 3 (2016). https://doi.org/10.1186/s40345-015-0043-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40345-015-0043-z