Abstract
Background
Information regarding the movement ecology of horse-eye jack Caranx latus throughout the Caribbean is limited despite their prevalence. Passive acoustic telemetry was used to infer movement patterns of seven adult C. latus within Buck Island Reef National Monument (BIRNM), a no-take marine protected area (MPA) northeast of St. Croix, U.S. Virgin Islands. In addition, a preliminary exploration of detections recorded outside of BIRNM was used to gain knowledge of the potential for larger scale movements. Ascertaining long-term movement patterns, including residency, mobility, and identifying core activity spaces can play a considerable role in how MPAs, like BIRNM, are adapted to meet the needs of mobile species.
Results
High residency index values were observed for individual C. latus within the BIRNM array (mean ± SE: 0.913 ± 0.04, range 0.75–1.0) across the 17 months monitored. Most fish were also detected on receivers located outside BIRNM. An observed to expected detection ratio revealed that despite high residency, only 9.6% of expected transmissions were detected based on the average tag transmission rate. Network analysis revealed high individual connectivity with many of the receivers inside BIRNM and a large number of core use receivers (mean: 10.7, range 6–14) within individual networks.
Conclusions
Most C. latus were present in BIRNM at least twice per day, but were overall detected below the expected rates, demonstrating mobility, large core activity spaces and wide use of the acoustic array inside BIRNM and greater St. Croix shelf. How residency is inferred from acoustic telemetry detections, and interpreted for species with variable mobility, has important considerations for spatial management planning and telemetry analyses. For MPA development to meet the spatial requirements of species with mixed resident–mobile spatial ecology, detailed long-term movement data are required. Assessing residency in MPAs using acoustic telemetry should be formalized and carefully interpreted based on specific species, environmental conditions, and array configuration.
Similar content being viewed by others
Background
Animal movements have important ramifications for ecological processes as they often link disparate habitats and impact the pathways of energy flow, potentially in ways that strengthen ecosystem resilience [1, 2]. Furthermore, as spatial management has become more widely applied, so has the importance of considering how species movements expose them to differential fishing mortality, in particular when moving among areas with varying levels of protection [3]. Incorporating movement data from species of interest at the inception point of marine protected area (MPA) design or when considering boundary adjustment of an existing MPA is critical in ensuring that a necessary and sufficient space is protected [4]. Ultimately, data from which the spatial extent and patterns in fish movements can be inferred will strengthen our understanding of MPA efficacy for species and ecosystems.
Marine species are particularly challenging to protect through static spatial closures because of interspecific and ontogenetic changes in the scale of movement patterns. Within species or populations, individual behaviors can range between the extremes of true resident and transient characteristics [5, 6], often with individuals transitioning between the two types for parts of their life cycle [7]. Transitions can occur abruptly as when resident behavior is interrupted when individuals undertake migrations to form spawning aggregations before reverting to resident behavior [8, 9]. Further, morphological transformations that alter the physiology and ecology of individuals may result in a transition in movement type, such as the development of saltwater tolerance in juvenile anadromous fish followed by an emigration to saltwater ecosystems [10] or ontogenetic shifts in scale of home range and habitat use [11]. To address complexity and variability in movement dynamics across species, one of the first steps is to understand general patterns in long-term residency and space use [12]. Since successful spatial management requires knowledge of animal movements at different scales, it becomes important to ensure the combination of tools used to analyze movements are effective in accurately characterizing whether species are resident, mobile, or where they fall along the movement spectrum between these extremes.
Network analysis has become a useful tool for modeling animal movement, offering an alternative or complementary approach to traditional methods for analyzing acoustic telemetry data in fixed receiver arrays. For example, network analysis emphasizes movement corridors and links between core use areas and areas used infrequently [13,14,15]. Network methods can also explore connectivity across large geographic regions for migratory species and highlight unique movement strategies [16]. Understanding connections amongst receivers (inferred movement within an array) and identifying less frequently visited areas, often excluded from typical movement analysis (i.e., kernel utilization density estimators), is information managers can employ to adequately preserve space used by target species or infer important pathways between infrequently, but ecologically vital sites (i.e., spawning and foraging areas).
The horse-eye jack Caranx latus is a pelagic, widely distributed member of the Carangidae family found throughout the Caribbean [17]. In the western Atlantic, C. latus range from New Jersey to Brazil [18]. Although related to more economically valuable Carangids such as giant trevally C. ignobilis and crevalle jack C. hippos, C. latus are of minor commercial importance in the United States due to high levels of ciguatoxins [19]. In other parts of their range, such as the Southeast coast of Brazil, C. latus are relied on as a major source of income and food in artisanal fishing communities [20]. This species is also occasionally taken as bycatch, making up a small part of other Carangid fisheries [21] and is periodically targeted by recreational anglers as a gamefish. Despite regional use and high local abundances across its range, little information exists on reproduction, life-history characteristics, population trends, and long-term movement patterns. As both an important inshore and offshore mobile piscivorous predator [20, 22] ascertaining movement dynamics is a considerable step in understanding the role this species plays within the ecosystems it inhabits. Two conventional tagging studies were unsuccessful in recapturing or resighting tagged fish [23, 24]. Both studies attributed the lack of detection to C. latus being a highly mobile species with low tagging-site fidelity. Recently, one acoustically tagged C. latus demonstrated broad-ranging movements, a finding which supports interpretations from the previous two mark–recapture studies [17].
To assess the degree of mobility versus residency, identify key activity spaces, and determine whether Buck Island Reef National Monument (BIRNM), a no-take Caribbean MPA, provides coverage of primary activity spaces for C. latus, we used passive acoustic telemetry and multiple analytical techniques to (1) compare overall and monthly residency using published detection cutoff values and the expected detection ratio and (2) identify high use areas for individual C. latus by graphing spatial networks of core use receivers. We hypothesized that C. latus would be a relatively mobile species with low residency compared to other reef-associated species in the region.
Methods
Study area and array design
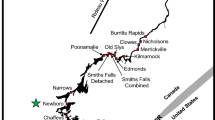
BIRNM is a no-take MPA located 1.5 km northeast of St. Croix, U.S. Virgin Islands (Fig. 1a, b) and is managed by the U.S. National Park Service (NPS; 25). The Monument was established in 1961 to preserve fringing reef habitat that surrounds an uninhabited island (Buck Island) from the southeast to the northwest creating a continuous lagoon habitat. The original boundary was expanded in 2001 to 77 km2 and new regulations were simultaneously implemented restricting all extractive activities within Monument boundaries [25]. In 2003, BIRNM became contiguous with St. Croix’s East End Marine Park (EEMP), a multi-use protected area with over 80% of 155 km2 open to fishing (Fig. 1b; [26]). Some regions within EEMP, for example Teague Bay, off the St. Croix mainland, have been designated as recreational areas which allow bait fish harvest, recreational guided fishing, and recreational shoreline take fishing. Following BIRNM’s shallow shelf break eastward, Lang Bank (LB), an 11.7 km2 seasonal no-take closure implemented in 1993 for a red hind Epinephelus guttatus spawning aggregation, provides some additional protection from December to February of each year and may be a spawning aggregation site for additional species (Fig. 1b; [8, 27]).
a Location of St. Croix, U.S. Virgin Islands within the greater Caribbean region. b Boundaries and acoustic receiver stations in Buck Island Reef National Monument (white circles), Lang Bank (white triangles), St. Croix’s East End Marine Park (white squares) and Teague Bay (white hexagons). The shallow water benthic habitat shapefile was obtained from NOAA Biogeography Branch. Unmapped deep-water habitat is represented by white
For this study, 78 VR2W acoustic receivers (69 kHz; Vemco, Nova Scotia, Canada) were deployed as fixed stations within BIRNM (depth of receivers, range 7–116 m, mean: 44.5 m) as part of a large collaborative acoustic network (Fig. 1b). In addition, three receivers were placed outside the eastern boundary of the Monument in EEMP, and five were located along the shallow shelf break out to LB, but not within the seasonal closure boundaries (Fig. 1b). The three receivers in EEMP and the five located along the shelf break were placed in areas open to fishing and these stations were present throughout the duration of the study period, from January 2016 to May 2017. In October (n = 13) and November (n = 3) 2016, additional receivers were installed near Teague Bay off the St. Croix mainland and supplemental receivers were installed within the LB closure area (n = 4; Fig. 1b). Finally, a seasonal array of 15 receivers was installed inside LB from December 2015 to April 2016. Data from the entire array were downloaded biannually via SCUBA and free diving by NPS employees, collaborators, and volunteers.
All receivers were anchored with either sand screws (0.91-m-long, 15-cm-diameter blades) or cement blocks, as determined by the underlying benthic habitat type [14]. BIRNM consists of a variety of habitat types distributed throughout the shallow shelf area in a patchy mosaic pattern [25] while benthic habitat type inside EEMP and LB is dominated by coral reef and colonized hardbottom, although habitat in Teague Bay is predominantly seagrass [26]. Each of these habitat types, as well as other environmental and study design factors, uniquely affects the ability of receivers to detect transmission signals from tagged fish. Range testing for a smaller subset of the current BIRNM array determined a 50% average detection probability at approximately 125 m [28].
Fish capture and tagging
On four separate tagging trips over three consecutive years (Table 1; Additional file 1), C. latus were captured inside BIRNM during day and dusk hours by trolling with recreational fishing gear using a variety of artificial lures, and at night by bottom jigging around a full moon event. No two fish were caught at the same location. Upon capture, fish were visually assessed to ensure there was no external physical trauma present. If this condition was met, individuals were implanted with a coded transmitter dependent on body size where tag type was assumed to not influence detection probability or differ between tag type (Vemco V9, V13, or V16, 69 kHz, approximate 632, 1299, 3650-day battery life, respectively; Table 1). Each transmitter was programmed with a varying dominant delay ping rate between 50 and 130 s (mean: 90 s) which reduced the risk of transmission collisions and were assumed to not influence detection probability. In addition, all tags had a 2-week interval of a quicker 15-s minimum ping rate and 45-s max ping rate (mean: 30 s) to maximize detection probability, set to start at either 30, 120, 210, or 300 days post-activation. Prior to tag implantation, fish were placed in a large tote of ambient seawater with 10-g l−1 of the anesthetic tricaine methanesulfonate (MS222) to induce stage 4 anesthesia (see [14] for a detailed description of tagging methodology). Once individuals displayed slowed gill movement and a loss of equilibrium, they were held in a supine position for the duration of the surgery. Halfway through the surgery, fresh seawater was added to initiate recovery. Each transmitter was disinfected with 70% isopropyl alcohol prior to being inserted anteriorly into the coelomic cavity through a small incision off the central mid-line between the pelvic and anal fins. The incision was closed with 2–3 simple interrupted sutures (Ethicon polydioxanone monofilament sterile absorbable FS-1, 24 mm reverse cutting needle sutures; Model PDS*II) and all surgeries lasted approximately 8 min. A small fin clip was sampled from the anal fin of each tagged fish for future genetic and stable isotope analyses and fork length was measured to the nearest centimeter. All individuals responded well to surgery and were briefly held over the side of the boat until they were strong enough to swim away within 200 m of the capture location.
Data analysis
From 2015 to 2017, eleven C. latus were tagged with acoustic transmitters and tracked within BIRNM. Fish that were recorded on receivers for less than 3 days and fish that had less than 1000 detections were removed to ensure individuals that either died, shed the tag post-release, or quickly emigrated from the array did not influence space-use analyses. Based on these criteria, three tagged fish were excluded from analyses (see Additional files 1, 2, 3). The smallest tagged C. latus (FL = 35.5 cm), a potentially immature individual, provided limited movement data, being only present in the array for 36 days and visiting four receivers. Due to inconclusive space-use patterns and a sudden departure from the system indicated by consistent detection data followed by an abrupt end of detections, this fish was not included in further analyses. The number of detections recorded over the duration of the study has the potential to influence ecological inferences [14]. Therefore, the two cutoffs (less than 3 days of detections and less than 1000 detections) were employed to reduce the potential for inaccurate interpretation of space-use patterns inside BIRNM. Detection data recovered from all receivers were corrected for time drift, then filtered for detections that occurred less than 15 s apart, based on the quickest tag ping rate (occurred during the rapid 2-week ping rate). Short ping transmissions (i.e., those that occurred < 15 s apart) were assumed to be spurious, attributed to echoes or simultaneous detections, and were removed.
Residency within the BIRNM array was represented using a residency index (RI). The RI was calculated by dividing the total number of days a fish was detected on any receiver within BIRNM by the maximum number of possible days the fish could have been detected (i.e., the period between the day of release and the last day the fish was detected; [29]). An individual was considered present if there were at least two detections per day [30]. RI values range from 0 (complete absence) to 1 (complete presence) and was calculated for each individual fish for the entire BIRNM array (i.e., overall residency). Monthly RI values for each fish were calculated based on the number of days the individual was detected at least twice divided by the total number of days in the month.
The ratio between the observed and expected (O/E) detections in a given day was also examined (based on the mean dominant delay of 90 s) and averaged monthly. For all tags, the rapid 2-week ping rate often overlapped neighboring months, thus the different expected values were calculated separately. When overlap occurred between months, the mean expected rapid rate (30 s) value was averaged with the mean expected dominant rate (90 s) to estimate monthly ratio. For reference, an O/E detection ratio was calculated for two synchronization or “sync” tags (range: 500–700 s; nominal delay: 600 s) within the array that were co-located with individual receivers, both located west of Buck Island. Available detection data from January 2016 to November 2016 for sync tags were filtered in the same way as animal tags. Both reference O/E detection ratios were calculated based on the observed amount of detections for the receiver it was co-located with (i.e., how well did the receiver detect the co-located sync tag).
Though additional receivers outside BIRNM were deployed at opportunistic times throughout the primary study period (January 2016 to May 2017), data collected were aggregated and used as a preliminary investigation to assess movement outside the BIRNM array. Detections observed on all receivers outside Monument boundaries (LB, EEMP and Teague Bay) were summed by month across the study period.
To examine broad-scale usage of the array by C. latus, network analysis (NA) as described by Finn et al. [31] was implemented using the igraph package [32]. Briefly, NA created individualized spatial graphs where stationary receivers were treated as network nodes with node size weighted according to the number of detections recorded at that location. Movements between nodes were represented by edges weighted by the amount of movement between two receivers. All receivers were placed in their actual (x, y) locations to facilitate interpreting the extent of space used within the BIRNM array. Edge arrows indicate directed movement pathways, with self-loop arrows representing detections occurring consecutively at the same receiver [31]. The ggnetworkmap function in the GGally package [33] and the ggmap package [34] were used to geographically plot individual fish movements in a network.
To test whether individual fish exhibited non-random movements, 10,000 random networks for each individual fish were generated using a bootstrap approach. Each new sequence of movements was based on the number of filtered detections retained for an individual fish and allowed the individual to be detected at any receiver (n = 78 BIRNM stations) throughout the sequence (i.e., a link rearrangement). A to/from matrix was constructed from the new random sequence creating a weighted movement list and thus a new random movement graph (i.e., random network). Network-level metrics (degree, betweenness, and closeness) were calculated for each new random movement graph (n = 10,000) to test against the original observed movement graph metrics using a one-sample Wilcoxon signed rank test (α = 0.05).
Core use receivers (CURs) were identified to define highly visited areas with the igraph package [32] following methodology of Becker et al. [14]. Centrality metrics based on degree value (also referred to as node strength: the total number of ingoing/outgoing movements from a receiver) were used to rank the receivers in an individual’s network, with receivers falling below the 50% identified as being CURs [14]. Centrality degree should be broadly comparable to other utilization techniques that estimate frequency of use, therefore we chose degree over betweenness and closeness centrality metrics to identify CURs for individual networks. CURs generated through NA were used as an alternative to conventional utilization density estimators as they provide a more holistic representation of individual space use [15]. All data processing and analyses were conducted in R statistical software version 3.3.3 [35].
Results
Seven C. latus were tracked within BIRNM from January 2016 to May 2017. Fish ranged in size from 45.0 to 82.0 cm fork length (FL; mean ± SD = 60.9 ± 11.9 cm; Table 1). Maturity for this species is presumed to occur at a mean FL between 35.0 and 40.0 cm [36], suggesting all fish monitored were mature adults. Individuals were present in the array from 114 to 477 days and accumulated moderate to large detection histories (range 6826–78,321; Table 1). Maximum time between successive detections on BIRNM receivers ranged from 0.82–14.7 days for individual fish (see Additional file 4).
Residency index values for the seven C. latus were high (mean ± SE = 0.913 ± 0.04, range = 0.75–1.0) for the entire BIRNM array. Monthly variation was evident, with decreasing RI values from January (RI = 1.0) to June (RI = 0.78) 2016 and increasing RI values from June (RI = 0.78) to November (RI = 1.0) of the same year (Fig. 2a). No evident trend marked differences in residency in the first half of 2017. Although the O/E detection ratio was typically less than 0.2 (< 20% of expected detections; mean ± SE: 9.62 ± 2.89%), it generally matched monthly RI patterns (Fig. 2b). As 2016 progressed, the number of detections recorded on receivers inside BIRNM decreased. However, the observed number of detections increased towards the end of year and into 2017, peaking in January and decreasing to May 2017. The O/E detection ratio for the two sync tags determined that 90.1% and 82.3% (mean: 86.2%) of all transmissions were recorded by the co-located receiver.
a Monthly residency index values averaged (± SE) across seven C. latus monthly from January 2016 (01/16) to May 2017 (05/17). b Observed to expected detection ratio at the mean delay ping rate (i.e., 90-s dominant delay averaged with the 30-s rapid rate when applicable) for each tag and averaged (± SE) per month. c Total number of detections (± SE) recorded on receivers outside of BIRNM. Vertical black line denotes the time when an additional 20 receivers were deployed near Teague Bay (n = 16) and Lang Bank (n = 4). Detection data for receivers outside of BIRNM were not available in May 2017. Numbers above the top panel (a) represent the number of fish averaged each month for all three panels
In total, 224 detections from six of the seven individual fish (range 3–91 detections) were recorded on receivers outside of Monument boundaries over the duration of the study (Table 1; see Additional files 5 and 6 for detailed detection data outside BIRNM). Fish were detected between 1 and 6 different days per month (mean: 2.11, median: 2) when they were detected outside BIRNM (see Additional file 7). Peak detections outside the MPA occurred March through June 2016 (45.5% of total detections) and began to peak again in March and April 2017 (32.6% of total detections; Fig. 2c).
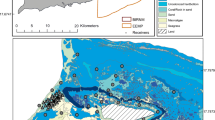
Observed network metrics for each individual fish were all significantly different than random (P < 0.001). Therefore, all networks were considered non-random and included in analyses. Individual spatial networks visually showed that C. latus frequently moved over large areas within the BIRNM array. However, different fish were detected more regularly over specific areas than others, indicating some level of inter-individual variation in space use (see Additional file 8 for all spatial plots). For example, three fish (Tags 17203, 23604, and 23608) showed a higher concentration of movement south of Buck Island while also using the deep-water receivers in the northeastern portion of the Monument (Tag 23608 shown in Fig. 3a). In contrast, movements of two fish (Tags 23601 and 23603) were concentrated in the northwestern side of Buck Island but also displayed occasional movements to the same deep-water receivers in the northeastern portion of the Monument (Tag 23603 shown in Fig. 3b).
Individual spatial graphs (a = 23608, b = 23603) showing connectivity and space use in BIRNM. The left panel show visited receivers (white dots with dark and light grey halos) with directed movement pathways (white lines). Receivers the fish did not visit, but had the potential to, are shown as the grey dots without a halo. The right column shows unipartite spatial plots of the same two fish (a = 23608, b = 23603). Grey lines connecting nodes are directed movement pathways. Note, this type of plot does not show the non-visited receivers. For both panels, dark nodes represent core use receivers (CURs) while light grey nodes are the remaining receivers each fish visited. Thickness of movement pathway lines is proportional to use frequency. Node size corresponds with amount of detections, with larger nodes indicating higher use. The red ‘x’s are the release locations
The number of CURs identified varied between individual C. latus (mean = 10.7, range = 6–14). One specific receiver was present as a CUR in all C. latus networks, although individuals shared between two and seven CURs (mean: 2.34, median: 2). There were some fish that had CURs exclusive in their own networks. Notably, release locations were near or surrounded by CURs in each individual spatial network.
Discussion
The seven C. latus used for analyses were detected on receivers across the entire spatial extent of the BIRNM array, with five fish displaying extensive use of the array and an ability to move over large distances. These fish demonstrated long-term fidelity to the BIRNM array, indicated by the lowest observed individual residency index of 0.75. When the expected maximum number of daily detections was compared to those observed, most individuals were not detected for much of the day (9.6% of expected detections were recorded). While missed detections result from smaller detection ranges for receivers in high-rugosity habitats [28], lack of receiver coverage in deep water and other environmental and study design effects, movement outside of BIRNM did occur and suggests fish were spending time outside the Monument (see Additional files 5, 6, 7). The dichotomy of high RI within the BIRNM array with low detections compared to what was expected, led to questions around the application of residency indices to inform spatial management and conservation approaches.
Characterizing residency index values for movement studies has become a common method used to assess the number of days an animal was detected (i.e., was present) within the acoustic receiver arrays. Many studies, regardless of the study animal, select a minimum of two detections per day to consider an animal present or ‘resident’ (e.g., [30, 37,38,39]). However, a residency index does not translate into amount of potential protection gained since an animal detected twice within minutes but then not detected again for long periods, could easily be resident to another undetected and potentially unprotected location [6]. We suggest that additional metrics, such as the O/E ratio or comparable metric, be included to infer residency and for determining species-specific differences in movement capabilities that directly influence conservation and management goals.
In the BIRNM array, two stationary sync tags had high O/E detection ratios (mean: 86.2%) for transmitters that were co-located (i.e., in close proximity) with a receiver. Determining temporal fluctuations in detection efficiency throughout BIRNM and amongst various depths and benthic habitat types would strengthen the interpretation of expected numbers of detections for all receivers in the array. By doing so, the estimated O/E detection ratios for animal tags could be refined across the study system and improve the accuracy of this metric in reflecting presence within the array as a probability of occurrence. Incorporating multiple metrics (RI and an O/E detection ratio or comparable metric) would help bridge the inference of residency to likelihood that a species occupied areas within the range of receivers over time. By providing O/E detection ratios, the residency and movements, the potential protection offered by MPAs can be assessed more completely.
Interpreting residency patterns relative to the level of protection achieved by MPAs can be enhanced by understanding detailed movement patterns, including those of other tagged species in the system. Using residency metrics and network analysis to visualize space use of C. latus revealed high individual residencies, low O/E detection ratios in the BIRNM array, and large core use areas compared with other co-occurring species (great barracuda Sphyraena barracuda: [6], yellowtail snapper Ocyurus chrysurus: [46]). For example, S. barracuda and O. chrysurus were rarely (only once for one O. chrysurus) detected on receivers outside of BIRNM over the same or longer time periods. Most C. latus were detected on receivers outside BIRNM, between one and six different days in any given month, demonstrating greater mobility that is affording individuals from different species varying levels of protection.
Although C. latus were present throughout the year in BIRNM, monthly fluctuations in residency and the O/E detection ratio were evident. Seasonal variation in abundance in another population of C. latus was documented through underwater visual observations off the coast of Belize, where fish exhibited peaks corresponding to spawning events in July and August along the shelf edge of the reef promontory and during April when courtship behavior was identified [40]. Additional receivers deployed outside of the BIRNM array (LB, EEMP, and Teague Bay) detected all but one fish, although time and individual use varied, and amount of detections were relatively low in comparison to the amount of detections recorded inside BIRNM. Detections outside of the array peaked around March and April in both years and suggest that boundary crossings and movement in general are more frequent during spring months. Therefore, it is possible that C. latus monitored in this study were exhibiting movements to courtship or spawning aggregation sites [40]. This interpretation is supported by the lowest monthly RI value in June 2016 and the lowest amount of detections recorded inside BIRNM during summer months, though this hypothesis warrants additional investigation given the limited number and distribution of receivers outside BIRNM.
The movement of most individuals outside BIRNM boundaries over time indicates that the current extent of the MPA does not envelop the entire space used by these fish. In some circumstances, large MPAs are not feasible so conservationists and managers look to protect small activity spaces important for spawning or other deliberate aggregations [41]. Based on CUR results from network analysis, it appears that core activity spaces of C. latus monitored were often close to capture (and release) locations and likely contained within BIRNM. However, due to the low number and distribution of receivers outside of BIRNM, core spaces might extend to areas with little to no receiver coverage. Some fish not included in analyses (4 of 11 tagged fish) may also have core spaces and entire home ranges outside the array, like barracuda [6]. Additional research is needed to refine activity space estimates, and to determine environmental drivers of movement outside BIRNM, which will clarify to what degree boundary crossings are being made. If C. latus or similar species were to require protection, additional regulations and more enforcement or protection during periods of substantial movement might be important conservation initiatives. Fish, such as C. latus, that form spawning aggregations outside protected areas are more vulnerable to overexploitation [42]. Future research should move towards developing quantitative comparative tools to determine occupancy inside and outside of MPAs and the optimal strategic array configuration to estimate exposure to fishing pressure. A balance between conservation and sustainable exploitation is needed to continue ensuring support for MPAs as a management tool [43].
Telemetry technology and the tools to quantify movement data are continuously improving and as a result there has been an upsurge in the number of studies highlighting species-specific movement patterns [44]. As additional data become available, better spatial management frameworks and designs can be applied with an ultimately higher chance of success in meeting their conservation goals [4]. As the integration of multi-species movements becomes increasingly beneficial in management decisions, the influence of geographic scale on the level of protection needs to be elucidated since it can play a role in structuring the ecosystem dynamics in and around MPAs. For example, individual S. barracuda have been observed establishing small core territories within BIRNM with high residencies, potentially attributed to increased protection from fishing pressure [6, 14]. Although the few C. latus monitored in this study had high site fidelity to the BIRNM array, they will not receive the same level of protection as S. barracuda that had no detections recorded outside BIRNM, due to recurrent transient movements between the different protected and non-protected areas.
Successful spatial management draws on the availability of movement data [4] and is especially critical when little ecological research has been conducted on a presumably high trophic niche predator. A growing number of studies highlight the importance of marine predators in shaping the foundation of coral reef ecosystems [2, 39, 45]. Given the mobility of C. latus and general fidelity to the BIRNM array, this species may play an integral role in influencing community structure and function, but may also act as a conduit of nutrient and energy transfer outside of the study area. Spatial distributions of marine predators and wide-ranging species can generally provide information necessary to ensure long-term protection of all species when using no-take MPA management approaches [39]. Since C. latus are a common reef fish throughout the Caribbean and likely exhibit similar movement patterns to other Carangids, specifics of their movement ecology and establishing a baseline understanding of spatial dynamics can aid in MPA management decisions. However, additional research on seasonal utilization patterns inferred with different residency metrics, specific delineation of habitat use, responses to abiotic conditions or extreme weather events, and lunar phase shifts in distribution might provide accompanying insights for improving management strategies of this species as well as other Carangids. Ultimately, research combining multiple movement studies on reef fish, sharks, sea turtles, and other organisms, all with unique residencies and movement patterns, will lead to a better understanding of the role MPAs play in conserving species and ecological communities.
Conclusions
Although this study has a small sample size of seven adult C. latus, it presents the most robust movement findings to date on this common Caribbean and greater Atlantic Ocean reef predator. Our observations substantiated the only previous telemetry study, with opportunistic data on one fish of comparable size, that showed high mobility and extensive use of the acoustic array [17]. Here, we were able to gain valuable insights on the movements of seven fish over an extended period (up to 17 months monitored), showing similar movement characteristics of high mobility and extensive use of the array. Simultaneous movement studies in BIRNM on S. barracuda and O. chrysurus documented little to no movement outside of the BIRNM array during the same time period [6, 46], thereby suggesting C. latus are a more transient species, perhaps occupying a role of both a resident and roaming predator.
High residency for most individual fish contrasted the periods of time C. latus were not being detected. Potential influences of missed detections could be attributed to receiver configuration or environmental variables affecting detection efficiency, rather than fish moving outside of BIRNM, resulting in some uncertainty in interpreting detections. Reconciling lack of detections and array configuration should be a priority consideration for all future telemetry studies. Yet, receivers located outside the BIRNM array showed frequent visitation of tagged C. latus suggesting that a portion of missed daily detections are attributable to movement outside of BIRNM. The longer and more consistent residency patterns of other species in BIRNM support this conclusion.
General conclusions were made regarding the spatial protection afforded by an MPA to a relatively mobile fish, with core activity spaces of C. latus mostly contained within BIRNM boundaries, but consistent movement to areas with seasonal or little protection. Providing multiple residency metrics and illuminating connectivity pathways of movements in relation to management boundaries are important considerations when developing effective spatial management plans. The formalization of how we assess residency based on species, array configuration, and ecosystem composition should be key considerations during the development phase with specific attention to how the data will be used to inform management decisions.
Availability of data and materials
The datasets used and/or analyzed during the current study or associated R code are available from the corresponding author on reasonable request.
Abbreviations
- MPA:
-
Marine protected area
- BIRNM:
-
Buck Island Reef National Monument
- NPS:
-
National Park Service
- EEMP:
-
East End Marine Park
- LB:
-
Lang Bank
- RI:
-
Residency index
- O/E:
-
Observed to expected detection ratio
- NA:
-
Network analysis
- CUR:
-
Core use receiver
References
Lundberg J, Moberg F. Mobile link organisms and ecosystem functioning: implications for ecosystem resilience and management. Ecosystems. 2003;6:87–98.
Papastamatiou YP, Meyer CG, Kosaki RK, Wallsgrove NJ, Popp BN. Movements and foraging of predators associated with mesophotic coral reefs and their potential for linking ecological habitats. Mar Ecol Prog Ser. 2015;521:155–70.
Murawski SA, Wigley SE, Fogarty MJ, Rago PJ, Mountain DG. Effort distribution and catch patterns adjacent to temperate MPAs. ICES J Mar Sci. 2005;62:1150–67.
Lea JSE, Humphries NE, von Brandis RG, Clarke CR, Sims DW. Acoustic telemetry and network analysis reveal the space use of multiple reef predators and enhance marine protected area design. Proc R Soc B. 2016;283:20160717.
O’Toole AC, Danylchuk AJ, Goldberg TL, Suski CD, Philipp DP, Brooks E, Cooke SJ. Spatial ecology and residency patterns of adult great barracuda (Sphyraena barracuda) in coastal waters of The Bahamas. Mar Biol. 2011;158:2227–37.
Becker SL, Finn JT, Novak AJ, Danylchuk AJ, Pollock CJ, Hillis-Starr Z, Lundgren I, Jordaan A. Coarse- and fine-scale acoustic telemetry elucidates movement patterns and temporal variability in individual territories for a key coastal mesopredator. Env Biol Fish. 2020;103:13–29.
Nemeth RS. Population characteristics of a recovering US Virgin Islands red hind spawning aggregation following protection. Mar Ecol Prog Ser. 2005;286:81–97.
Nemeth RS, Blondeau J, Herzlieb S, Kadison E. Spatial and temporal patterns of movement and migration at spawning aggregations of red hind, Epinephelus guttatus, in the U.S. Virgin Islands. Environ Biol Fish. 2007;78:365–81.
TinHan T, Erisman B, Aburto-Oropeza O, Weaver A, Vázquez-Arce D, Lowe CG. Residency and seasonal movements in Lutjanus argentiventris and Mycteroperca rosacea at Los Islotes Reserve, Gulf of California. Mar Ecol Prog Ser. 2014;501:191–206.
McCormick SD, Shrimpton JM, Zydlewski JD. Temperature effects on osmoregulatory physiology of juvenile anadromous fish. In: Wood CM, McDonald DG, editors. Global warming: implications for freshwater and marine fish. Cambridge: Cambridge University Press; 1997. p. 279–301.
Knip DM, Heupel MR, Simpfendorfer CA, Tobin AJ, Moloney J. Ontogenetic shifts in movement and habitat use of juvenile pigeye sharks Carcharhinus amboinensis in a tropical nearshore region. Mar Ecol Prog Ser. 2011;425:233–46.
Currey LM, Heupel MR, Simpfendorfer CA, Williams AJ. Sedentary or mobile? Variability in space and depth use of an exploited coral reef fish. Mar Biol. 2014;161:2155–66.
Lédée EJI, Heupel MR, Tobin AJ, Knip DM, Simpfendorfer CA. A comparison between traditional kernel-based methods and network analysis: an example from two nearshore shark species. Animal Behav. 2015;103:17–28.
Becker SL, Finn JT, Danylchuk AJ, Pollock CG, Hillis-Starr Z, Lundgren I, Jordaan A. Influence of detection history and analytic tools on quantifying spatial ecology of a predatory fish in a marine protected area. Mar Ecol Prog Ser. 2016;562:147–61.
Jacoby DMP, Freeman R. Emerging network-based tools in movement ecology. Trends Ecol Evolut. 2016;31:301–14.
Griffin LP, Brownscombe JW, Adams AJ, Boucek RE, Finn JT, Heithaus MR, Rehage JS, Cooke SJ, Danylchuk AJ. Keeping up with the Silver King: using cooperative acoustic telemetry networks to quantify the movements of Atlantic tarpon (Megalops atlanticus) in the coastal waters of the southeastern United States. Fish Res. 2018;205:65–76.
Farmer NA, Ault JS. Accounting for detection gaps when evaluating reef fish habitat use in an acoustic array. Can J Fish Aquat Sci. 2018;75:375–88.
Berry FH, Smith-Vaniz WF. Carangidae. In: Fischer W, Bianchi G, Scott WB, editors. FAO species identification sheets for fishery purposes. Eastern central Atlantic; fishing areas 34, 47 (in part), vol. 1. Rome: FAO; 1981. p. 215–96.
Vernoux J, Lewis RJ. Isolation and characterization of Caribbean ciguatoxins from the horse-eye jack (Caranx latus). Toxicon. 1997;35:889–900.
Rombenso AN, Bowzer JC, Moreira CB, Sampaio LA. Culture of Caranx species [Horse-eye Jack Caranx latus (Agassiz), Blue Runner Caranx crysos (Mitchill), and Crevalle Jack Caranx hippos (Linnaeus)] in near-shore cages off the Brazilian coast during colder months. Aquac Res. 2014;47:1–4.
Smith-Vaniz WF, Chiappa Carrara X, Robins RH, Munroe T, Abad-Uribarren A. Caranx latus. The IUCN Red List of Threatened Species. 2015;e.T191829A78743665. Downloaded on 12 November 2019.
Silvano R. Feeding and interspecific feeding associations of Caranx latus in a subtropical reef. Environ Biol Fish. 2001;60(4):465–70.
Randall JE. Tagging reef fishes in the Virgin Islands. Proc Gulf Caribb Fish Inst. 1962;14:201–41.
Chapman MR, Kramer DL. Movements of fishes within and among fringing coral reefs in Barbados. Environ Biol Fish. 2000;57:11–24.
Pittman SJ, Hile SD, Jeffrey CFG, Caldow C, Kendall MS, Monaco ME, Hillis-Starr Z. Fish assemblages and benthic habitats of Buck Island Reef National Monument (St Croix, US Virgin Islands) and the surrounding seascape: a characterization of spatial and temporal patterns. Silver Spring: NOAA Technical Memorandum NOS NCCOS 71; 2008.
Pittman SJ, Dorfman DS, Hile SD, Jeffrey CFG, Edwards MA, Caldow C. Land-sea characterization of the St. Croix East End Marine Park, U.S. Virgin Islands. Silver Spring: NOAA Technical Memorandum NOS NCCOS 170; 2013.
Schärer-Umpierre MT, Mateos-Molina D, Appledoorn R, Bejarano I, Hernández-Delgado EA, Nemeth R, Nemeth MI, Valdés-Pizzini M, Smith TB. Marine managed areas and associated fisheries in the US Caribbean, vol 69. In: Johnson ML, Sandell J, editors. Advances in marine biology. Oxford: Academic Press; 2014. p. 129–52.
Selby TH, Hart KM, Fujisaki I, Smith BJ, Pollock CJ, Hillis-Starr Z, Lundgren I, Oli MK. Can you hear me now? Range-testing a submerged passive acoustic receiver array in a Caribbean coral reef habitat. Ecol Evol. 2016;6:4823–35.
Afonso P, Abecasis D, Santos RS, Fontes J. Contrasting movements and residency of two serranids in a small Macaronesian MPA. Fish Res. 2016;177:59–70.
Lédée EJI, Heupel MR, Tobin AJ, Simpfendorfer CA. Movements and space use of giant trevally in coral reef habitats and the importance of environmental drivers. Anim Biotelemetry. 2015;3:6.
Finn JT, Brownscombe JW, Haak CR, Cooke SJ, Cormier R, Gagne T, Danylchuk AJ. Applying network methods to acoustic telemetry data: modeling the movements of tropical marine fish. Ecol Mod. 2014;293:139–49.
Csardi G, Nepusz T. The igraph software package for complex network research. Inter Journal Complex Syst. 2006;1695:1–9.
Schloerke B, Crowley J, Cook D, Hofmann H, Wickham H, Briatte F, Marbach M, Thoen E. GGally: extension to ggplot2. 2014. R package version 1.3.2. https://CRAN.R-project.org/package-GGally.
Kahle D, Wickham H. ggmap: spatial Visualization with ggplot2. R J. 2013;5:144–61.
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2017. http://www.R-project.org/.
Munro JL. Caribbean coral reef fishery resources. 2nd ed. Manila: ICLARM Studies and Reviews; 1983. p. 1–276.
Espinoza M, Lédée EJI, Simpfendorfer CA, Tobin AJ, Heupel MR. Contrasting movements and connectivity of reef-associated sharks using acoustic telemetry: implications for management. Ecol Appl. 2015;25:2101–18.
Wolfe BW, Lowe CG. Movement patterns, habitat use and site fidelity of the white croaker (Genyonemus lineatus) in the Palos Verdes Superfund Site, Los Angeles, California. Mar Environ Res. 2015;109:69–80.
Filous A, Friedlander A, Wolfe B, Stamoulis K, Scherrer S, Wong A, Stone K, Sparks R. Movement patterns of reef predators in a small isolated marine protected area with implications for resource management. Mar Biol. 2017;164:1–16.
Heyman WD, Kjerfve B. Characterization of transient multi-specie reef fish spawning aggregations at Gladden Spit, Belize. Bull Mar Sci. 2008;83:531–51.
Gell F, Roberts C. Benefits beyond boundaries: the fishery effects of marine reserves. Trends Ecol Evolut. 2003;18:448–55.
Grüss A, Robinson J, Heppell SS, Heppell SA, Semmens BX. Conservation and fisheries effects of spawning aggregation marine protected areas: what we know, where we should go, and what we need to get there. ICES J Mar Sci. 2014;71(7):1515–34.
Meyer CG, Holland KN, Papastamatiou YP. Seasonal and diel movements of giant trevally Caranx ignobilis at remote Hawaiian atolls: implications for the design of marine protected areas. Mar Ecol Prog Ser. 2007;333:13–25.
Hussey NE, Kessel ST, Aarestrup K, Cooke SJ, Cowley PD, Fisk AT, Harcourt RG, Holland KN, Iverson SJ, Kocik JF, Mills Fleming JE, Whoriskey FG. Aquatic animal telemetry: a panoramic window into the underwater world. Science. 2015. https://doi.org/10.1126/science.1255642.
Harborne AR, Selwyn JD, Lawson JM, Gallo M. Environmental drivers of diurnal visits by transient predatory fishes to Caribbean patch reefs. J Fish Biol. 2017;90:265–82.
Novak AJ, Becker SL, Finn JT, Pollock CJ, Hillis-Starr Z, Jordaan A. Scale of biotelemetry data influences ecological interpretations of space and habitat use in yellowtail snapper. Mar Coast Fish. 2020. https://doi.org/10.1002/mcf2.10119.
Acknowledgements
We thank current and former NPS employees (Ian Lundgren, Tessa Code, and Nathaniel Hanna Holloway), collaborators, and the many volunteers for supporting the fieldwork portion of this project and for downloading and managing the acoustic array in St. Croix. This work would not have been possible without the collaboration and contributions from the USCAN group. Lastly, we would like to thank the Jordaan Lab at the University of Massachusetts Amherst and two anonymous reviewers for the many thoughtful comments to earlier versions of this manuscript.
Funding
Funding for this research was provided by the National Park Service, Puerto Rico Sea Grant (Project Number: R-101-2-14), and University of Massachusetts Amherst.
Author information
Authors and Affiliations
Contributions
AJN, SLB, CGP, AJD, and AJ conceived the ideas and designed methodology; AJN, SLB, CGP, ZH, and AJ collected the data; AJN and JTF analyzed the data; AJN and AJ led the writing of the manuscript. All authors contributed to the drafts and gave final approval for publication. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All capture and tagging methods were approved under University of Massachusetts Amherst’s IACUC no. 2013-0031. Research conducted within BIRNM was approved by NPS under study no. BUIS-00058 and individual research collection permit nos. BUIS-2013-SCI_0003 and BUIS-2014-SCI-0006.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Tagging and detection information for the four individual C. latus that were not included in analyses.
Additional file 2.
Spatial plots for the 4 horse-eye jack Caranx latus (Tags 19663, 23591, 23592, and 45878) that were not used in analyses due to limited spatial inference, low detection histories, and were only present in the array for a relatively short period of time (see Additional files 1 and 3 for tagging/detection details). Note that CUR assignment was not done for these fish. Red ‘x’ marks release location.
Additional file 3.
Detection histories for all C. latus tagged within the BIRNM acoustic receiver array. Asterisks denote the four fish with insufficient detection histories that were excluded from all analyses.
Additional file 4.
Delta time (Δt) represents the time between a recorded detection and the next recorded detection for each individual fish only on BIRNM receivers. For example, Tag 23601 had a max Δt of 14.3 days, meaning that the maximum amount of time between two successive detections was over two-weeks from one detection to the next. Average Δt is the mean time in minutes between successive detections.
Additional file 5.
Total number of individual fish detections recorded on receivers (n = 20) outside of Buck Island Reef National Monument (BIRNM) from January 2016 to May 2017. There were no detections recorded in January 2016 and detection data for receivers outside BIRNM were not available for May 2017. Note, Tag 19678 did not have any detections recorded on receivers outside of the Monument.
Additional file 6.
Total number of C. latus (n = 6, no outside detections from Tag 19678) detections recorded on receivers outside of Buck Island Reef National Monument (BIRNM) either in East End Marine Park (EEMP), Lang Bank (LB), or Teague Bay (TB) from January 2016 to May 2017. Note, there were no detections recorded in January 2016 and detection data was not available for May 2017.
Additional file 7.
Number of days in each month when individual C. latus were detected on either East End Marine Park (EEMP), Lang Bank (LB), or Teague Bay (TB) receivers. For example, in June 2016, Tag 23603 was detected on outside receivers on 6 different days within the month. Dashed lines indicate the tag had not been deployed yet. Note, Tag 19678 did not have any detections recorded on receivers outside of the Monument
Additional file 8.
Individual spatial graphs of all seven tagged horse-eye jack C. latus used for analyses. Dark nodes represent core use receivers (CURs) while light grey nodes are the remaining receivers each fish visited. Note, this type of plot does not show the non-visited receivers. Grey lines connecting nodes are directed movement pathways and thickness of lines is proportional to frequency of use. Node size corresponds with amount of detections, with larger nodes indicating higher use. The red ‘x’ in each graph is the release location. Spatial graphs for Tags 23603 and 23608 were selected as examples for Fig. 3 in the article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Novak, A.J., Becker, S.L., Finn, J.T. et al. Inferring residency and movement patterns of horse-eye jack Caranx latus in relation to a Caribbean marine protected area acoustic telemetry array. Anim Biotelemetry 8, 12 (2020). https://doi.org/10.1186/s40317-020-00199-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40317-020-00199-8