Abstract
Background
Ocean water temperature is changing as a result of anthropogenic influences on the marine environment. Highly mobile marine ectotherms, such as sea turtles, may be particularly susceptible to these changes. However, our current understanding of location-specific thermal tolerances, especially at coastal foraging or over-wintering areas, is limited. Human-induced changes, such as thermal effluent from power plants, appear to have a suite of influences on species that reside in affected areas. Here, we describe a study of green turtle habitat use related to changing water temperature at a coastal foraging site that has recently experienced a power plant closure, leading to a transition to cooler ambient water temperature. We used a combination of active and passive acoustic telemetry to monitor green turtle distribution in relation to water temperature in this dynamic thermal environment.
Results
Both before and after closure of the power plant, turtles were distributed in significantly warmer waters than surrounding environments during winter months (December–February). Turtles in winter were rarely detected in water temperatures lower than 14.5 °C. Body size was negatively correlated with water temperature after closure of the power plant, with larger turtles found in cooler waters, while smaller turtles remained within warmer areas. There was not a significant relationship between body size and water temperature before closure of the power plant as water temperature was more constant during operation.
Conclusions
Green turtles in San Diego Bay experienced a shift in water temperature following the loss of thermal effluent from a power plant. The effects of this shift were particularly evident during winter months, when ambient water temperatures were coolest. Water temperatures in the southern region of San Diego Bay were significantly warmer during winter before the closure of the power plant, and turtles were detected in significantly warmer water. Turtles in San Diego Bay may associate with or seek out thermal refugia, when possible, to avoid low water temperatures. The cold water temperature inactivity threshold for East Pacific green turtles may be lower than previously thought. There was a significant negative relationship between turtle size and water temperature after power plant closure. East Pacific green turtles exhibit clear responses in habitat use to changes in water temperature at a foraging site near the edge of their geographic range.
Similar content being viewed by others

Background
Globally and locally, water temperatures are changing at unprecedented rates and these changes are likely already impacting animals [6, 20, 42]. Although considerable uncertainty remains as to how exactly marine ecosystems will be affected by global temperature change [9], understanding how marine ectotherms respond to thermal dynamics is a necessary element for effective conservation and management. Marine ectotherms reliant on particular thermal conditions for proper physiological and behavioral functions are particularly susceptible to changes in environmental temperature.
Marine turtles represent one such group of marine ectotherms whose relationship with water temperature has been particularly well studied. Environmental temperature directly affects rates of marine turtle metabolism and other physiological processes, i.e., circulation, respiration, feeding and digestion, osmoregulation and pH balance [17, 19, 33]. As environmental temperature increases, and in turn body temperature, so does the rate of these bodily processes [17, 33]. Likewise, metabolic and physiological processes of marine turtles, like other ectotherms, slow at lower environmental temperatures [52, 59]. For adult marine turtles at foraging habitats, water temperature is a key environmental factor that influences behavior. Marine turtles have been found to decrease activity levels and become torpid in response to water temperatures below 15 °C as a means to direct energy to basic physiological maintenance; metabolic rates, heart rate and respiration rates are all lower in cooler water temperatures [18, 46, 48].
For marine turtles, temperature changes will likely affect local and migratory movement, as well as the availability of adequate resources at breeding and foraging areas [16, 20, 42]. Marine turtles may be able to contend with changes in the thermal environment by expanding their ranges and/or utilizing thermal refugia. However, new areas may not have the appropriate food resources or the environmental conditions previous habitats offered. Access to thermal refugia is likely most important for marine megafauna at the boundaries of their range [22, 50] where they must contend with thermal conditions outside of their preferred thermal optima [30]. As a result, these populations may be able to tolerate greater variability in water temperature and/or may acclimate to variability by changing their behavior.
Size also plays an important role in distribution related to water temperature. Larger organisms maintain a higher degree of thermal inertia and are more equipped to contend with cooler water temperatures due to their reduced surface area to volume ratio. Because of their large body mass marine turtles are able to maintain stable core body temperature through metabolic heat production [5, 38, 52]. This thermal inertia allows diving ectotherms to maintain internal body temperature warmer than the external environment for extended periods of time—further increasing dive duration and/or depth [33, 37, 45]. However, thermal inertia is ineffective at maintaining core body temperature even in larger turtles after prolonged exposure to water temperatures below 8–10 °C [35, 41, 61].
The general thermal ecology of marine turtles has been fairly well described. Species-specific temperature preferences have been found in both pelagic and coastal waters [14, 15, 46, 47, 50–52]. Marine turtle species experience different thermal conditions across their ranges; hence, understanding location-specific tolerance is crucial. Aggregations in temperate marine ecoregions [49], especially near the thermal extremes of a given species, are more susceptible to extreme water temperatures approaching potentially lethal levels, i.e., 8–10 °C [4].
One major anthropogenic influence on local marine environments is thermal effluent from power plants, which use once-through cooling (OTC) systems. OTC power stations discard waste heat, a by-product of the plant cooling process, into nearby aquatic environments, thus altering the thermal conditions of the environment. These plants are most commonly found in temperate climates, where ambient environmental water temperature is low enough to be utilized for the cooling process [23, 28]. Average water temperature discharged from OTC power stations between 1996 and 2005 was 37 °C (±6.5–6.8 °C) and 9.5–10 °C (±4.8–5.0 °C) higher than ambient temperatures in summer, when ambient temperatures are highest [28]. Since the early 1970s, studies of mobile aquatic organisms have demonstrated physiological and behavioral changes across taxa in response to the heated effluent from power plants [12, 50, 55, 57]. Marine turtle species in Brazil, Chile and the USA have also demonstrated high aggregations in the thermal effluent areas of industrial plants [10, 11, 57, 7, 13, 43, 56].
Understanding the thermal ecology of marine turtles in the natural environment is critical to the management of these species. Although considerable work has been done in the laboratory studying responses by marine turtles to temperature change [36, 39, 52, 59], the results of these studies may have limited application to how ectothermic vertebrates contend with shifts in environmental temperature in situ, where other environmental variables are also factors [2]. Because of the shifts away from OTC power plants in the USA [8], these plant closures have provided an opportunity to study how marine ectotherms such as marine turtles respond behaviorally to shifts in environmental temperature.
We used acoustic telemetry to monitor the response of green turtles (Chelonia mydas) to temperature in the northern extreme of their foraging aggregation [10, 11]. The behavior and movements of green turtles were described before and after closure of an OTC fossil-fuel power plant. We also explore how this relationship varies with turtle size. Although local and global scale changes may be driven by different factors, changes to the thermal environment at the local level provide insights as to how animals may react to longer-term changes in global environmental temperature.
Methods
Study site
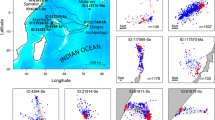
San Diego Bay (SDB) is a narrow, 22.5-km-long natural harbor near the US–Mexico border along the west coast of the USA that is the terminus of three watersheds encompassing over 660 km2 (Fig. 1). SDB is bordered by several municipalities, including San Diego (population: 1.3 million), Chula Vista (population: 257,000), National City (population: 60,000) and Coronado (population: 24,000). The area affected by thermal effluent from the South Bay Power Plant (SBPP; Dynegy, Inc.) was located in the southern section of SDB, known as South Bay, in the 2300-acre San Diego Bay National Wildlife Refuge [58]. South Bay is designated as the region south of the Sweetwater Channel in San Diego Bay (Fig. 1). The SBPP was in operation from the 1960s until its full decommissioning on December 31, 2010. A foraging aggregation of green turtles has been documented in SDB since the 1800s, with ongoing research on these turtles beginning in the 1970s [32, 53]. From the 1960s, green turtles in SDB were almost exclusively observed in South Bay, near the power plant effluent area and adjacent eelgrass beds, and this area is recognized as the home range for this aggregation (Fig. 1; [10, 11, 26, 27, 31, 53]).
San Diego Bay is located near the border of the USA and Mexico, in Southern California (inset). Green turtles in San Diego Bay primarily inhabit the southern portion of the Bay–South Bay [34]
Turtle capture
Turtles were captured in years 2009–2012 during months November–May (before closure; 2009–2010) and December–June (after closure; 2011–2012) in the effluent and intake areas of the SBPP following protocol outlined in Eguchi et al. [10] and Lemons et al. [24]. Sampling methods were adjusted slightly and extended until June after the closure of the SBPP.
Entanglement nets (100 m long by 5 m deep, mesh size 0.6 m stretched) were placed in the water and were continuously monitored from land. Nets were physically checked for entanglement at 30-min intervals or more frequently when turtles or other organisms were observed in the net, following established protocols for turtle capture in this area [10, 11, 26, 27]. Nets were placed in the effluent area, the intake channel and open-water areas adjacent to the SBPP (Fig. 1). Upon capture, sex of adult turtles was determined, if possible, based on the presence or absence of male secondary sexual characteristics: elongated tail extending beyond the carapace edge, curvature of the front claws and softened plastron [60]. Life stage of turtle was categorized as juvenile (juvenile and subadult) or adult, based on straight carapace length (SCL): juveniles and subadults <80 cm and adults ≥80 cm [1, 25]. Turtles were weighed, tagged, fitted with telemetry devices and released back into San Diego Bay near the site of capture within 60 min.
Acoustic telemetry
Both active (via boat-based survey) and passive (via underwater receiver stations) acoustic telemetry were used to monitor green turtle movement. Tagged turtles were equipped with an ultrasonic transmitter (Sonotronics, Inc., Tucson, Arizona, CT Series). To reduce hydrodynamic drag [21], transmitters were affixed to the rearmost lateral scute of each turtle using fiberglass mesh and resin, following a modified procedure from Balazs et al. [3]. Each transmitter had a unique combination of frequency, pulse burst and pulse burst interval. Active tracking was conducted using an omnidirectional hydrophone (Sonotronics, TH-2), a directional hydrophone (Sonotronics, DH-4) and an ultrasonic receiver (Sonotronics, USR-08) from aboard a 17-ft Boston Whaler 170 Montauk (“Wanda”) with an 85 horsepower outboard motor. Submersible ultrasonic receivers (Sonotronics, SUR) were used for passive tracking.
A turtle detection was defined as any location where an acoustic receiver (either active or passive) positively detected a tagged turtle. Data were collected actively via grid surveys and passively via SUR stations, each of which represented different spatial and temporal scales (Fig. 2). The combination of active and passive telemetry allowed for near-constant monitoring of turtle movement across the home range of this population.
Active monitoring
Active tracking of turtles occurred via a semi-monthly boat-based survey across SDB. The range of frequencies for all tagged turtles (35–40 kHz) was scanned via hydrophone and receiver using a 500-m latitude/longitude grid and covering the expanse of SDB (Fig. 2a). When a tagged turtle was detected, a directional hydrophone was employed to exact a more accurate location of the turtle. A turtle was considered in close proximity (approximately 10 m) when the acoustic signal could be heard loudly at the receiver’s lowest gain setting, or if the turtle was sighted [26, 27]. While actively tracking, water temperature was monitored using a handheld multiparameter instrument calibrated using ice water (556MPS, YSI Incorporated, Yellow Springs, Ohio) and data were collected at each grid point on every survey, regardless of whether a turtle was detected.
Passive monitoring
For passive tracking, turtles were detected by autonomous ultrasonic receivers (SURs). The range of detection for the SURs was up to approximately 100 m, based on range tests from a previous study in SDB [27]. SURs (n = 10) were deployed throughout South Bay in SDB: the intake channel, effluent area and eelgrass pastures (Fig. 1). An additional set of SURs (n = 5) were deployed following closure of the SBPP to extend the spatial range of possible turtle detection beyond the vicinity of the SBPP. The SURs were deployed in South Bay, as in previous studies [26, 27], with the addition of several SURs in central and northerly locations in SDB (Fig. 2b). When a tagged turtle was within the detection range of the SUR, the date and time were recorded. Attached to each SUR was a temperature logger (HOBO Water Temperature Pro v2, Onset Computer Corporation, Bourne, MA), which monitored water temperature at intervals of 3–5 min, depending on site; temperatures were recorded more frequently (3-min intervals) in the effluent area of the SBPP to capture changes in temperature dependent on plant operation. These data permitted water temperature to be matched with turtle detections at SUR locations, as well as creating a thermal profile at each site.
Analyses
Water temperature
To determine whether turtle behavior in winter months changed in response to a decrease in water temperature, we use data on ambient water temperature and turtle distribution. We used a t test to compare changes in the mean ambient water temperature before (2009–2010) to after (2011–2012) the plant closure during winter months (December–February), when ambient water temperature was coldest. We used ArcGIS 10.3 (ESRI, Inc., Redlands, CA) to map water temperatures in south San Diego Bay before and after SBPP closure. Kriging was used to interpolate among data to create an estimated surface of winter water temperature.
Turtle detections and associated thermal conditions
Turtle detections and water temperature collected during winter months were compared before and after the SBPP decommissioning. A binary logistic regression was used to determine the relationships between turtle detection/non-detection and potential predictors: SBPP operational status (before/after), water temperature and the interaction between them.
Association between size and thermal conditions
We used a generalized linear model (GLM) to determine whether there was a relationship between continuous variables of turtle size (weight) and water temperature (at detection), before SBPP closure and after SBPP closure. Turtle size data were collected for 21 individual turtles before SBPP closure and 26 individual turtles after SBPP closure.
Statistical analyses were performed using SYSTAT 13 [54], SAS software [44] and R [40] and evaluated at significance levels of alpha = 0.05. Mean values and standard errors (±SE) are reported.
Results
Tagged turtles
A total of 35 individuals were tagged and tracked during this study. Of these, 9 turtles were monitored only before the SBPP closure, 14 turtles were monitored only after the SBPP closure, and 12 turtles were monitored both before and after the SBPP closure (Fig. 3). The study group was comprised of 14 female turtles, 13 male turtles and 8 turtles that were juvenile/unknown sex. During SBPP operation, turtles aggregated in the effluent area (outfall) and nets were almost exclusively placed in this channel. Following the SBPP closure, turtle capture in the outfall area was inconsistent as turtles ceased to aggregate in the absence of the thermal effluent. Consistent successful turtle captures occurred almost exclusively in the intake channel following SBPP closure. Tag retention varied among individuals with a minimum of 1 day and a maximum of 396 days. Weights of tagged turtles ranged from 18 to 192 kg; when an individual was captured more than once, the weights were averaged across the study period (Fig. 3).
Water temperature
Average water temperatures in winter months were not significantly different between 2009 and 2010 (before SBPP closure) and were not significantly different between 2011 and 2012 (after SBPP closure). Therefore, we grouped temperature based on SBPP operational status: before (2009, 2010) and after (2011, 2012). Both before and after SBPP closure, the warmest water temperatures in South Bay were recorded in the effluent area (Fig. 4). Mean winter water temperature in the effluent area of the SBPP was significantly colder after closure of the SBPP (t = −8.945, df = 976, p < 0.001). Mean winter water temperature during SBPP operation was 17.8 °C (±1.9 SE), and mean winter water temperature after SBPP closure was 16.6 °C (±2.1 SE).
Turtle detections and associated thermal conditions
Turtles in this study were detected exclusively in the South Bay region of SDB: in and around the SBPP intake channel, effluent area and adjacent eelgrass beds (Fig. 1). Results from the binary logistic regression revealed that water temperatures at the locations of turtle detections were significantly warmer than where turtles were not detected, both before (Z = 2.569, p = 0.010) and after SBPP closure (Z = 5.477, p < 0.001). Before SBPP closure, the mean water temperature where turtles were detected was 18.0 °C (±0.1 SE) with a range of 14.5–25.6 °C (Fig. 5a). Mean water temperature was 17.4 °C (±0.2) at locations where turtles were not detected, with a range of 11.3–29.1 °C (Fig. 5a). After SBPP closure, the mean water temperature at turtle detections was 17.0 °C (±0.1 SE) with a range of 12.5–22.4 °C (Fig. 5b). Mean water temperature was 15.9 °C (±0.2) with a range of 11.54–21.86 °C at locations where turtles were not detected (Fig. 5b).
Association between size class and thermal conditions
The GLM revealed no significant relationship between mean water temperature at turtle detection locations and turtle size before SBPP closure (F = 0.324; df = 1; p = 0.569; y = −0.003x + 20.134). However, following the closure of the SBPP turtle size had a significant negative relationship with mean water temperature. Larger turtles were found in the coldest waters and smaller turtles in the warmest waters (F = 4.858; df = 1; p = 0.028; y = −0.011x + 17.934).
Discussion
In San Diego Bay, we found that green turtles responded to shifts in water temperature by inhabiting warmer than average areas in winter months. Water temperatures in San Diego Bay during winter frequently dropped to levels that are physiologically challenging for green turtles; however, tagged turtles were not detected in water temperatures <12.5 °C. This temperature is lower than that suggested for green turtle survival, as Seminoff [46] recorded an approximate minimum water temperature at detection of about 15 °C of green turtles in the nearby Gulf of California.
Turtles in San Diego Bay utilized the thermal effluent from the SBPP as a means of thermal refuge when water temperatures were at or below the inactivity threshold for this population. Turtles aggregated in the effluent area when water temperatures were cooler (at night; in winter) and then would move out into adjacent eelgrass pastures for foraging during the day and in summer [27]. However, after the SBPP closure, resident turtles were still able to find pockets of warm water, despite the loss of thermal effluent. Although not considered here, it is also possible that turtles in SDB and other foraging sites altered their activity levels as well as their spatial distribution (i.e., changing patterns of resting or diving) in response to temperature shifts [29].
Green turtles in San Diego Bay are some of the largest on record in the Eastern Pacific Ocean, potentially an indirect result of the year-round warmer water temperatures during SBPP operation; turtles were able to forage year-round instead of entering torpor as is typical in other foraging populations [10, 11]. Prior to the closure of the SBPP, turtles of all sizes were distributed in similar average water temperatures. However, after closure of the SBPP, we found a negative relationship between sea turtle size and water temperature.
Conclusions
Understanding the relationship of marine megafauna and water temperatures is critical given the ongoing and rapid shifts in local and global thermal conditions [30]. These environmental changes will continue to impact resident and migratory animals in coastal environments [6]. Because logistical constraints make monitoring highly mobile species with complex life histories problematic, biotelemetry plays an important role in characterizing marine species responses to changing thermal conditions in the ocean. Human-induced changes to water temperature at the local level provide a relevant, model system to monitor behavioral and physiological responses to temperature shifts that can help predict likely responses to marine temperature shifts for long-lived, highly mobile marine megafauna.
Green turtle presence in San Diego Bay preceded the operation of the SBPP [53], and our research demonstrates the turtles’ continued use of this foraging area even with the decrease in winter water temperatures. The habitat use of turtles was affected by the power plant operation with turtles aggregated in the effluent area. Following the plant decommissioning, we observed a shift in habitat use in response to the loss of warm-water effluent. Our findings suggest that foraging turtles can identify and associate with thermal refugia and that these areas may be outside of the former local home range for this aggregation [26]. MacDonald et al. [26] found that green turtle home range in San Diego Bay was limited to waters within the San Diego Bay National Wildlife Refuge, where turtles were afforded protection in the form of limited boat traffic and speed restrictions [58]. Turtles venturing northerly in San Diego Bay to associate with possible thermal refugia will encounter greatly increased commercial and recreational boating activity from the US Navy, cruise ships, cargo ships and recreational users. Continued monitoring of movement and habitat use of this aggregation is necessary to describe how foraging turtles, like those found in San Diego Bay, will continue to respond to thermally dynamic environments and other direct human impacts (e.g., boat strikes, debris entanglement, fisheries bycatch and contamination). Conservation and management of this foraging aggregation will be critical, especially as the ecosystem in San Diego Bay recovers in the years following the power plant closure.
Other studies have demonstrated similar aggregations of marine turtles in the thermal effluent of power plants that utilize once-through cooling systems [10, 11, 57, 7, 13, 43, 56]. However, to the best of our knowledge, this study is the first to document turtle response during both power plant operation and in the time immediately following plant decommissioning. As energy needs continue to change and new technology eliminates the need for once-through cooling systems [8], these power plant closures will become increasingly common. Power plant closures provide an experimental in situ opportunity to explore the response of a large vertebrate marine species to changing thermal conditions. In light of current and future changes to the thermal environment from anthropogenic influences, behavioral plasticity of coastal organisms will play an important role in whether these organisms can acclimatize to increased thermal variability. Characterizing the responses of coastal fauna to rapid shifts in thermal conditions addresses a gap in ecological knowledge—understanding how populations of long-lived marine vertebrates will be affected by a thermally dynamic environment that is changing at a rapid rate.
Abbreviations
- GLM:
-
generalized linear model
- OTC:
-
once-through cooling
- SBPP:
-
South Bay Power Plant
- SCL:
-
straight carapace length
- SDB:
-
San Diego Bay
- SUR:
-
submersible ultrasonic receiver
References
Amorocho DF, Reina RD. Feeding ecology of the east Pacific green sea turtle Chelonia mydas agassizii at Gorgona National Park, Colombia. Endanger Species Res. 2007;3:43–51.
Angilleta MJ Jr, Steury TD, Sears MW. Temperature, growth rate, and body size in ectotherms: fitting pieces of a life-history puzzle. Integr Comp Biol. 2004;44:498–509.
Balazs GH, Miya RK, Beavers SC. Procedures to attach a satellite transmitter to the carapace of an adult green turtle, Chelonia mydas. In: Keinath JA, Barnard BE, Musick JA, Bell BA, editors. Proceedings of the 15th annual symposium on sea turtle biology and conservation. NOAA Tech. Memo. NMFS-SEFSC-537. 1996; p. 21–26.
Bostrom BL, Jones TT, Hastings M, Jones DR. Behaviour and physiology: the thermal strategy of leatherback turtles. PLoS ONE. 2010;5(11):e13925.
Cossins AR, Bowler K. Temperature biology of animals. New York: Chapman and Hall; 1987. p. 63–6.
Dingle H. Migration: the biology of life on the move. Oxford: Oxford University Press; 2014.
Donoso M, Dutton PH. Forage area identified for green turtles in northern Chile. In: Proceedings from the 20th annual symposium on sea turtle biology and conservation. NOAA Technical Memorandum NMFS-SEFSC-477. US Department of Commerce (274); 2000.
Dorjets V. Many newer power plants have cooling systems that reuse water. United States Energy Information Administration; 2014. http://www.eia.gov/todayinenergy/detail.cfm?id=14971. Accessed 9 July 2016.
Edwards M, Richardson AJ. Impact of climate change on marine pelagic phenology and trophic mismatch. Nature. 2004;430:881–4.
Eguchi T, Seminoff JA, LeRoux RA, Dutton PH, Dutton DL. Abundance and survival rates of green turtles in an urban environment: coexistence of humans and an endangered species. Mar Biol. 2010;157(8):1869–77.
Eguchi T, Seminoff JA, LeRoux RA, Prosperi D, Dutton DL, Dutton PH. Morphology and growth rates of the green sea turtle (Chelonia mydas) in a northern—most temperate foraging ground. Herpetologica. 2012;68(1):76–87.
Gibbons JW, Sharitz RR. Thermal ecology: environmental teachings of a nuclear reactor site. Bioscience. 1981;31(4):293–8.
Guerra-Correa C, Guerra-Castro C, Bolandos P, Silva A, Garfias P. Sea turtle congregations in discrete temperate shoreline areas in cold northern Chilean coastal waters. In: Proceedings of the 27th annual symposium on sea turtle biology and conservation. Miami, FL: US Department of Commerce NOAANMFS Southeast Fisheries Science Center; 2008.
Hawkes LA, Broderick AC, Godfrey MH, Godley BJ. Investigating the potential impacts of climate change on a marine turtle population. Glob Change Biol. 2007;13:923–32.
Hawkes LA, Broderick AC, Coyne MS, Godfrey MH, Godley BJ. Only some like it hot—quantifying the environmental niche of the loggerhead sea turtle. Divers Distrib. 2007;13:447–57.
Hawkes LA, Broderick AC, Godfrey MH, Godley BJ. Climate change and marine turtles. Endanger Species Res. 2009;7(2):137–54.
Hochachka PW, Somero GN. Biochemical adaptation: mechanism and process in physiological evolution. New York: Oxford University Press, Inc.; 2002.
Hochscheid S, Bentivegna F, Bradhai MN, Hays GC. Overwintering behaviour in sea turtles: dormancy is optional. Mar Ecol Prog Ser. 2007;340:287–98.
Jackson DC. Life in a shell: a physiologist’s view of a turtle. Cambridge: Harvard University Press; 2011.
James MC, Davenport J, Hays GC. Expanded thermal niche for a diving vertebrate: a leatherback turtle diving into near-freezing water. J Exp Mar Biol Ecol. 2006;335:221–6.
Jones TT, Van Houtan KS, Bostrom BL, Ostafichuk P, Mikkelsen J, Tezcan E, Carey M, Imlach B, Seminoff JA, Rands S. Calculating the ecological impacts of animal-borne instruments on aquatic organisms. Methods Ecol Evol. 2013;4(12):1178–86.
Laist DW, Taylor C, Reynolds JE III. Winter habitat preferences for Florida manatees and vulnerability to cold. PLoS ONE. 2013;8(3):e58978.
Langford TEL. Thermal discharges and pollution. In: Thorpe SA, Turekian KK, editors. Encyclopaedia of ocean sciences, vol. 6. New York: Elsevier Science Ltd; 2001. p. 2933–40.
Lemons G, Lewison R, Komoroske L, Gaos A, Lai CT, Dutton P, Eguchi T, LeRoux R, Seminoff JA. Trophic ecology of green sea turtles in a highly urbanized bay: insights from stable isotopes and mixing models. J Exp Mar Biol Ecol. 2011;405(1):25–32.
Limpus CJ, Reed PC. The green turtle, chelonia mydas, in Queensland: a preliminary description of the population structure in a coral reef feeding ground. In: Grigg G, Shine R, Ehmann H, editors. Biology of Australasian frogs and reptiles. Sydney: Royal Zoological Society of New South Wales; 1985. p 47–52.
MacDonald BD, Lewison RL, Madrak SV, Seminoff JA, Eguchi T. Home ranges of East Pacific green turtles, Chelonia mydas, in a highly urbanized temperate foraging ground. Mar Ecol Prog Ser. 2012;461:211–21.
MacDonald BD, Madrak SV, Lewison RL, Seminoff JA, Eguchi T. Fine scale diel movement of the east Pacific green turtle, Chelonia mydas, in a highly urbanized foraging environment. J Exp Mar Biol Ecol. 2013;443:56–64.
Madden N, Lewis A, Davis M. Thermal effluent from the power sector: an analysis of once-through cooling system impacts on surface water temperature. Environ Res Lett. 2013;8(3):035006.
Madrak SV. Influence of temperature on habitat use by East Pacific green turtles (Chelonia mydas) in an urbanized environment. Dissertation. San Diego State University. San Diego, California, UC Davis, Davis, California. Proquest. 2016; 11154, 15867.
McClellan CM, Brereton T, Dell’Amico F, Johns DG, Cucknell A-C, Patrick SC, Penrose R, Ridoux V, Solandt J-L, Stephen E, Votier SC, Williams R, Godley BJ. Understanding the distribution of marine megafauna in the English channel region: identifying key habitats for conservation within the busiest seaway on Earth. PLoS ONE. 2014;9(2):e89720.
McDonald D, Dutton P. Fibropapillomas on sea turtles in San Diego Bay, California. Mar Turt Newsl. 1990;51:9–10.
McDonald D, Dutton P, Mayer D, Merkel K. Review of the green turtles of south San Diego Bay in relation to the operations of the SDG&E South Bay Power Plant. San Diego: San Diego Gas & Electric Co; 1994.
McNab BK. The physiological ecology of vertebrates: a view from energetics. Ithaca: Cornell University Press; 2002. p. 121–9.
Merkel and Associates. San Diego Bay 2011 eelgrass survey. Prepared for: Naval Facilities Engineering Command Southwest, Port of San Diego, San Diego, California, USA; 2011.
Milton S, Lutz P. Natural and human impacts on sea turtles. Oil and sea turtles. Biology, planning, and response. NOAA OR&R technical document 2003 27-34.
Moon D-Y, Mackenzie DS, Owens DW. Simulated hibernation of sea turtles in the laboratory: I. Feeding, breathing frequency, blood pH, and blood gases. J Exp Zool. 1997;278:372–80.
Neill WH, Stevens ED. Thermal inertia versus thermoregulation in “warm” turtles and tunas. Science. 1974;184:1008–10.
Paladino Frank V, O’Connor Michael P, Spotila James R. Metabolism of leatherback turtles, gigantothermy, and thermoregulation of dinosaurs. Nature. 1990;344(6269):858–60.
Prange HD. Energetics of swimming of a sea turtle. J Exp Biol. 1976;64:1–12.
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/ (2015). Accessed 3 Dec 2015.
Roberts K, Collins J, Paxton CH, Hardy R, Downs J. Weather patterns associated with green turtle hypothermic stunning events in St. Joseph Bay and Mosquito Lagoon, Florida. Phys Geogr. 2014;35(2):134–50.
Robinson RA, Crick HQP, Learmonth JA, Maclean IMD, Thomas CD, Bairlein F, Forchhammer MC, Francis CM, Gill JA, Godley BJ, Harwood J, Hays GC, Huntley B, Hutson AM, Pierce GJ, Rehfisch MM, Sims DW, Santos MB, Sparks TH, Stroud DA, Visser ME. Travelling through a warming world. Endanger Species Res. 2009;7:87–99.
Sarmiento-Devia RA, Harrod C, Pacheco AS. Ecology and conservation of sea turtles in Chile. Chelonian Conserv Biol. 2015;14(1):21–33.
SAS Software. SAS Institute Inc., Cary, NC, USA.
Sato K. Body temperature stability achieved by the large body mass of sea turtles. J Exp Biol. 2014;217(20):3607–14.
Seminoff JA. Biology of the East Pacific green turtle, Chelonia mydas agassizii, at a warm temperate feeding area in the Gulf of California, México. Ph.D. thesis, Tucson: University of Arizona; 2000.
Shillinger GL, Swithenbank AM, Bailey H, Bograd SJ, Castelton MR, Wallace BP, Spotila JR, Paladino FV, Piedra R, Block BA. Vertical and horizontal habitat preferences of post-nesting leatherback turtles in the South Pacific Ocean. Mar Ecol Prog Ser. 2011;422:275–89.
Southwood A, Avens L. Physiological, behavioral, and ecological aspects of migration in reptiles. J Comp Physiol B. 2010;180(1):1–23.
Spalding MD, Fox HE, Allen GR, Davidson N, Ferdaña ZA, Finlayson M, Halpern BS, Jorge MA, Lombana A, Lourie SA, Martin KD, McManus E, Molnar J, Recchia CA, Robertson J. Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. Bioscience. 2007;57(7):573–83.
Spotila JR, Foley RE, Schubauer JP, Semlitsch RD, Crawford KM, Standora EA, Gibbons JW. Opportunisitic behavioral thermoregulation of turtles, Pseudomys scripta, in response to microclimatology of a nuclear reactor cooling reservoir. Herpetologica. 1984;40(3):299–308.
Spotila JR, Standora EA. Environmental constraints on the thermal energetics of sea turtles. Copeia 1985;3:694–702.
Spotila JR, O’connor MP, Paladino FV. Thermal biology. In: Musick JA, Lutz PL, editors. The biology of sea turtles. Boca Raton: CRC Press; 1997. p. 297–314.
Stinson ML. Biology of sea turtles in San Diego Bay, California, and the northeastern Pacific Ocean, Master’s thesis. San Diego: San Diego State University; 1984.
SYSTAT version 13. Systat Software, Inc., San Jose, California, USA.
Teixeira TP, Neves LM, Araujo FG. Effects of a nuclear power plant thermal discharge on habitat complexity and fish community structure in Ilha Grande Bay, Brazil. Mar Environ Res. 2009;68:188–95.
Torezani E, Baptistotte C, Mendes SL, Barata PCR. Juvenile green turtles (Chelonia mydas) in the effluent discharge channel of a steel plant, Espirito Santo, Brazil, 2000-2006. J Mar Biol Assoc UK 2010;90(2):233–46.
Turner-Tomaszewicz C, Seminoff JA. Turning off the heat: impacts of power plant decommissioning on green turtle research in San Diego Bay. Coast Manag. 2012;40(1):73–87.
USFWS (United States Fish and Wildlife Service). https://www.fws.gov/refuge/San_Diego_Bay/about.html (2012). Accessed 7 Nov 2016.
Wallace BP, Jones TT. What makes marine turtles go: a review of metabolic rates and their consequences. J Exp Mar Biol Ecol. 2008;356:8–24.
Wibbels T. Diagnosing the sex of sea turtles in foraging habitats. In: Eckert KL, Bjorndal KA, Abreu-Grobois FA, Donnelly M, editors. Research and management techniques for the conservation of sea turtles, vol 4. Blanchard, Pennsylvania, USA: IUCN/SSC Marine Turtle Specialist Group Publication No. 4; 1999.
Witherington BE, Ehrhart LM. Hypothermic stunning and mortality of marine turtles in the Indian River Lagoon System, Florida. Copeia 1989;3:696–703.
Authors’ contributions
This study was conducted as part of the doctoral dissertation of SVM in the Joint Doctoral Program in Ecology through San Diego State University and University of California, Davis. SVM developed methodology, conducted acoustic telemetry and monitoring, performed the statistical analyses and drafted the manuscript. RLL secured funding for the research, participated in study design, helped to draft the manuscript and served as academic advisor and dissertation committee chair for SVM. JAS participated in study design and field research, assisted in securing funding, provided edits for the manuscript and served on the dissertation committee of SVM. TE participated in study design and field research, assisted in securing funding and provided guidance in statistical analyses and edits for the manuscript. The study was conceived by JAS and TE and further developed by RLL and SVM. All authors read and approved the final manuscript.
Acknowledgements
The authors wish to acknowledge members of the Conservation Ecology Lab at San Diego State University and members of the Marine Turtle Ecology and Assessment Program. We thank the following individuals for their tremendous support in the field: J. Bredvik, J. Brower, S. Coates, C. Coppenrath, A. D’Amico, A. Gaos, S. Graham, T. Grimes, D. Ho, C. Jackson, G. Lemons, N. Magana, D. Mahoney, B. MacDonald, R. Moens, N. Nourn, K, Ritchie, A. Miller, D. Prosperi, J. Okuyama, C. Turner-Tomaszewicz and the entire NOAA-NMFS team that assisted with turtle capture. Our gratitude is also extended to R. LeRoux for the invaluable assistance in sonic transmitter attachment, permitting and coordination of turtle capture.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analyzed in this study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
All research and animal handling were carried out under National Marine Fisheries Service Permit #1591 and #16803 and were in compliance with IACUC protocol at San Diego State University.
Funding
Financial and logistical support was provided by San Diego State University, San Diego State University Research Foundation, NOAA Service Southwest Fisheries Science Center, Unified Port of San Diego, the US Navy, Sonotronics Inc. and South Bay Power Plant LLC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Madrak, S.V., Lewison, R.L., Seminoff, J.A. et al. Characterizing response of East Pacific green turtles to changing temperatures: using acoustic telemetry in a highly urbanized environment. Anim Biotelemetry 4, 22 (2016). https://doi.org/10.1186/s40317-016-0114-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40317-016-0114-7